Key Points
Adding venetoclax to FluBu2 reduced-intensity conditioning transplant did not impair engraftment or induce excessive graft-versus-host disease.
Monitoring measurable residual disease by ultra-sensitive duplex sequencing revealed complex clonal dynamics before and after transplant.
Abstract
Adding the selective BCL-2 inhibitor venetoclax to reduced-intensity conditioning chemotherapy (fludarabine and busulfan [FluBu2]) may enhance antileukemic cytotoxicity and thereby reduce the risk of posttransplant relapse. This phase 1 study investigated the recommended phase 2 dose (RP2D) of venetoclax, a BCL-2 selective inhibitor, when added to FluBu2 in adult patients with high-risk acute myeloid leukemia (AML), myelodysplastic syndromes (MDS), and MDS/myeloproliferative neoplasms (MPN) undergoing transplant. Patients received dose-escalated venetoclax (200-400 mg daily starting day −8 for 6-7 doses) in combination with fludarabine 30 mg/m2 per day for 4 doses and busulfan 0.8 mg/kg twice daily for 8 doses on day −5 to day −2 (FluBu2). Transplant related–toxicity was evaluated from the first venetoclax dose on day −8 to day 28. Twenty-two patients were treated. At study entry, 5 patients with MDS and MDS/MPN had 5% to 10% marrow blasts, and 18 (82%) of 22 had a persistent detectable mutation. Grade 3 adverse events included mucositis, diarrhea, and liver transaminitis (n = 3 each). Neutrophil/platelet recovery and acute/chronic graft-versus-host-disease rates were similar to those of standard FluBu2. No dose-limiting toxicities were observed. The RP2D of venetoclax was 400 mg daily for 7 doses. With a median follow-up of 14.7 months (range, 8.6-24.8 months), median overall survival was not reached, and progression-free survival was 12.2 months (95% confidence interval, 6.0-not estimable). In patients with high-risk AML, MDS, and MDS/MPN, adding venetoclax to FluBu2 was feasible and safe. To further address relapse risk, assessment of maintenance therapy after venetoclax plus FluBu2 transplant is ongoing. This study was registered at clinicaltrials.gov as #NCT03613532.
Introduction
Cytogenetic and molecular risk profiles identify patients with myelodysplastic syndromes (MDS) or acute myeloid leukemia (AML) at high risk of disease relapse and short overall survival (OS) even after allogeneic hematopoietic cell transplantation (allo-HCT).1,2 Persistent genomic evidence of measurable residual disease (MRD) is another determinant of disease relapse.3-6 Although myeloablative conditioning strategies may have the potential to overcome these obstacles, not all patients are candidates due to advanced age or medical comorbidities, and thus reduced-intensity conditioning (RIC) approaches are required.7 However, increasing the intensity of conditioning therapy is inadequate to overcome the negative impact of select high-risk mutations, especially TP53.2 Optimizing the antileukemic activity of an RIC chemotherapy regimen for patients with these high-risk disease features is one strategy to prevent posttransplant relapse.
BH3 profiling AML myeloblasts at diagnosis has identified subpopulations with decreased mitochondrial apoptotic priming status (readiness to undergo apoptosis) that may contribute to disease relapse.8,9 Myeloblasts with relatively low apoptotic baseline priming status exhibit BCL-2 dependence, and this vulnerability can be exploited with venetoclax, a highly selective, orally bioavailable BCL-2 inhibitor.8,9 Based on high response rates and improved OS, venetoclax was approved in combination with low-dose cytarabine10 or hypomethylating agents for the treatment of patients with newly diagnosed AML ineligible for intensive chemotherapy.11 We hypothesized that the addition of venetoclax to RIC chemotherapy could reduce the risk of posttransplant relapse. Here, we report the safety and preliminary efficacy of adding venetoclax to fludarabine and busulfan (FluBu2) chemotherapy in patients undergoing transplant for high-risk MDS, MDS/myeloproliferative neoplasms (MDS/MPN), and AML.
Methods
Study participants
This phase 1 clinical trial was conducted at the Dana-Farber Cancer Institute and Brigham and Women’s Hospital (Boston, MA). The study was approved by the Dana-Farber Cancer Institute Institutional Review Board and was conducted in accordance with the principles of the Declaration of Helsinki. This study was registered at clinicaltrials.gov as #NCT03613532. All patients provided written informed consent before enrollment.
Patients were enrolled from October 2018 through January 2020. Eligible patients were at least 18 years old and had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 to 2, adequate organ function, an available 8 of 8 HLA-matched related or unrelated donor with peripheral blood stem cells as source, and a diagnosis of one of the following: (1) high-risk AML defined as adverse risk per European LeukemiaNet (ELN) criteria,12 secondary (history of antecedent hematologic malignancy or therapy related) or secondary-type ontogeny,13 or persistent MRD by multiparameter flow cytometry (≥0.1%) despite morphologic remission14,15 ; (2) high-risk MDS defined as International Prognostic Scoring System intermediate-2 or high at diagnosis, therapy-related, or mutation in TP53 or in the RAS pathway2 ; or (3) high-risk chronic myelomonocytic leukemia or MDS/MPN-unclassifiable defined by the presence of trisomy 8, chromosome 7 abnormalities, or complex karyotype16 or a mutation in ASXL1.17 Due to recent frontline venetoclax combination therapy approval in elderly patients with AML, the protocol was subsequently amended to allow prior venetoclax exposure. Patients with MDS or MDS/MPN were required to enter the study with ≤10% marrow blasts, and those with AML were required to be in complete remission/complete remission with incomplete hematologic recovery (CR/CRi) (<5% marrow blasts) according to morphologic examination. Persistent flow or next-generation sequencing (NGS) MRD on screening marrow was allowed but not required. Patients with a history of prior allo-HCT or eligible to receive a myeloablative transplant were excluded.
Study design and treatment
The primary objective was to determine the safety, maximum tolerated dose (MTD)/recommended phase 2 dose (RP2D), and schedule of venetoclax in combination with RIC chemotherapy, FluBu2, for patients with high-risk AML, MDS, and MDS/MPN overlap syndromes undergoing allo-HCT. The secondary objectives included estimation of OS, progression-free survival (PFS), CR rate, nonrelapse mortality (NRM), cumulative incidence of relapse, and acute and chronic graft-versus-host-disease (GVHD). Following the determination of MTD/RP2D using a standard 3 + 3 design, a 10-patient dose expansion cohort was used to confirm safety. Patients who received at least 1 dose of venetoclax on trial were considered evaluable for all study end points.
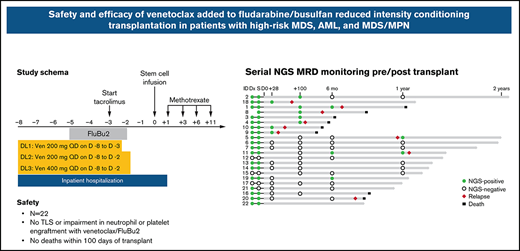
Patients were hospitalized from day −8 until at least day 1 for monitoring of tumor lysis syndrome (TLS). Venetoclax was administered without intra-patient ramp-up starting on day −8 (dose level [DL] 1, 200 mg per day on days −8 through −3 [6 doses]; DL2, 200 mg per day on days −8 through −2 [7 doses]; and DL3, 400 mg per day on days −8 through −2 [7 doses]). FluBu2 consisted of fludarabine 30 mg/m2 per day administered as a bolus intravenous infusion over ∼30 minutes once a day on days −5 through −2 and busulfan 0.8 mg/kg administered intravenously twice daily (∼12 hours apart) over 3 hours on days −5 through −2 for a total of 8 doses. Allopurinol was given on days −8 through −2. Peripheral blood stem cells were infused on day 0 (target dose of 5 × 106 CD34+ cells/kg). GVHD prophylaxis included tacrolimus (beginning on day −3, target trough 5-10 ng/mL) and methotrexate (5 mg/m2 on days 1, 3, 6, and 11). Tacrolimus was tapered beginning on day 100 at the treating physician’s discretion. Post-HCT cyclophosphamide, antithymocyte globulin, or T-cell depletion was not allowed. Granulocyte colony–stimulating factor (5 µg/kg per day) was administered subcutaneously the day after the last methotrexate dose until the absolute neutrophil count (ANC) was ≥0.5 × 109/L for at least 2 consecutive days. Antibiotic and antiviral prophylaxis were given according to institutional guidelines. Posttransplant maintenance therapy was not planned but was permitted in an isolated BCR-ABL mutated AML case.
The dose-limiting toxicity (DLT) period began from the first dose of venetoclax (day −8) to day 28 posttransplant (37 days). A DLT was defined as an adverse event (AE) definitely, probably, or possibly related to the study treatment (graded by using National Cancer Institute Common Toxicity Criteria for Adverse Events, version 5.0) including any treatment-related death, primary graft failure, delay in neutrophil engraftment (defined as failure to achieve an ANC ≥0.5 × 109/L by day 28 on at least 2 separate occasions >2 days apart by day 28), grade 4 or higher TLS or nonhematologic organ toxicity, or grade 3 or higher veno-occlusive disease. Common and expected transplant-related complications such as grade 3 to 4 mucositis or acute GVHD were not considered DLT events.
Safety and response assessments
Acute and chronic GVHD events were graded at each study visit.18,19 Response assessments were performed following ELN criteria for AML,12 International Working Group criteria for MDS,20 and the proposed international consortium for MDS/MPN21 at screening and at day 100, 6 months, and 12 months after transplantation.
Sequencing and mutational analysis
Clinical and research-level NGS assays were performed on samples collected at screening (pretransplant) and posttransplant. The clinical 88 gene targeted sequencing assay (sensitivity estimated to be 3%) was performed in a Clinical Laboratory Improvement Amendments–certified laboratory in real time.22 A research-level duplex NGS assay was used in screening (pretransplant) and in posttransplant MRD surveillance samples (59 bone marrow, 11 peripheral blood) allowing for more sensitive detection of persistent variants (supplemental Table 1).
The supplemental Materials provide additional methods.
Statistical analysis
Clinical characteristics and safety and laboratory data were summarized descriptively. Wilcoxon-rank-sum tests or Wilcoxon signed-rank tests were used as appropriate. The maximum grade for each type of AE was recorded for each patient. OS and PFS were estimated by using the Kaplan-Meier method, and the log-rank test was used for group comparison. Cumulative incidence of NRM and relapse were estimated in the competing risks framework treating each other as a competing event. Cumulative incidence of acute and chronic GVHD were also estimated in the competing risks framework treating relapse or death without developing GVHD as a competing event. The Gray test was used for group comparison of cumulative incidences.23 All P values were two-sided at the significance level of .05 unless otherwise stated. All analyses were performed by using SAS 9.4 (SAS Institute, Inc., Cary, NC), and R version 3.6.1 (the CRAN project, www.cran.r-project.org).
Results
Patient and disease characteristics
The characteristics of all 22 patients are detailed in Table 1 and Figure 1. Three patients in each cohort were treated at DL1 and DL2, and six patients were treated at DL3 during dose escalation. An additional 10 patients were enrolled into an expansion cohort at the highest planned DL (ie, DL3). There were 7 patients with AML (6 in CR and 1 in CRi), 13 patients with MDS (4 in CR, 2 in marrow CR with hematologic improvement [HI], 3 in marrow CR without HI, and 4 with excess blasts), and 2 patients with MDS/MPN (1 in CR and 1 with excess blasts). Five patients with AML had pretransplant bone marrow aspirate samples assessed by using multiparametric flow cytometry. Two patients with AML had flow MRD <0.02%, and 3 had flow MRD >0.02%. Six patients had therapy-related MDS/AML after treatment of lymphoid malignancies (27%). Three (14%) of 22 patients were previously treated with venetoclax. The ECOG PS was 2 in 10 patients (45%) and an Hematopoietic Cell Transplantation–specific Comorbidity Index (HCT-CI) score ≥4 in 12 patients (55%). Pretransplant TP53 mutations were detected in 12 patients (55%).
Baseline disease and genetic profiles of study patients. Comutation plot of diagnostic mutations amenable to MRD tracking. Columns represent individual patients, and rows represent clinical variables or the presence of mutation(s) identified at diagnosis or mutations at screening with VAF ≥2%. This VAF cutoff is suggestive of a diagnostic mutation that was not confirmed at diagnosis due to lack of diagnostic sample or technical assay differences. Asterisk represents a BCR-ABL–mutated case (detected by reverse transcriptase polymerase chain reaction).
Baseline disease and genetic profiles of study patients. Comutation plot of diagnostic mutations amenable to MRD tracking. Columns represent individual patients, and rows represent clinical variables or the presence of mutation(s) identified at diagnosis or mutations at screening with VAF ≥2%. This VAF cutoff is suggestive of a diagnostic mutation that was not confirmed at diagnosis due to lack of diagnostic sample or technical assay differences. Asterisk represents a BCR-ABL–mutated case (detected by reverse transcriptase polymerase chain reaction).
Safety
All planned venetoclax doses were administered without interruption. The frequency and grade of toxicities were similar to those observed with FluBu2 alone.23 No patients experienced a DLT at any dose, and thus an MTD was not reached. Venetoclax 400 mg daily for 7 doses starting days −8 through −2 was identified as the RP2D.
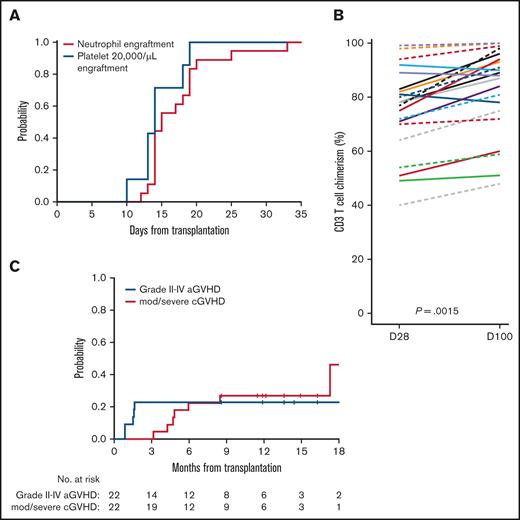
The ANC did not decline below 0.5 × 109/L in 4 patients (18%). Among 18 patients with conditioning-induced neutropenia, the median time to neutrophil recovery was 15 days (range, 12-33 days) (Figure 2A). The platelet count did not decrease below 20 000/μL in 14 patients (64%). In the remaining 8 patients, the median time to achieve a platelet level >20 000/μL was 14 days (range, 10- 19 days). The day 28 and day 100 median donor-derived chimerism values for leukocytes were 98% (range, 85%-100%) and 99% (range, 69%-100%), CD33+ granulocytes were 100% (range, 97%-100%) and 100% (range, 94%-100%), and CD3 T cells were 78% (range, 40%-99%), and 89% (range, 48%-100%), respectively (Figure 2B). Among 22 patients, the median marrow chimerism at day 100 was 98% (range, 53%-100%). No patients experienced primary or secondary graft failure.
Engraftment, donor chimerism, and GVHD rate after venetoclax plus FluBu2 allo-HCT. (A) No evidence for impairment in neutrophil (red) and platelet (blue) engraftment. (B) CD3 T-cell donor chimerism was evaluated at days 28 and 100. In the T-cell chimerism plot, individual patients’ chimerism are connected by lines at days 28 and 100. The black solid line connects 2 medians at days 28 and 100 (P = .0015). (C) Risk for acute (blue) and chronic (red) GVHD were plotted. No grade IV acute GVHD (aGVHD) events occurred. cGVHD, chronic GVHD; D, day; mod/sev, moderate/severe.
Engraftment, donor chimerism, and GVHD rate after venetoclax plus FluBu2 allo-HCT. (A) No evidence for impairment in neutrophil (red) and platelet (blue) engraftment. (B) CD3 T-cell donor chimerism was evaluated at days 28 and 100. In the T-cell chimerism plot, individual patients’ chimerism are connected by lines at days 28 and 100. The black solid line connects 2 medians at days 28 and 100 (P = .0015). (C) Risk for acute (blue) and chronic (red) GVHD were plotted. No grade IV acute GVHD (aGVHD) events occurred. cGVHD, chronic GVHD; D, day; mod/sev, moderate/severe.
The most common nonhematologic grade 3 AEs regardless of attribution included alanine aminotransferase increase (n = 3), diarrhea (n = 3), oral mucositis (n = 2), and maculo-papular rash (n = 4) (Table 2). Grade 3 febrile neutropenia was observed in 2 patients (9%) and grade 4 sepsis in 1 patient (5%). During the DLT period, no new bacterial, viral, or fungal infections were reported. There was no evidence of TLS or veno-occlusive disease. No deaths occurred within 100 days of transplant.
Acute GVHD occurred in 12 of 22 patients (grade I, n = 7; grade II, n = 4; grade III, n = 1). The cumulative incidence of grade II to IV acute GVHD events at 6 months was 23% (95% confidence interval [CI], 8-42) (Figure 2C). One patient experienced grade III acute GVHD involving the skin and gut at day 55, which was complicated by grade IV acute kidney injury. No grade IV acute GVHD event was observed. The cumulative incidence of moderate and severe chronic GVHD at 1 year was 27% (95% CI, 11-47).
Outcomes
Overall, 9 (41%) of 22 patients had morphologic relapse (defined as ≥5% blasts in the marrow or peripheral blood), including 3 (14%) before day 100. In this conditioning chemotherapy trial, there was no planned posttransplant antileukemic treatment. One patient with known BCR-ABL mutation was permitted to continue post–allo-HCT ponatinib maintenance therapy. Early intervention for persistent NGS-detected MRD or falling chimerism was left to investigator discretion and commonly involved tapering immune suppression when clinically feasible. Two of the 9 patients who had morphologic relapse later achieved second remission (CR2; one patient after tapering immunosuppression and one patient after chemotherapy plus donor lymphocyte infusion). Four of the 5 patients with AML in morphologic CR at day 100 with available flow MRD had negative test results (<0.02%) (supplemental Table 2). Of the 3 patients who received a venetoclax-based combination regimen (decitabine/venetoclax) before trial, all had TP53-mutated disease (1 t-MDS and 2 AML) and 2 ultimately had morphologic relapse on study.
The median follow-up time among 14 surviving patients was 14.7 months (range, 8.6-24.8). For the entire cohort, OS and PFS at 1 year was 67% (95% CI, 43-83) and 53% (95% CI, 31-72), respectively (Figure 3A). Median OS has not been reached, and median PFS was 12.2 months (95% CI, 6.0-not estimable). The cumulative incidence of relapse and NRM at 1 year were 37% (95% CI, 17-57) and 9.4% (95% CI, 1.5-27), respectively (Figure 3B). Of the 8 patients who died, 6 deaths (27%) were attributed to relapsed disease and 2 (9%) from transplant-related complications, including one from GVHD on day 183 and one due to multiple causes (failure to thrive, grade I skin GVHD, and pulmonary embolism) on day 267.
Outcomes after venetoclax plus FluBu2 allo-HCT. Kaplan-Meier estimate and log-rank test of probability of OS (blue) and PFS (red) (A) and probability of NRM (blue) and relapse (red) (B). 95% CIs are reported for each outcome. Probability of OS (C) and PFS (D) in relation to ECOG PS of 0 to 1 (blue) vs ECOG PS of 2 (red).
Outcomes after venetoclax plus FluBu2 allo-HCT. Kaplan-Meier estimate and log-rank test of probability of OS (blue) and PFS (red) (A) and probability of NRM (blue) and relapse (red) (B). 95% CIs are reported for each outcome. Probability of OS (C) and PFS (D) in relation to ECOG PS of 0 to 1 (blue) vs ECOG PS of 2 (red).
Univariable analysis was performed to identify risk factors for OS and PFS. ECOG PS was the only factor that was associated with OS and PFS (P = .0032 and P = .0002 for ECOG PS 2 vs 0-1, respectively) (Figure 3C-D). Other factors, including age, sex mismatch, CR or CR/CRi status, DRI (Disease Risk Index) score, HCT-CI, cytomegalovirus serostatus, diagnosis, disease status, marrow blast percentage at study entry, or number of mutations, were not significantly associated with OS or PFS. Although limited by sample size, a trend toward poor outcome was suggested in cases with a pretransplant TP53 mutation compared with those without (supplemental Figure 1A-B).
Posttransplant MRD surveillance
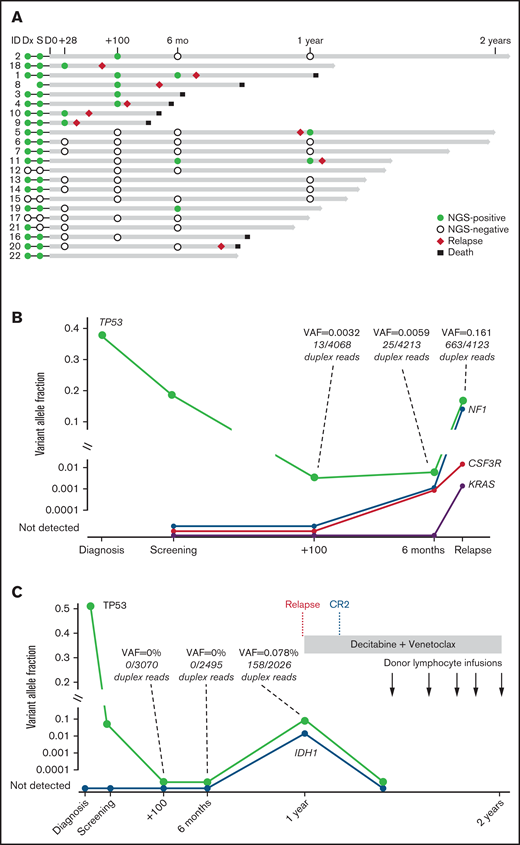
Serial banked pre/post–allo-HCT samples were sequenced by using duplex unique molecular identifier–tagged targeted NGS to quantify abundance of somatic mutations before and after venetoclax plus FluBu2 transplantation. As expected in this cohort of high-risk myeloid malignancies, most patients (18 of 22 patients [82%]) had persistent mutations detectable at time of transplantation (Figure 4A); individual patient mutations pre/post-transplant are shown in supplemental Table 3). Seven (78%) of nine patients who experienced morphologic relapse (≥5% blasts) had a detectable pretransplant TP53 mutation. In total, 8 patients were MRD positive at day 28 or day 100 (median variant allele fraction [VAF], 0.0053; range, 0.0008-0.0332), of whom 6 eventually relapsed. In contrast, just 3 (21.4%) of 14 patients who were MRD negative at these early time points eventually relapsed, and all 3 had TP53 mutations. Sequencing identified new mutations acquired at time of relapse (Figure 4B) and mutation clearance after transplant, including in TP53 (Figure 4C). Patient-level responses, including NGS status and available flow MRD status, are detailed in supplemental Table 1.
Serial genetic MRD monitoring before and after transplant. (A) NGS swimmer plot. Each bar represents a patient, with the length of the gray bars indicating follow-up time. Symbols indicate NGS status at serial time points, relapse, and death. Patients arranged according to NGS status at days 28/100, and then by follow-up time. Mutation VAF, relapse, and post-relapse course in patients 1 and 5 are shown. (B) Patient 1 had a baseline mutation in TP53, relapsed >6 months’ posttransplant, and acquired new mutations in NF1, CSF3R, and KRAS. (C) Patient 5 was another TP53-mutated patient who relapsed but later achieved durable CR2 after salvage therapy (decitabine and venetoclax) followed by donor lymphocyte infusion. TP53 VAF and read level support indicated at time points leading up to and including relapse. CR2 indicates timepoint of second complete remission achieved after two cycles of decitabine and venetoclax.
Serial genetic MRD monitoring before and after transplant. (A) NGS swimmer plot. Each bar represents a patient, with the length of the gray bars indicating follow-up time. Symbols indicate NGS status at serial time points, relapse, and death. Patients arranged according to NGS status at days 28/100, and then by follow-up time. Mutation VAF, relapse, and post-relapse course in patients 1 and 5 are shown. (B) Patient 1 had a baseline mutation in TP53, relapsed >6 months’ posttransplant, and acquired new mutations in NF1, CSF3R, and KRAS. (C) Patient 5 was another TP53-mutated patient who relapsed but later achieved durable CR2 after salvage therapy (decitabine and venetoclax) followed by donor lymphocyte infusion. TP53 VAF and read level support indicated at time points leading up to and including relapse. CR2 indicates timepoint of second complete remission achieved after two cycles of decitabine and venetoclax.
BH3 profiling
We questioned if measuring cellular readiness for apoptosis by measuring cytochrome c release via BH3 profiling predicted relapse. There were insufficient myeloblasts for study, but BH3 profiling of monocyte populations from 12 patients (8 MDS, 1 MDS/MPN, and 3 AML) suggested mitochondrial sensitivity to venetoclax (unadjusted P = .07, one-sided) might associate with lack of relapse by day 100 (supplemental Figure 2).
Discussion
The majority of individuals diagnosed with MDS/AML are aged >60 years and have high-risk disease. These patients typically require RIC, which is associated with a high risk of relapse often within a few months after transplantation before full development of a graft-versus-leukemia effect.24 Thus, RIC conditioning strategies need to be optimized to prevent early relapses.25 In an effort to more effectively address residual disease before transplantation to prevent early relapses and allow time for graft-versus-leukemia to develop, we combined venetoclax with a standard RIC conditioning regimen for MDS and AML based on strong preclinical rationale. Although benefit may be extended to other prognostic groups, as a first step we specifically focused enrollment on patients at the highest risk of relapse despite RIC allo-HCT, including patients with adverse-risk AML according to ELN criteria, TP53 or RAS-pathway mutated MDS, and ASXL1-mutated or complex karyotype MDS/MPN. Antiapoptotic proteins are known to mediate resistance to chemotherapy. Thus, combining venetoclax with busulfan was hypothesized to eliminate residual disease. Indeed, our preclinical in vitro studies identified synergistic cell killing with combination venetoclax and busulfan in human AML cell lines, justifying our approach (supplemental Figure 3).
This first report of our phase 1 study of venetoclax plus FuBlu2 conditioning found that the combination is safe with an RP2D of 400 mg/d of venetoclax for 7 doses starting day −8 of the FluBu2 regimen. The addition of venetoclax did not increase risk of infection, impair donor engraftment, or induce excessive acute or chronic GVHD. Based on the limited clinical activity of single-agent venetoclax and preclinical combination data, we purposefully designed the treatment schedule to maximize overlap of venetoclax and conditioning chemotherapy.26 A venetoclax dose exceeding 400 mg/d, which is the optimal venetoclax dose with hypomethylating agents in terms of response and risk-benefit ratio, was not tested in the dose escalation schema to avoid excessive gastrointestinal toxicity immediately posttransplant and because biological activity was observed at this dose.27 Our goal was to combine venetoclax with FluBu2 to add selective antileukemic activity rather than broadly increase intensity for marrow ablation. Although we enrolled a relatively sick patient population with 55% of the patients having an HCT-CI score ≥4, no DLTs were observed, and the MTD of venetoclax plus FluBu2 was not reached. With the now prominent role of venetoclax in the older AML treatment landscape, which occurred during the conduct of this study, it would be difficult to limit enrollment to venetoclax-naive patients for an RIC allo-HCT study. We have insufficient data (n = 3) to conclude whether prior venetoclax exposure decreases the potential benefit of venetoclax-based conditioning chemotherapy, and this topic deserves further study.
Venetoclax plus FluBu2 conditioning provided durable disease control in a cohort of high-risk patients as defined by ontogeny and genetics. With a median follow-up of 14.7 months, 11 (50%) of 22 patients remain alive and without morphologic evidence of leukemia since study entry. Median OS has not yet been reached. Although the presence of a TP53 mutation was still associated with high risk of relapse, 6 of 12 TP53-mutated patients (50%) are alive, including 3 without disease recurrence after transplant.
In this study, we additionally performed serial MRD-depth NGS assessments before and after venetoclax plus FluBu2 transplant. We found that ultra-sensitive duplex sequencing in the post–allo-HCT setting revealed the kinetics of early mutation clearance, identified relapse-specific mutations, and provided dynamic surveillance of poor-risk mutations. Persistent genetic MRD at day 28 and day 100 identified all but 3 cases that ultimately relapsed posttransplant. Thus, day 28 or day 100 is suspected to be the critical time point to consider early withdrawal of immunosuppression or initiation of posttransplant maintenance therapy for MRD-positive patients to prevent disease relapse. Dynamic MRD evaluation further identified patients with pretransplant TP53 mutations who became MRD negative after venetoclax plus FluBu2 and remain in clinical remission, suggesting some patients with poor-risk mutations may not require additional posttransplant intervention.
The current study represents an important step in the effort to optimize transplant conditioning for higher risk patients undergoing RIC allogeneic transplants. Additional interventions could be combined with venetoclax-based conditioning to further reduce relapse risk, including early withdrawal of immune suppression, donor lymphocyte infusion, or maintenance therapy with targeted agents such as sorafenib in cases of FLT3-mutated AML.28,29 Posttransplant maintenance with hypomethylating agent therapy, which is hypothesized to regulate the graft-versus-leukemia effect and graft-versus-host disease effects, is feasible but insufficient to reduce relapse risk.30–32 Because patients with persistent posttransplant MRD in our study still had an increased risk of relapse, we have amended the trial to separately assess the safety and efficacy of posttransplant maintenance therapy with hypomethylating agents and venetoclax following venetoclax plus FluBu2 transplantation (#NCT03613532).
Our conditioning approach may need to be modified as the therapeutic landscape changes for MDS and AML before transplantation. Venetoclax may induce MCL1 dependence in residual myeloblasts, and thus targeting other antiapoptotic proteins may be necessary with recent changes to frontline AML therapies for older patients (ie, venetoclax plus hypomethylating agent).11,33 Our data contribute to the growing body of evidence supporting the use of a venetoclax add-on strategy to a chemotherapy backbone.34–37 The addition of venetoclax to other RIC (including fludarabine/melphalan) and myeloablative conditioning regimens for patients with high-risk disease undergoing transplant might also be of interest to reduce relapse risk and requires investigation to confirm safety.38,39 Although not assessed in this trial, and we are waiting for BMT CTN 1703 results, we anticipate that the addition of posttransplant cyclophosphamide for GVHD prophylaxis may require additional safety assessment if given after the venetoclax/FluBu2 conditioning regimen.40,41 Biomarkers such as BH3 profiling assays may help to identify potential responders to venetoclax-based treatment strategies. In the pretransplant setting, BH3 profiling of myeloblasts was not feasible due to low cell numbers. Although the significance of BH3 profiling of non-myeloblasts is not clear, the apoptotic priming status of progeny of the malignant clone such as monocytes in MDS and MDS/MPN cases may provide insight into initial venetoclax sensitivity. Thus, further exploration of the priming response to BH3 peptides in different myeloid subsets as a predictor of long-term response to venetoclax plus FluBu2 in a larger data set is warranted.
Our study shows that venetoclax can be added safely to a FluBu2-based RIC chemotherapy regimen in patients with high-risk AML, MDS, and MDS/MPN. The results of our study, showing an encouraging OS rate, and evidence of genetic MRD clearance, suggest that the addition of venetoclax to FluBu2 may improve posttransplant outcomes in high-risk patients. Given the feasibility of administering this regimen, venetoclax plus RIC chemotherapy may be an optimal backbone to explore additional posttransplant interventions to prevent disease relapse.
Acknowledgments
Research reported in this publication was supported by the Ted and Eileen Pasquarello Tissue Bank in Hematologic Malignancies and the National Cancer Institute (NCI) of the National Institutes of Health (NIH) under award numbers CA066996 and K08CA245209, and NIH/NCI SPORE in Myeloid Malignancies grant 1P50CA206963. This work was also supported by grants from the Frederick A. Deluca Foundation. Genentech provided trial and drug (venetoclax) support.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or NCI.
Authorship
Contribution: J.S.G., H.T.K., C.S.C., A.L., R.J.S., and J.H.A. designed the study; J.S.G., C.S.C., J.B., M.G., V.T.H., J.K., S.N., R.R., R.S., F.L., and R.J.S. enrolled and treated the patients; J.R., H.Q.K., D.P., and T.M. processed and performed biomarker assays; J.S.G., H.T.K., H.M.M., C.S.C., J.R., G.F., H.Q.K., D.P., T.M., R.M.S., D.J.D., A.L., R.C.L, R.J.S., and J.H.A. analyzed and interpreted the data and created the figures; A.S.K., F.L., and J.R. performed pathology review; and J.S.G., H.T.K., H.M.M., R.C.L., R.J.S., and J.H.A. drafted the manuscript. All authors contributed to data collection, writing and revision of the manuscript, and approval of the final version.
Conflict-of-interest disclosure: J.S.G. reports grants from and serves on advisory boards for AbbVie, Astellas, and Takeda; and receives grants/research funding from Roche Genentech, Pfizer, Prelude, and AstraZeneca. C.S.C. serves on the advisory board/consults for Mesoblast, Syndax, Incyte, CareDx, and Editas; received personal fees (education) from Janssen, Omeros, Mallinckrodt, and Pfizer; and serves on the Data and Safety Monitoring Board for AlloVir, Da Volterra, Pluristem, and BioLineRx. J.K. serves on board and consults for Mallinckrodt, EMD Serono, Merck, Cugene, Biolojic Design, Gentibio, Nekonal, Equilibrium, and Amgen; and receives grants/research support from BMS, Miltenyi, Regeneron, Equillium, and Clinigen. R.R. receives grant support from CRISPR Therapeutics; and serves on the advisory board for Glycostem. A.S.K. serves on boards/consulted for LabCorp, Inc.; and received personal fees and grants from the Multiple Myeloma Research Foundation. D.J.D. consults for AbbVie, Amgen, Agios, Autolus, Blueprint, Forty-Seven, Glycomimetics, Incyte, Jazz, Kite, Novartis, Pfizer, Servier, and Takeda. R.M.S. reports grants and personal fees from AbbVie, Agios, and Novartis; personal fees from Actinium, Argenx, Astellas, AstraZeneca, BioLineRx, Celgene, Daiichi Sankyo, Elevate, GEMoaB, Janssen, Jazz, MacroGenics, Otsuka, Pfizer, Hoffmann–La Roche, Stemline, Syndax, Syntrix, Syros, Takeda, and Trovagene; and grants from Arog. D.J.D. receives grant/research funding from AbbVie, Novartis, Blueprint, and Glycomimetics. J.H.A. serves on the Data and Safety Monitoring Board for CSL Behring and Janssen and on the scientific advisory board for Merck, SNIPR Biome, and Pharmacosmos. A.L. serves on the scientific advisory board of Flash Therapeutics, Dialectic Therapeutics, and Zentalis Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Jacqueline S. Garcia, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: Jacqueline_garcia@dfci.harvard.edu.
References
Author notes
Presented in abstract form at the 62nd annual meeting of the American Society of Hematology, San Diego, CA, 5 December 2020, and the 61st annual meeting of the American Society of Hematology, Orlando, FL, 7 December 2019.
Deidentified individual participant data that underlie the reported results will be made available for approved use by the study authors. Proposals for access should be sent to jacqueline_garcia@dfci.harvard.edu. Complete trial cohort-level data will be published on clinicaltrials.gov at the conclusion of the trial.
The full-text version of this article contains a data supplement.