Key Points
A simplified frailty score predicts survival and toxicity in older patients with DLBCL and can inform treatment-intensity decisions.
Full-dose R-CHOP is not superior to R-miniCHOP in older unfit DLBCL patients, whereas older fit patients likely benefit from full-dose R-CHOP.
Abstract
Patients with diffuse large B-cell lymphoma (DLBCL) have a median age of 70 years. Yet, empirical knowledge about the treatment of older patients is limited because they are frequently excluded from clinical trials. We aimed to construct a simplified frailty score and examine survival and treatment-related mortality (TRM) according to frailty status and treatment intensity in an older real-world population with DLBCL. All patients aged ≥70 years diagnosed with DLBCL between 2006 and 2016 in southeastern Norway (N = 784) were included retrospectively and divided into training (n = 522) and validation (n = 262) cohorts. We constructed and validated a frailty score based on geriatric assessment variables and examined survival and TRM according to frailty status and treatment. The frailty score identified 3 frailty groups with distinct survival and TRM, independent of established prognostic factors (2-year overall survival [OS]: fit, 82%; unfit, 47%; frail, 14%; P < .001). For fit patients, full-dose R-CHOP (initial dosage >80%) was associated with better survival than attenuated R-CHOP ([R-miniCHOP]; 2-year OS: 86% vs 70%; P = .012), also in adjusted analyses. For unfit and frail patients, full-dose R-CHOP was not superior to R-miniCHOP, whereas an anthracycline-free regimen was associated with poorer survival in adjusted analyses. A simplified frailty score identified unfit and frail patients with a higher risk for death and TRM, which can aid treatment-intensity decisions in older patients with DLBCL. In this study, fit patients benefited from full-dose R-CHOP, whereas unfit and frail patients had no benefit from full-dose R-CHOP over R-miniCHOP. An online calculator for assessment of the frailty score is available at https://wide.shinyapps.io/app-frailty/.
Introduction
The most common subtype of non-Hodgkin lymphoma, diffuse large B-cell lymphoma (DLBCL), has a median age at diagnosis of 70 years.1 Since the introduction of anti-CD20 antibody treatment >2 decades ago, survival has improved substantially for patients younger than 70 years. In contrast, survival improvement has been modest for older patients.2
Standard treatment for patients aged 60 to 80 years is R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone), which was established through randomized trials.3,4 For patients aged ≥80 years, the attenuated R-CHOP "R-miniCHOP" has been suggested as standard treatment based on a phase 2 clinical trial.5 However, many older patients will not tolerate standard treatment, and further dose reductions take place or low-intensity treatment is often administered. Because most clinical trials exclude all older patients or unfit older patients, treatment protocols are based on data from limited and selected patient cohorts.6,7 Thus, choosing the right treatment intensity can be challenging, especially for unfit patients.
Frailty status determined by a geriatric assessment (GA) predicts survival and treatment-related toxicity in older patients with cancer,8-10 and consensus recommendations suggest using a GA to guide treatment decisions.11-14 Despite these recommendations, GA has not been widely implemented into routine clinic practice or clinical trials.
To the best of our knowledge, no published randomized trial has compared treatment intensity levels for older unfit patients with DLBCL. Only a few prospective nonrandomized studies have examined treatment intensity and survival according to frailty status,8,15-17 and the evidence base for guiding treatment decisions according to frailty status in DLBCL is scarce. Because randomized trials are challenging to perform in this patient population, representative cohort studies examining treatment and outcome according to frailty status are needed.18 The Italian Lymphoma Foundation (FIL) recently conducted a large prospective trial examining outcome according to a simplified GA (sGA).19 However, survival and toxicity according to treatment intensity in the different frailty groups were not examined in detail, and the prognostic value of the sGA was not shown for R-CHOP–treated patients, toxicity, or when adjusted for known prognostic factors. The prospective nature of the study design will also not be fully representative, especially for unfit and frail patients.
We report survival and treatment-related mortality (TRM) according to frailty status and treatment intensity from a large retrospective population-based study of patients with DLBCL aged ≥70 years. To assign patients’ frailty status, we constructed and validated a frailty score based on GA variables obtained from medical records and applied this retrospectively.
Methods
Study design
This is a population-based cohort study in which all patients were identified through the Cancer Registry of Norway (CRN). The CRN has an estimated 98.8% completeness on cancer diagnosis in Norway based on accumulated information from pathology reports, discharge hospital diagnosis and death certificates,20 and treating physicians prospectively report a range of clinical and treatment features to the CRN, including Eastern Cooperative Oncology Group performance status (ECOG PS) and International Prognostic Index (IPI) score. The registry receives data on patients’ vital status from the Norwegian Population Registry.
Additionally, we retrieved detailed information from clinical records of all patients to quality-check data, collect missing data, and obtain information not routinely reported to the CRN. Review of clinical records was performed by 4 physicians (K.T.I., M.A.M., M.R., and H.H.) with help from study nurses. The study was approved by the Norwegian Regional Committees for Medical and Health Research Ethics (REK 2017/1861) and Data Protection Officers at all participating hospitals. The study was conducted in accordance with the Declaration of Helsinki. Individual participant data will not be shared.
Patients
All patients aged ≥70 years diagnosed with DLBCL in the period from 2006 to 2016 in southeastern Norway, with the exception of patients at the Hospital of Southern Norway, were included initially. We excluded patients with primary central nervous system lymphoma or prior lymphoproliferative disease (a concurrent diagnosis of indolent lymphoma or chronic lymphocytic leukemia was allowed), as well as patients diagnosed after death.
Frailty score development
We aimed to create a simplified frailty score that could classify patients into 3 frailty groups based on validated GA variables that can be scored with high quality from data routinely collected in clinical practice. To allow for temporal validation,21 we divided the study cohort into training and validation cohorts based on the time of diagnosis. Candidate variables were selected to cover functional status, comorbidity, nutrition, and age, all key elements of a GA,11,22 and included a modified Katz Activities of Daily Living (ADL),23 the Charlson Comorbidity Index (CCI),24 body mass index (BMI), albumin, the Geriatric Nutritional Risk Index (GNRI),25 and age ≥ 85 years. The GNRI is an adaption of the well-known Nutritional Risk Index26 for older subjects and has shown prognostic significance in patients with cancer, including older patients with DLBCL.27,28 Age ≥ 85 years was chosen as a frailty indicator based on review of the literature. The prevalence of clinical frailty has been described to increase sharply after 85 years,29 and this cutoff has been suggested as a frailty indicator in the literature22,30 and in an expert position paper on older patients with DLBCL.12 The cutoff is also in line with clinical experience that few patients older than 85 years of age will tolerate full-dose R-CHOP treatment. ADL was scored as “dependent” if the patient had limitations in any of the 6 categories, lived in an institution, or received help from home nursing. The cutoffs for GNRI (absent/low, moderate, severe), CCI (0-1, 2, ≥3), albumin (<36 g/L), and BMI (< 25 kg/m2) were based on cutoffs used in the literature and clinical reasoning, with some adjustments to our data set to create sufficiently large groups. The groupings were then examined for associations with OS in the training cohort to evaluate their suitability. The frailty model was created by including all candidate variables in a multivariate Cox regression model for OS in the training cohort, removing variables in a backward stepwise selection process with a 5% significance level as stopping criteria. Further details are provided in supplemental Methods.
Definition of treatment groups
Treatment intensity was divided into 4 categories: full-dose R-CHOP, attenuated R-CHOP, anthracycline-free regimen, and no chemotherapy. Patients were assigned to a category based on “planned treatment,” here defined by the regimen and dosing given at the first cycle. Doxorubicin was used to define R-CHOP dosage, and the cutoff for attenuated R-CHOP was set at an initial doxorubicin dose ≤80% of the standard dose (50 mg/m2) based on cutoffs used in the literature,31-35 clinical reasoning, and the distribution of dose reductions in our cohort. Further details are provided in supplemental Methods.
Outcome variables
Main outcomes were OS and progression-free survival (PFS). Secondary outcome was TRM. Date of death was retrieved from the CRN, whereas information on relapse/progression and TRM was retrieved from clinical records. TRM was defined as deaths occurring during or shortly after treatment, likely caused by acute treatment toxicity. Deaths occurring at a later stage were also registered as TRM if documented in clinical records as a likely result of long-term toxicity. All patients were followed until death or were censored at the end of follow-up (1 December 2017). Further details are provided in supplemental Methods.
Statistical analysis
OS was defined as time from diagnosis to death from any cause or censoring, and PFS was defined as time from diagnosis to progression, relapse, death from any cause, or censoring. Kaplan-Meier estimates were calculated for PFS and OS, and the log-rank test was used to compare curves. Median follow-up was estimated with the reverse Kaplan-Meier method.36,37 Hazard ratios (HRs) for OS and PFS were estimated using Cox regression, and cumulative incidence and subdistribution HRs for TRM were estimated with competing-risk methods.38,39 Missing values were imputed using the multivariate imputation by chained equations (MICE) method.40 Harrell’s C Index41 was calculated to evaluate the predictive performance of the frailty classification. Sensitivity analyses in the form of E-values were calculated to assess how robust the observed association between treatment intensity and survival was to potential unmeasured confounding.42 Baseline characteristics were compared using the χ2 or Fisher’s exact test, when indicated. A P value < .05 was considered significant, and confidence intervals (CIs) were calculated with a 95% confidence level. All analyses were performed using STATA version 16 (StataCorp). Further details are provided in supplemental Methods.
Results
Patient characteristics
Through the CRN, we initially identified 889 patients who met the inclusion criteria. We excluded 9 patients with uncertain histology, 33 patients with primary central nervous system lymphoma, 51 patients who were diagnosed after death, and 12 patients who were lost to follow-up; the final study cohort consisted of 784 patients (supplemental Figure 1). For frailty score development, the final study cohort (N = 784) was divided into a training cohort (522 patients diagnosed between 2009 and 2016) and a validation cohort (262 patients diagnosed between 2006 and 2009). Median follow-up was 4.3 years (interquartile range [IQR], 2.4-5.9) for the training cohort, 9.8 years (IQR, 8.9-11.1) for the validation cohort, and 5.8 years (IQR, 3.2-8.7) for the total cohort. Patient baseline characteristics are shown in Table 1.
A simplified frailty classification predicts survival and TRM
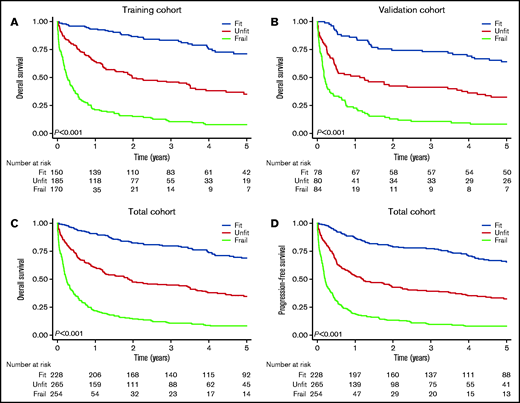
Based on the frailty model development shown in supplemental Methods, the final model consisted of ADL, CCI, GNRI, and age ≥ 85 years (Table 2). Weights for the GA variables were obtained by rounding the HR to the closest 0.5. The frailty score was then created by multiplication of the rounded HRs, producing a frailty score ranging from 1 to 20. An increasing frailty score was associated with decreasing OS (log-rank for trend, P < .001; supplemental Figure 2), and the cutoffs for fit (1), unfit (1.5-3.0), and frail (>3) were based on distribution of the score, clinical reasoning, and Kaplan-Meier curves for OS. With these cutoffs, patients had to have impairment in ≥2 GA domains to be defined as frail; a patient was not classified as frail based on age alone. The definition of fit also allowed patients up to 85 years. In the total cohort, we were able to classify 747 of the 784 patients (95%) as fit (n = 228, 31%), unfit (n = 265, 35%) or frail (n = 254, 34%) (supplemental Figure 1).
The frailty grouping demonstrated prognostic utility in the training and validation cohorts (Figure 1). Applied to the total cohort, the 2-year OS was 82% (95% CI, 77-87) for fit patients, 47% (95% CI, 41-53) for unfit patients, and 14% (95% CI, 10-19) for frail patients (P < .001; Figure 1). The 2-year cumulative incidence of TRM following R-CHOP was 4.7% (95% CI, 2.4-8.2) for fit patients, 13% (95% CI, 8.7-18) for unfit patients, and 24% (95% CI, 14-35) for frail patients (supplemental Figure 3). The frailty classification also predicted survival when analyses were restricted to patients receiving R-CHOP (P < .001; supplemental Figure 4).
Survival by frailty group. Overall survival by frailty group in the training cohort (A), the validation cohort (B), and the total cohort (C). (D) PFS by frailty group in the total cohort.
Survival by frailty group. Overall survival by frailty group in the training cohort (A), the validation cohort (B), and the total cohort (C). (D) PFS by frailty group in the total cohort.
Frail and unfit patients were more often older and had higher IPI score, Ann Arbor stage, and ECOG PS than the fit patients and were more often the recipients of anthracycline-free regimens or did not receive chemotherapy (supplemental Table 1). Nevertheless, the frailty grouping retained its prognostic utility for survival and TRM, also when adjusted for age group, IPI score, Ann Arbor stage, and ECOG PS and when restricted to patients receiving chemotherapy (Table 3; supplemental Table 2) or R-CHOP (supplemental Table 3). Harrell’s C Index for 2-year OS for the model restricted to patients receiving chemotherapy was 0.71 for the unadjusted model and 0.77 for the adjusted model, indicating a robust model. Additionally, the frailty classification retained prognostic utility when stratified for age group, IPI score, and Ann Arbor stage (supplemental Figures 5-7). An online calculator for assessment of the frailty score is available at https://wide.shinyapps.io/app-frailty/.
Next, we analyzed survival and TRM according to treatment intensity and stratified by frailty group. Comparisons were performed at 2 years because most patients who received anthracycline-free chemotherapy were not alive beyond this point (Figure 2). Additionally, we expected that this would limit dilution of a possible treatment effect due to nonlymphoma-related deaths.
Survival by treatment intensity level in fit, unfit and frail patients. OS and PFS for different treatment intensity levels in fit patients (A-B), unfit patients (C-D), and frail patients (E-F).
Survival by treatment intensity level in fit, unfit and frail patients. OS and PFS for different treatment intensity levels in fit patients (A-B), unfit patients (C-D), and frail patients (E-F).
Fit patients benefit from full-dose R-CHOP
In the fit group, treatment with full-dose R-CHOP was associated with better survival than attenuated R-CHOP (2-year OS, 86% vs 70%, P = .012; 2-year PFS, 85% vs 63%, P = .002; Figure 2). The difference remained significant when adjusted for IPI score, Ann Arbor stage, sex, and time period in a Cox regression model (Table 4). More often, fit patients receiving attenuated R-CHOP were older and had heart disease and ECOG PS ≥ 2 than did patients receiving full-dose R-CHOP (supplemental Table 4). Of note, 21 patients aged 80 to 84 years received full-dose R-CHOP; when adding age ≥ 80 years and heart disease to the multivariate model, the risk of death remained higher with attenuated R-CHOP vs full-dose R-CHOP (2-year OS: HR, 2.59; 95% CI, 1.12-6.00; P = .026; E-value, 3.25; 2-year PFS: HR, 2.24; 95% CI, 1.06-4.72; P = .034; E-value, 2.88). When ECOG PS was added to the model, the survival difference was no longer significant. The risk of TRM was not significantly different between full-dose R-CHOP and attenuated R-CHOP (supplemental Table 5). Eight fit patients received an anthracycline-free regimen (rituximab, cyclophosphamide, vincristine, prednisone). The majority were ≥80 years of age and had received radiation, and 3 of them later received R-CHOP. Six patients did not receive chemotherapy. They all were older, had limited disease, and had obtained complete remission after radiation and/or surgery.
Unfit patients benefit from R-CHOP, but attenuated R-CHOP is sufficient
For unfit patients, survival following full-dose R-CHOP was not superior to attenuated R-CHOP (2-year OS, 58% vs 53%; P = .347; 2-year PFS, 53% vs 51%, P = .617; Figure 2; supplemental Figure 8). After adjusting for IPI score, Ann Arbor stage, sex, and time period in a Cox regression model, there was still no difference in the risk of death between full-dose R-CHOP and attenuated R-CHOP as a dichotomous variable (Table 4) and as a continuous variable (2-year OS: HR, 0.99; 95% CI, 0.98-1.00; P = .122; 2-year PFS: HR, 0.99; 95% CI, 0.98-1.00; P = .169). Unfit patients receiving full-dose R-CHOP were younger and had a more advanced Ann Arbor stage than did patients receiving attenuated R-CHOP (supplemental Table 6). After adding age ≥ 80 years, heart disease, and ECOG PS ≥ 2 to the multivariate model, there was still no difference in the risk of death between full-dose R-CHOP and attenuated R-CHOP (2-year OS: HR, 1.29; 95% CI 0.78-2.13; P = .319; 2-year PFS: HR, 1.13; 95% CI, 0.70-1.84; P = .614). The same was observed when the adjusted model was stratified for age older or younger than 80 years (data not shown). The risk of TRM was also similar for full-dose R-CHOP and attenuated R-CHOP (supplemental Table 5).
Importantly, unfit patients receiving an anthracycline-free regimen had significantly poorer survival than did those who received attenuated R-CHOP (2-year OS, 27% vs 53%; P = .001; 2-year PFS, 20% vs 51%; P < .001; Figure 2). The risk of death for the anthracycline-free group remained higher than for the full-dose R-CHOP group and the attenuated R-CHOP group when adjusted for IPI score, Ann Arbor stage, sex, and time period in a Cox regression model (Table 4). Unfit patients who received an anthracycline-free regimen were older and more often had heart disease than did patients who received attenuated R-CHOP (supplemental Table 6). Even so, the risk of death remained higher for patients receiving an anthracycline-free regimen than for patients treated with attenuated R-CHOP when adding age ≥ 80 years, heart disease, and ECOG PS ≥ 2 to the multivariate model (2-year OS: HR, 2.22; 95% CI, 1.33-3.71, P = .002; E-value, 2.86; 2-year PFS: HR, 2.57; 95% CI, 1.57-4.20; P < .001; E-value, 3.23). This was also observed when the analysis was restricted to patients aged ≥80 years (data not shown). However, we did not observe a difference in the risk of TRM (supplemental Table 5). Unfit patients not receiving chemotherapy had significantly poorer survival than did patients receiving an anthracycline-free regimen when adjusted for IPI score, Ann Arbor stage, sex, and time period in a Cox regression model (data not shown).
Frail patients have poor survival but some derive benefit from attenuated R-CHOP
Only 15 of the frail patients received full-dose R-CHOP; compared with attenuated R-CHOP there was no difference in survival (Figure 2; Table 4). Frail patients receiving anthracycline-free chemotherapy had poorer survival than did those receiving attenuated R-CHOP (2-year OS, 12% vs 28%; P = .009; 2-year PFS, 11% vs 26%; P = .007; Figure 2). The inferior survival of the anthracycline-free group remained significant when compared with the attenuated R-CHOP group (Table 4) and the total R-CHOP group (full dose and attenuated combined) in a Cox regression model adjusted for IPI score, Ann Arbor stage, sex, and time period (2-year OS: HR, 1.70; 95% CI, 1.15-2.53; P = .008; E-value, 2.24; 2-year PFS: HR, 1.70; 95% CI, 1.15-2.51; P = .008; E-value, 2.24). More often, patients in the anthracycline-free group were older and had heart disease compared with those who received R-CHOP (supplemental Table 7). After adjusting for age ≥ 80 years, heart disease, and ECOG PS ≥ 2 in the multivariate model, the difference between R-CHOP and anthracycline-free chemotherapy remained significant (2-year OS: HR, 1.64; 95% CI, 1.07-2.52; P = .023; E-value, 2.17; 2-year PFS: HR, 1.62; 95% CI, 1.06-2.47; P = .026; E-value, 2.14). However, there was no difference in the risk of TRM between the groups (supplemental Table 5). Frail patients who did not receive chemotherapy had significantly poorer survival than did those receiving an anthracycline-free regimen, also when adjusted for IPI score, Ann Arbor stage, sex, and time period in a Cox regression model (data not shown).
Discussion
We used a large population-based cohort of 784 patients with DLBCL aged ≥70 years to develop a stratification tool based on established geriatric frailty indicators. The resulting frailty score robustly predicted survival and TRM independent of established prognostic factors, also in R-CHOP–treated patients, and is easy to apply in daily oncological practice. Compared with the other regimens, R-CHOP was associated with superior survival in all frailty groups, without a significant increase in TRM. Although full-dose R-CHOP was associated with superior survival in fit patients, it was not better than R-miniCHOP in the unfit and frail groups.
A full GA, as well as simplified versions, has the ability to predict survival and toxicity in older patients with hematologic malignancies.8-10,43,44 FIL recently conducted a large prospective trial in which they propose an sGA, consisting of functional status, comorbidity, and age > 80 years, for older patients with DLBCL.19 Dissimilarities between this study and their study include the prospective design and larger training cohort in the latter. Additionally, their proposed Elderly Prognostic Index, consisting of sGA, IPI, and hemoglobin, has been validated externally, as opposed to our temporal validation. However, our retrospective population-based design with near-complete coverage is likely more representative, especially for unfit and frail patients. Additionally, we demonstrate that the frailty score is prognostic for survival and toxicity independent of established prognostic factors, also in R-CHOP–treated patients, whereas sGA’s ability to predict survival and toxicity in adjusted analyses and R-CHOP–treated patients has not been demonstrated. The fit group identified by the sGA had similar survival as our fit group, despite a stricter age cutoff for fit in their study (<80 years vs <85 years), although there were more patients with Ann Arbor stage I/II disease in our fit group (53% vs 34%). In contrast, the frail group had better survival than in our study (2-year OS, 40-50% vs 14%), despite more patients with Ann arbor stage III/IV disease (68% vs 60%). This could be due to our population-based design for which we managed to include more severely frail patients. Moreover, the inclusion of nutritional status in our frailty scoring may have improved the accuracy of patient stratification.
An important limitation of our study is selection bias in treatment allocation, which could overestimate treatment benefits. We tried to minimize this by stratifying patients by frailty status, comparing treatment groups that were likely to be most homogenous, defining treatment intensity by initial dosage, and adjusting for potential confounders. However, adjustment for all possible confounders is impossible in an observational design. Nevertheless, sensitivity analyses were performed on the adjusted Cox models that imply that considerable unmeasured confounding would be needed to explain away the observed survival differences, especially in the unfit group. Here, relatively high E-values, together with a substantial observed survival difference, support the finding that R-CHOP was superior to anthracycline-free chemotherapy in older unfit patients. Insufficient statistical power could be a reason for the lack of observed survival difference for R-CHOP dosage in unfit and frail patients. However, in unfit patients, a relatively large cohort, together with a selection bias that would most likely draw in the direction toward better survival for full-dose R-CHOP, strongly support our finding that full-dose R-CHOP is not superior to attenuated R-CHOP in older unfit patients. The lack of difference in the risk of TRM within frailty groups could also be due to insufficient power, and long-term TRM could be underreported.
Randomized trials in older patients with DLBCL, who likely make up a high proportion of fit patients, have also demonstrated good survival and acceptable toxicity with full-dose R-CHOP.3,4,45 To our knowledge, there is only 1 published randomized trial in older fit patients with DLBCL defined by a GA.46 The investigators did not find any difference in survival or toxicity between full-dose R-CHOP and a less intensive regimen consisting of rituximab, cyclophosphamide, epirubicin, vinblastine and prednisone; however, the latter regimen consisted of a relatively high epirubicin dose that was equivalent to two thirds of doxorubicin, 50 mg/m2. In 2 prospective cohort studies by FIL,43,47 R-CHOP at a >70% dose was associated with better survival than was less intensive therapy in fit patients. Furthermore, in a retrospective study of a likely fit population older than 80 years, patients had an excellent outcome, which was comparable to that in younger patients, when treated with full-dose R-CHOP.34 Collectively, these results support our finding that older fit patients treated with R-CHOP have a relatively good prognosis and that full-dose R-CHOP could be superior to attenuated R-CHOP. Consensus reports recommend full-dose R-CHOP for fit patients younger than 80 years and attenuated R-CHOP for fit patients older than 80 years.14,48 Our data indicate that fit patients up to 85 years of age may benefit from full-dose R-CHOP; however, because of the retrospective design and a data set that is limited for this patient group (53 fit patients aged 80-84 years of whom 21 received full-dose R-CHOP), these patients must be followed cautiously if given the full dose, and dose modifications must be made promptly when deemed necessary
To our knowledge, there are no published randomized trials comparing treatment intensity for older unfit patients defined by a GA. Two phase 2 trials with ofatumumab/R-miniCHOP in patients aged ≥80 years,5,49 which likely included unfit and fit patients, demonstrated 2-year OS rates of 59% to 65% with acceptable toxicity. A halted phase 2 trial15 with obinutuzumab-miniCHOP for unfit patients aged ≥65 years demonstrated a 2-year OS of 68%. In the study by FIL,19,47 unfit patients had similar OS following full-dose R-CHOP (≥70%) and attenuated R-CHOP (<70%) but poorer survival following an anthracycline-free regimen, although the results were not adjusted for possible confounders. Registry-based and smaller retrospective studies8,31-33,50 report better outcomes with an anthracycline-containing regimen, also for older patients and for patients with comorbidity. None of these studies stratified their analyses according to frailty status.
Unfit patients receiving anthracycline-free regimens had inferior survival in our study, and the majority of these were 80 to 84 years of age. Many clinicians may hesitate to give intensive treatment to this group simply because of their age. Additionally, some clinical guidelines recommend giving therapy that is less intensive than R-CHOP to unfit patients.48 Of note, this study and the studies mentioned above indicate that many of these patients are undertreated with an anthracycline-free regimen, and unfit patients should receive attenuated R-CHOP when possible. The similar survival rates following attenuated and full-dose R-CHOP in unfit patients suggest that these patients may have an increased half-life of drugs or drug metabolites, making attenuated R-CHOP a sufficient regimen.
Most frail patients in our study did not receive chemotherapy or they received an anthracycline-free regimen, and all treatment groups had relatively poor survival. Nevertheless, some frail patients seem to benefit from R-CHOP. The majority of frail patients who received R-CHOP were <80 years of age without heart disease and with a moderate/severe GNRI. Our finding suggests that the majority of frail patients were not candidates for R-CHOP, but some patients may be classified as frail primarily as a result of the clinical impact of lymphoma; these patients can benefit from R-CHOP treatment. The use of prephase treatment (steroids alone or combined with vincristine) can improve patients’ status, making them suitable for R-CHOP.4
To the best of our knowledge, this is the largest published study investigating survival and toxicity according to frailty status and treatment in older patients with DLBCL. The population-based design with near-complete coverage, detailed and quality-checked data, long follow-up, and few missing data allowed the development and validation of a robust frailty classification that is especially suited for a real-world older population with DLBCL. Additional strengths of the frailty score include integration of nutritional status, a classification that does not identify patients as frail because of age alone, and the ability to classify patients up to 85 years of age as fit. Because the frailty score is based on data that are routinely collected in clinical practice, the additional time needed to calculate the score is only a few minutes with the use of an online calculator (https://wide.shinyapps.io/app-frailty/). The retrospective design did not allow inclusion of all elements of a complete GA, but our objective was to create a frailty classification that is also feasible to apply retrospectively; we managed to identify 3 distinct groups with the sGA. The observed outcomes were estimated without structured GA interventions, which could have reduced treatment toxicity.51,52 The large cohort and detailed data also allowed comparison of more homogenous treatment groups and adjustment for possible confounders.
In this large population-based cohort of older DLBCL patients, we show that a frailty classification that is easily applicable in everyday clinical practice is a strong predictor for survival and TRM, independent of established prognostic factors and treatment, and could help to inform treatment decisions in older patients with DLBCL. R-CHOP was associated with superior survival in all frailty groups, without increased TRM. Full-dose R-CHOP was associated with better survival than attenuated R-CHOP in fit patients. Importantly, full-dose R-CHOP was not superior to attenuated R-CHOP in unfit and frail patients. Our results support existing evidence on the importance of anthracycline in treating DLBCL, also in older, unfit, and even some frail patients. Furthermore, R-miniCHOP seems to be a sufficient regimen in unfit and frail patients, possibly sparing them the undue toxicity associated with full-dose treatment.
Our results support that age alone should not be a contraindication for R-CHOP treatment in clinical practice and underscore the importance of an objective GA for individualizing treatment intensity in this patient group. However, caution should be exercised when recommending full-dose R-CHOP for fit patients older than 80 years of age because of the retrospective design and limited number of patients in this category in our study. Our proposed frailty score and the observed survival benefits from individualized R-CHOP dosing warrant further validation in a prospective setting.
Acknowledgments
The authors thank Astrid Bergrem (Lovisenberg Hospital), Abdulkarim Hilli (Diakonhjemmet Hospital), Martin Ruppert and Sameer Bhargava (Bærum Hospital), and Janicke Nilsson and Øyvind Øverli for assistance with assembling data from local hospitals. They also thank Tom Børge Johannesen (Cancer Registry of Norway) for assistance and useful input and Ole Christian Lingjærde for help with developing the online frailty calculator.
This work was supported by grants from the South-Eastern Norway Regional Health Authority and the Norwegian Cancer Society.
Authorship
Contribution: H.H., M.B., E.B.S., S.R., and K.T.I. designed the study; K.T.I., H.H., M.A.M., M.R., L.S.R., D.A.B., L.F.R., and M.S. collected clinical data; K.T.I., H.H., M.B., E.B.S., S.R., M.B.J., and K.L. analyzed and interpreted the data; K.T.I. created all tables and figures, as well as wrote the manuscript with input from all coauthors; and all authors edited and approved the final version of the manuscript.
Conflict-of-interest disclosures: The authors declare no competing financial interests.
Correspondence: Harald Holte, Department of Oncology, Oslo University Hospital, Post Box 4953 Nydalen, 0424 Oslo, Norway; e-mail: hhe@ous-hf.no.
References
Author notes
Presented in abstract form at the (virtual) Nordic Lymphoma Plenary Meeting, 5 November 2020.
Requests for data sharing may be submitted to Harald Holte (hhe@ous-hf.no).
The full-text version of this article contains a data supplement.