Key Points
A subset of patients receiving ≥12 months of VEN-based therapy can experience durable treatment-free remission after ceasing therapy.
The risk of relapse, duration of relapse-free survival, and overall survival were similar to a cohort of patients continuing therapy until progression.
Abstract
The clinical benefit of adding venetoclax (VEN) to hypomethylating agents or low-dose cytarabine in older and/or unfit patients with newly diagnosed acute myeloid leukemia (AML) has been confirmed in phase 3 studies. With the increased uptake of VEN-based therapies for patients with AML, a pertinent question is whether treatment can be safely ceased among patients who have achieved sustained remission. We hypothesized that a proportion of patients opting to cease therapy may benefit from a treatment-free remission (TFR) period without indefinite treatment. We report the retrospective outcomes of 29 patients in remission for a minimum of 12 months on VEN-based therapy, with 55% continuing therapy until disease progression and 45% electively ceasing treatment (STOP). With follow-up exceeding 5 years, we observed a median TFR lasting 45.8 months among the STOP cohort, with >50% of patients still in sustained remission at the data cutoff. The risk of relapse and duration of relapse-free and overall survival were similar between the 2 cohorts. Factors favoring sustained TFR within the cohort included NPM1 and/or IDH2 mutation at diagnosis, complete remission without measurable residual disease, and at least 12 months of VEN-based combination therapy prior to treatment cessation.
Introduction
Use of venetoclax (VEN) in combination with either azacitidine or low-dose cytarabine (LDC) has led to substantial improvements in clinical response and overall survival (OS) among older and/or unfit patients with newly diagnosed acute myeloid leukemia (AML).1,2 In the VIALE-A trial, the median duration of complete remission (CR) was 17.5 months, with a 70% likelihood of sustained remission at 18 months if the patient had achieved CR or CR with incomplete recovery and was measurable residual disease (MRD) negative.1,3 Beyond 18 months, subsequent events were less common, with a remission plateau emerging. A similar remission plateau is evident after 18 months in patients receiving VEN-LDC.4 Therefore, for patients receiving VEN-based therapy, an important question is whether cessation of therapy could be considered to realize the quality-of-life benefits associated with treatment-free remission (TFR).
Practical burdens associated with indefinite therapy include vulnerability to treatment-related toxicities, psychosocial fatigue from prolonged therapy, and health–economic costs related to hospital resource use and drug procurement.5 We sought to evaluate the natural history and clinical/genetic characteristics of patients with AML and durable remission after at least 12 months of VEN-based therapy according to whether treatment was continued or electively ceased.
Methods
Patients were eligible if they were ≥65 years with newly diagnosed AML, received frontline therapy with VEN combined with either a hypomethylating agent (HMA) or LDC for at least 12 months, and were in first CR without prior allogeneic stem cell transplant.4,6 To enhance treatment homogeneity, the cohort was limited to patients previously enrolled in clinical trials at the 3 participating centers.4,6 Patients were stratified between 2 treatment approaches: a “STOP” cohort if therapy was electively ceased while in remission and a “CONT” cohort in which therapy was continued until relapse. Additional information is provided in the supplemental Appendix.
Results and discussion
Between November 2014 and August 2018, the Alfred Hospital (AH), MD Anderson Cancer Center (MDACC), and University of Colorado (UC) enrolled a total of 146 patients to VEN-based therapy for newly diagnosed older patients with AML not eligible for allogeneic stem cell transplant (supplemental Figure 1). A total of 29 patients (20%) received at least 12 months of VEN-combination therapy (13/36 [33%], 11/45 [24%], and 5/65 [8%] from AH, MDACC, and UC, respectively). Of the 29 patients treated for at least 12 months and in continuous remission, 13 patients (45%) had treatment electively ceased, whereas 16 (55%) continued therapy until disease progression, forming the STOP and CONT cohorts, respectively. Treatments received included VEN-HMA in 20 (69%) and VEN-LDC in 9 (31%). Baseline characteristics were comparable between the 2 cohorts (Table 1; supplemental Figure 2). Median age was 74 years (range, 65-80). The proportion of patients with adverse European LeukemiaNet 2017 risk was 46% and 43% in STOP and CONT, respectively.7 Best response of CR was achieved in most patients: 92% in STOP and 81% in CONT. The STOP cohort was more frequently treated with prior VEN-LDC.
The median follow-up time was 61.8 and 58.0 months for the STOP and CONT cohorts, respectively (data cut 31 January 2021). As expected, the median duration of therapy received in the STOP cohort was significantly shorter than in the CONT group (19.3 vs 28.8 months; P = .01). Similarly, the STOP cohort was exposed to fewer treatment cycles (median 17 vs 28 cycles; P = .03). The main documented reasons for treatment cessation in the STOP cohort were patient request (46%) and medical reasons (54%). Patient requests centered on quality-of-life factors (50%) or wanting to reduce the burden associated with injections, monitoring, and pill-taking (50%). The medical reasons for ceasing therapy were recurrent neutropenia (2/7 [29%]), ophthalmological issues (2/7 [25%]), recurrent urinary tract infections (1/7 [13%]), bleeding due to thrombocytopenia (1/7 [13%]), and renal failure (1/7 [13%]).
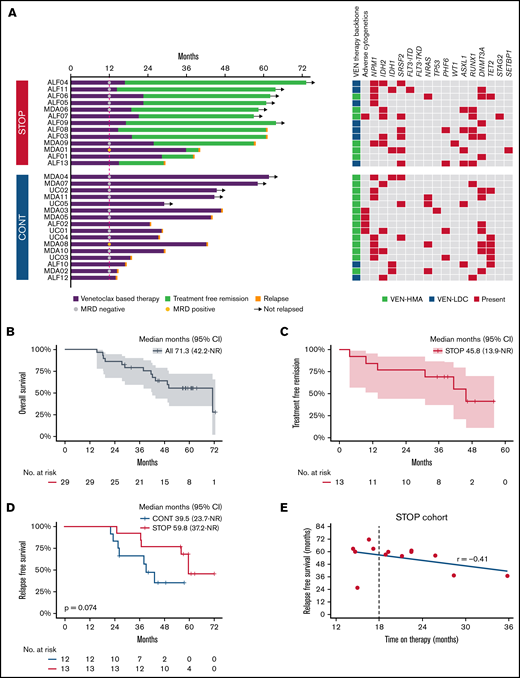
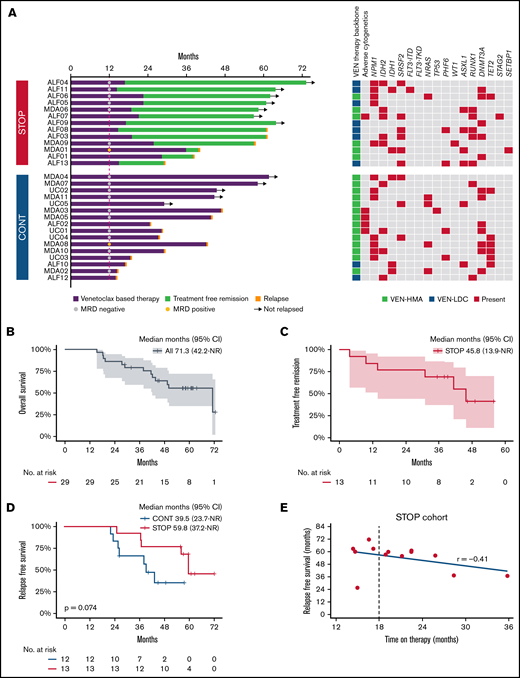
The median OS for the entire cohort was 71.3 months, with 79.2% alive at 3 years and an estimated OS of 55.5% at 5 years (Figure 1B). Median OS in the STOP group was 71.3 months (95% CI, 41.6 to not reached), compared with 43.7 months (95% CI, 19.4 to not reached) in the CONT cohort (P = .023) (supplemental Figure 3A). During the observation period, 46% and 69% of patients subsequently relapsed in the STOP and CONT cohorts, respectively. A landmark survival analysis at the median duration of therapy in the STOP cohort (19.3 months) was performed to exclude lead-time bias in patients relapsing early in the CONT cohort, thus excluding 4 patients in CONT who relapsed prior to 19.3 months. No significant difference in either median relapse-free survival (RFS) (59.8 vs 39.5 months; P = .074) ((Figure 1D) or OS (median 71.3 vs 50.2 months; P = .15) (supplemental Figure 3B) was evident between STOP vs CONT cohorts, respectively. Patients in the STOP cohort benefitted from a median TFR of 45.8 months (95% CI, 13.9 to not reached) (Figure 1C). At the time of the data cut, 54% of patients (7/13) in the STOP cohort remained in remission. Notably, there was no correlation between the duration of prior therapy received after at least 12 months of VEN-combination and RFS (P = .45; Figure 1E; supplemental Figure 4).
Analysis of patients receiving VEN-based therapy for at least 12 months. (A) Natural history among patients receiving VEN-based therapy for at least 12 months following a STOP or CONT therapy strategy. Bars indicate exposure to VEN-based therapy, MRD status at 12 months, TFR in the STOP cohort, relapse events (red cap), ongoing remission (arrows), treatment received, and baseline cytogenetic/genetic mutation profile. (B) OS in the entire cohort from commencement of VEN-based therapy. (C) Kaplan-Meier curve showing TFR in the STOP cohort. (D) RFS in the STOP and CONT cohorts landmarked at 19.3 months from day 1 of therapy. (E) Correlation between duration of therapy received and RFS.
Analysis of patients receiving VEN-based therapy for at least 12 months. (A) Natural history among patients receiving VEN-based therapy for at least 12 months following a STOP or CONT therapy strategy. Bars indicate exposure to VEN-based therapy, MRD status at 12 months, TFR in the STOP cohort, relapse events (red cap), ongoing remission (arrows), treatment received, and baseline cytogenetic/genetic mutation profile. (B) OS in the entire cohort from commencement of VEN-based therapy. (C) Kaplan-Meier curve showing TFR in the STOP cohort. (D) RFS in the STOP and CONT cohorts landmarked at 19.3 months from day 1 of therapy. (E) Correlation between duration of therapy received and RFS.
Next we assessed variables associated with longer OS (Figure 1A). Among patients surviving ≥3 years, 13/19 (68%) had either an NPM1 or IDH2 mutation, of whom 11 of 12 (92%) with available MRD assessment had undetectable MRD (supplemental Table 1). Among the NPM1/IDH2 mutant subgroup, 10/18 (56%) remained in remission by the data cut, compared with 2/11 (18%) with other genotypes (supplemental Figure 5). Among patients in the STOP cohort who remained in TFR at the data cut, 6/7 (86%) had an NPM1 or IDH2 mutation and achieved CR without MRD (Figure 1A).
At relapse, cytogenetic evolution was observed in 29% (17% in STOP and 36% in CONT) whereas molecular evolution was evident in 65% (67% in STOP and 55% in CONT) (supplemental Figure 6A). Interestingly, 73% (11/15 with available remission molecular data) of relapsing patients had a preleukemic DTA (DNMT3A, TET2, or ASXL1) mutation present during remission compared with 29% (2/7) among nonrelapsing patients. Upon relapse, salvage therapy was attempted in 5/6 (83%) STOP and 6/11 (55%) CONT patients. A total of 6 patients in the STOP group relapsed and 3 were retreated with the same VEN regimen as administered originally, including a dose ramp-up and tumor lysis prophylaxis, with 2/3 responding and achieving second remission. Subsequent relapse occurred after a period of 94 and 191 days. None of the patients in the CONT cohort were retreated with VEN-based therapies. The median time from relapse to death was 9.8 vs 3.8 months in STOP vs CONT cohorts, respectively (P = .81) (supplemental Figure 6B).
An analysis of 14 089 patients ≥66 years diagnosed with AML between 2001 and 2013 revealed that 43% to 60% of patients received no active AML therapy.8 The introduction of VEN-based therapy will likely reshape perceptions regarding treatment expectations among older AML populations. In VIALE-A, 50% receiving VEN-azacitidine were alive at 12 months.1 For these patients, an increasingly important question was “Should I continue treatment until progression, or is it safe to stop therapy at some stage?” In this observational study, we analyzed a cohort of older AML patients in remission and receiving VEN-based therapy ≥12 months. With a median follow-up time exceeding 5 years from AML diagnosis, we observed that patients ceasing VEN-based combination therapy after receiving at least 12 months of therapy could experience a median TFR of almost 4 years. Patients ceasing VEN-based therapy received almost half the number of treatment cycles compared with those continuing therapy until progression. In terms of risk, we could not identify a difference in relapse risk or OS compared with patients continuing therapy. However, the STOP group represents a highly selected population: 92% had achieved CR, 92% had intermediate cytogenetic risk, and 86% were MRD negative at time of treatment cessation. Of patients in the STOP group with ongoing TFR, 86% were originally NPM1 and/or IDH2 mutation positive and MRD negative at treatment cessation. In the STOP group, RFS duration was similar, regardless of whether prior therapy duration was for 12 to 18 months or >18 months. Based on these observations, we conclude that a durable TFR is possible among a select group of patients with either NPM1 or IDH2 mutation who have received VEN-based therapy for ≥12 months and are MRD negative at time of treatment cessation. One limitation of this report is our attempt to limit selection bias by restricting cohort eligibility at participating centers to patients enrolled in a clinical trial. Confirmation of these findings in a broader patient population is therefore required, ideally in the context of a prospective, randomized, discontinuation study.
Acknowledgments
This work was supported by grants from the Australian National Health and Medical Research Council 1162809 (A.H.W.), US Lymphoma Society, Specialized Center of Research [SCOR] grant 7015-18 (A.H.W.), Medical Research Future Fund grant 1141460 (A.H.W.), and a Scholar in Clinical Research funding from the Leukemia and Lymphoma Society (D.A.P).
Authorship
Contribution: C.C.C., A.H.W., C.D.D., and M.Y.K. conceived and designed the study; A.H.W., I.S.T., C.D.D., M.Y.K., and D.A.P. provided patients; C.C.C., D.H., A.K., and I.S.T. collected and assembled data; C.C.C. and A.H.W. analyzed and interpreted data; C.C.C. and A.H.W. wrote the manuscript; and all authors contributed to critical revision of the manuscript and approved the final version of the manuscript.
Conflict-of-interest disclosure: A.H.W. is an employee of the Walter and Eliza Hall Institute, which receives milestone and royalty payments related to venetoclax, and has received research funding from Servier and AbbVie. C.D.D. received research funding from AbbVie, Agios, Astex, ImmuneOnc, Daiichi Sankyo, Celgene/BMS, Calithera, and Loxo Oncolog and has served as a consultant/advisory board member for AbbVie, Agios, ImmunoOnc, Daiichi Sankyo, Celgene/BMS, Cleave, Invitae, GSK, Servier, and Notable Labs. D.A.P. received research funding from AbbVie and served as a consultant and advisory board member for AbbVie and Genentech. M.Y.K. received research funding from AbbVie, Genentech, F. Hoffman La-Roche, Eli Lilly, Cellectis, Calithera, Ablynx, Stemline Therapeutics, Agios, Ascentage, AstraZeneca, Rafael Pharmaceutical, Sanofi, and Forty-Seven; served as a consultant/advisory board member for AbbVie, Genentech, F. Hoffman La-Roche, Stemline Therapeutics, Amgen, Forty-Seven, Kisoji, and Janssen Pharmaceuticals; and, outside the submitted work, holds US patent 7795305 B2 CDDO (compounds and combination therapies) with royalties paid to Reata Pharm, a patent combination therapy with a mutant IDH1 inhibitor and a BCL-2 inhibitor licensed to Eli Lilly, and holds a patent 62/993 166 combination of an MCL-1 inhibitor and midostaurin, uses and pharmaceutical compositions thereof pending to Novartis. The remaining authors declare no competing financial interests.
Correspondence: Andrew H. Wei, Peter MacCallum Cancer Centre and Royal Melbourne Hospital, 305 Grattan St, Melbourne, VIC 3000, Australia; e-mail: andrew.wei@petermac.org.
References
Author notes
Requests for data sharing may be submitted to Andrew H. Wei andrew.wei@petermac.org.
The full-text version of this article contains a data supplement.