Key Points
First-line treatment with CPX-351 or the combination of venetoclax and azacitidine resulted in similar overall survival.
Early mortality was also similar; infection, neutropenic fever, and inpatient length of stay were higher with CPX-351.
Abstract
CPX-351 and venetoclax and azacitidine (ven/aza) are both indicated as initial therapy for acute myeloid leukemia (AML) in older adults. In the absence of prospective randomized comparisons of these regimens, we used retrospective observational data to evaluate various outcomes for patients with newly diagnosed AML receiving either CPX-351 (n = 217) or ven/aza (n = 439). This study used both a nationwide electronic health record (EHR)-derived de-identified database and the University of Pennsylvania EHR. Our study includes 217 patients who received CPX-351 and 439 who received ven/aza. Paitents receiving ven/aza were older, more likely to be treated in the community, and more likely to have a diagnosis of de novo acute myeloid leukemia. Other baseline covariates were not statistically significantly different between the groups. Median overall survival (OS) for all patients was 12 months and did not differ based on therapy (13 months for CPX-351 vs 11 months for ven/aza; hazard ratio, 0.88; 95% confidence interval, 0.71-1.08; P = .22). OS was similar across multiple sensitivity analyses. Regarding safety outcomes, early mortality was similar (10% vs 13% at 60 days). However, documented infections were higher with CPX-351 as were rates of febrile neutropenia. Hospital length of stay, including any admission before the next cycle of therapy, was more than twice as long for CPX-351. In this large multicenter real-world dataset, there was no statistically significant difference in OS. Prospective randomized studies with careful attention to side effects, quality of life, and impact on transplant outcomes are needed in these populations.
Introduction
Acute myeloid leukemia (AML) is the most common acute leukemia in adults in the United States with a median age of 68 years at diagnosis.1 In real-world data sets, fewer than half of older adults are offered traditional intensive therapy,2,3 and prognosis with nonintensive alternative therapy is poor.4 Outcomes in older adults with AML are dismal—historically median overall survival (OS) for adults age 65 to 75 was 6.9 months,5 with a 5-year OS < 5%.6
In the last 5 years, several new regimens have been approved, but none have been compared head-to-head. CPX-351, a dual-drug liposomal encapsulation of cytarabine and daunorubicin that delivers a synergistic 5:1 molar ratio, has shown superior OS compared with standard-of-care cytarabine plus daunorubicin chemotherapy (7 + 3 regimen) in fit, older patients with newly diagnosed secondary AML.7 Venetoclax and azacitidine (ven/aza), a combination of an oral B-cell lymphoma 2 inhibitor and hypomethylating agent (HMA), was superior to a HMA alone in older adults with either secondary AML or de novo AML who were deemed to be unfit for intensive chemotherapy and had not received prior HMAs.8
Although these trials contained some overlapping age-based inclusion criteria, the patient populations are not identical. Thirty-four percent of patients in the pivotal CPX-351 trial had received a prior HMA and would have been excluded from the VIALE-A ven/aza pivotal trial.7 Similarly, 60% of patients in the VIALE-A trial were over the age of 75 and would have been excluded from the CPX-351 pivotal trial.8 Myelodysplasia-related changes were not assessed so it is unclear how many of the 75% of patients with de novo AML would have met inclusion criteria for the CPX-351 trial. Long-term survival on the pivotal CPX-351 study was primarily associated with receipt of transplant, which was performed in 34% of patients in the CPX-351 trial,7 whereas patients on VIALE-A were treated until intolerance or progression, and 10% of patients were transplanted.8 Furthermore, trials do not fully capture the real-world decisions facing clinicians, as patients outside of clinical trials tend to be older, less physiologically fit with more comorbidities, and have worse survival.9,10
Increasingly, clinicians face the choice between CPX-351 and ven/aza in front-line AML, especially for older adults who often have complex comorbidities. Randomized studies in older adults have failed to show an advantage of low-intensity options like azacitidine and low-dose cytarabine vs traditional intensive induction therapy with anthracyclines and higher-dose cytarabine.11,12 Allogenic transplant after achievement of remission appears to be linked to improvement in OS, but the optimal induction regimen for older adults is unclear. There is emerging evidence of successful allogeneic transplant after initial treatment with ven/aza,13 and recent observational studies comparing venetoclax and azacitidine to 7 + 3 also show similar survival with transplant.14 Comparative data for CPX-351 vs ven/aza is lacking. Clinicians often rely on local practice patterns, hypotheses about molecular subsets that may benefit from 1 type of treatment, or other subjective factors to choose induction therapy.
Given the lack of a comparative, prospective trial, we conducted a retrospective observational study comparing the effectiveness of CPX-351 vs ven/aza with respect to OS of newly diagnosed adult patients with AML. We hypothesized that CPX-351 would result in superior OS compared with ven/aza because of the historical superiority of intensive chemotherapy overall less intensive options.4 Our analysis attempts to overcome the limitations of prior studies namely small sample size exacerbated by missing data, selection bias resulting in imbalanced covariates and failure to integrate both clinical comorbidities and detailed genetic features of disease through use of a national multicenter electronic medical record data repository and by applying analytical methods such as multiple imputation and inverse probability of treatment weighting. We also performed exploratory analyses assessing response to therapy and key safety outcomes including early mortality.
Methods
Study design and data sources
This report follows the Strengthening the Reporting of Observational Studies in Epidemiology Statement guidelines.15,16 Two sources were used for patient cohorts in this retrospective study. First, we queried the University of Pennsylvania Health System Electronic Medical Records database (HUP), which provides longitudinal data spanning both inpatient and outpatient settings at 5 hospitals including an academic medical center in Philadelphia, a rural hospital in Lancaster, PA, and 3 community hospitals in urban/suburban Philadelphia.
The second source was Flatiron Health, a nationwide electronic health record (EHR)-derived de-identified database. The Flatiron Health database is a longitudinal database, comprising de-identified patient-level structured and unstructured data, curated via technology-enabled abstraction.17,18 During the study period, the de-identified data originated from approximately 280 cancer clinics (∼800 sites of care). No patients from HUP are included in this database.
The University of Pennsylvania Institutional Review Board approval of the study protocol was obtained prior to study conduct and included a waiver of informed consent.
Eligibility
For both data sources, all patients who received CPX-351 or ven/aza as initial therapy (primary exposure) and had a diagnosis of AML (International Classification of Diseases-9 codes 205.0, 205.2, 205.3, 205.8, 205.9 or International Classification of Diseases-10 codes C92.0, C92.3, C92.5, C92.Z) with documented 20% blasts in bone marrow or peripheral blood were included. HUP charts were manually reviewed to confirm patients received CPX-351 or ven/aza as initial therapy. Any patients with acute promyelocytic leukemia, mixed phenotype or ambiguous lineage acute leukemia, pregnancy, or did not receive CPX-351 or ven/aza as initial therapy were excluded. Given a robust sample size with ven/aza, other combinations with venetoclax were not included to preserve a homogenous treatment effect. The time period for inclusion was diagnosis January 2017 to January 2021 with data cutoff of 30 April 2021.
Outcomes
The primary outcome was OS, defined as time from diagnosis to death or censored at end of study period at 30 April 2021. Secondary outcomes were remission rate (complete response [CR], CR + complete response with incomplete blood count recovery [CRi]; assessed according to the Revised International Working Group Criteria for AML; supplemental Table 7f), remission duration, and event-free survival (time since treatment initiation to date of induction failure, relapse from CR + CRi, or death from any cause; supplemental Table 7f). Safety outcomes were early (30- and 60-day) mortality and documented infections. HUP data allowed chart review for all patients, and febrile neutropenia and total inpatient hospital days before cycle 2 of therapy (“length of stay”) were exploratory outcomes. Anticipated potential effect modifiers or confounders were collected as covariates including, at time of diagnosis: demographic factors (age, race, ethnicity, practice setting), clinical factors (hematopoietic comorbidity index,19 including albumin and lactate dehydrogenase (LDH)20 and other comorbidities including history of myelodysplastic syndrome and prior HMA treatment; supplemental Table 7c), disease severity (baseline blood counts, bone marrow blast counts, European LeukemiaNet (ELN) cytogenetic risk groups [supplemental Table 7a], myelodysplastic-related cytogenetics [supplemental Table 7b], and FLT3, IDH, T53, ASXL1, RUNX1, TET2, and NPM1 mutational status).
Power
Before initial data collection, power calculation showed that 456 cases, allocated 2:1 between ven/aza and CPX-351, would have 80% power to detect a hazard ratio of 0.70 for OS from diagnosis to death or censoring at end of study period, assuming an event rate of 40% at 1 year7,8 with the use of a log-rank test at a 2-sided significance level of 0.05. Post hoc power calculation with 656 cases allocated 2:1 the minimally detected hazard ratio is 0.78.
Statistical methods
Descriptive statistics were calculated for baseline characteristics for the 2 treatment groups. For continuous variables, means and standard deviations for symmetric distributions and medians and interquartile ranges for skewed distributions and frequencies for categorical or ordinal variables with t test and χ2 test to compare continuous and categorical variables are reported. Primary outcome of OS was assessed using Kaplan-Meier method for median OS, the log rank test was used to assess survival differences between groups, and multivariable Cox proportional hazards regression analysis was used to estimate association between covariates and OS with hazard ratio (HR) and 95% confidence intervals (CIs). Patients were further stratified by age and cytogenetics to check for effect modification given prior work.5,21 Proportional hazards assumption was assessed using Schoenfeld residuals. Subgroups were examined in univariate analyses. Loss to follow-up or last visit more than 3 months before data cutoff without documented death was 8% (8.4% for CPX-351 and 8.8% for ven/aza; P = .88) and assumed to be missing at random.22 All tests were 2-sided, with a significance level of .05. All statistical analyses were performed in Stata.23
Sensitivity analyses
This study uses several sensitivity analyses to address common pitfalls of cohort studies. We conducted a restriction analysis where we included only patients receiving ven/aza or CPX-351 who met the original inclusion criteria for the CPX-351 pivotal trial: age between 60 and 75 years, therapy-related myeloid neoplasm, myelodysplastic-related cytogenetics or prior history of MDS with or without HMA exposure, and no central nervous system disease or core binding factor AML.7 Missing data can severely limit multivariable Cox proportional hazards regression analysis, which relies on complete cases, thus introducing bias and limiting power. We therefore also conducted multiple imputation, filling in missing values in multiple variables iteratively by using chained equations, a sequence of univariate imputation methods with fully conditional specification of prediction equations. Thirty imputations were used, with regression for continuous lab values, logistic regression for binary variables, and ordinal logistic regression for ordinal variables.24,25 Furthermore, to address confounding by indication, baseline differences between treatment groups were adjusted for, after multiple imputation, with inverse probability treatment weighting, a propensity score-based approach to estimate marginal treatment effects. The treatment effect estimates from each imputed dataset were then combined to obtain an overall estimate.26,27
Results
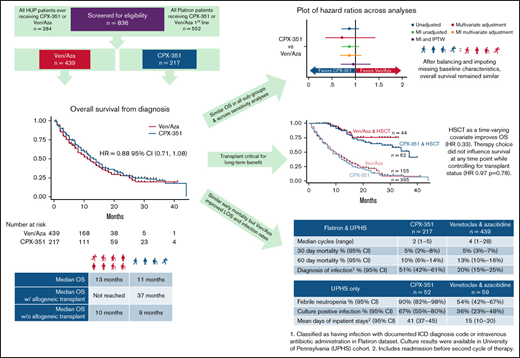
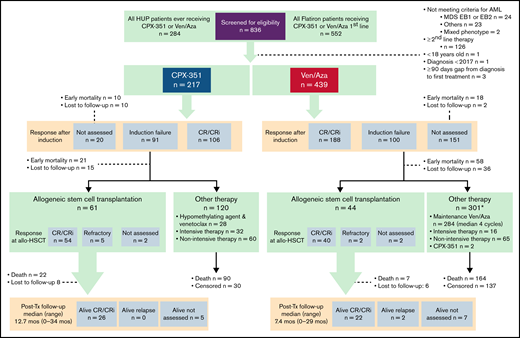
Patient population
A total of 439 patients received ven/aza (59 from HUP, 380 from Flatiron Health) and 217 received CPX-351 (52 from HUP, 165 from Flatiron Health; Figure 1). Response after cycle 1 of therapy was assessed in 93% of HUP patients and 70% of Flatiron Health patients. Average follow-up time was 10.3 months (13.4 months for CPX-351 and 8.8 months for ven/aza), with 237 deaths in the ven/aza group and 145 deaths in the CPX-351 group. Selected adverse events of early mortality and infection were evaluable in all patients. Febrile neutropenia and hospital length of stay were evaluable only for HUP patients.
Patient flow diagram. MDS EB, myelodysplastic syndrome with excess blasts.
Several patient baseline characteristics differed between groups receiving ven/aza vs CPX-351. Patients receiving ven/aza were older (median age, 75 vs 65 years; P < .001), more likely to have Medicare (29% vs 20%; P = .047), have diagnosis recorded as de novo AML vs secondary or therapy-related disease (52% vs. 29%; P < .001), and be treated in the community rather than an academic center (66% vs 52%; P < .001). All other baseline covariates—including ELN cytogenetic risk groups, high-risk mutations (TP53, ASXL1, RUNX1), FLT3, IDH, hematopoietic cell transplantation–specific comorbidity index (HCT-CI; supplemental Table 7c)—were balanced different between groups (Table 1; supplemental Tables 1a and 1b for baseline characteristics within each data source). Missing values in the HUP dataset were <5% for all baseline covariates (except for myelodysplasia-related cytogenetic changes); Flatiron Health dataset missing values ranged from 0% to 56% for covariates (eg, sex and baseline LDH, respectively).
OS
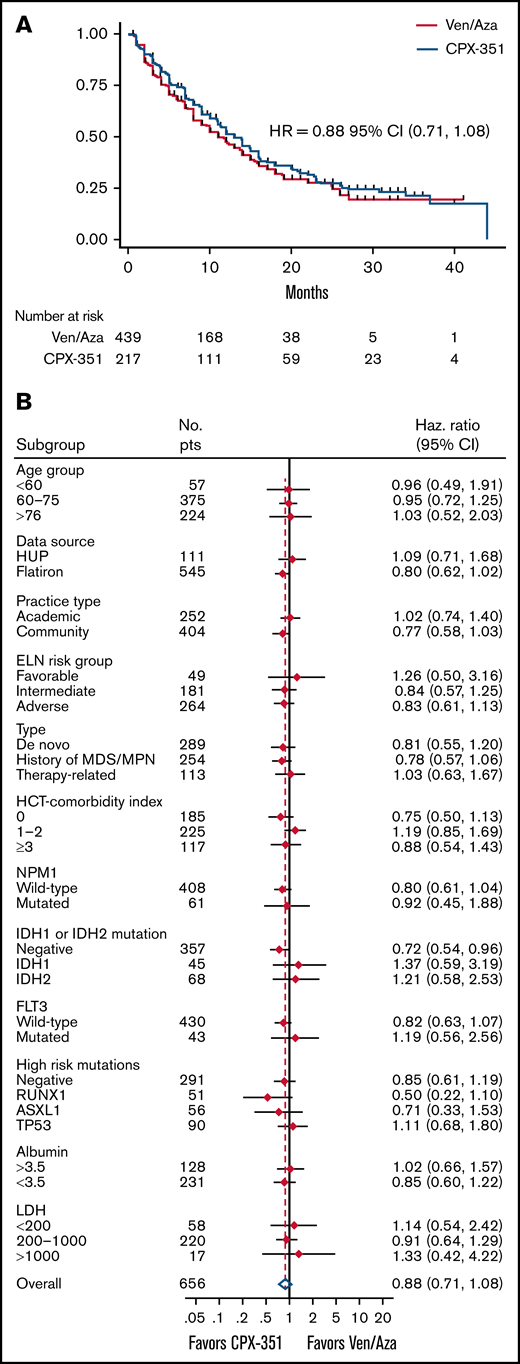
Median OS (mOS) was 12 months for all patients and 13 months for CPX-351 vs 11 months for ven/aza (HR, 0.88; 95% CI, 0.71-1.08; P = .22; Figure 2A; supplemental Figure 1). There were 144 deaths in the CPX-351 group (144 of 217, 66%) and 236 deaths in the ven/aza group (236 of 439, 54%). One-year OS was 51% (95% CI, 0.44-0.58) vs 48% (95% CI, 0.42-0.53), and 2-year OS was 28% (95% CI, 0.21-0.34) vs 28% (95% CI, 0.22-0.34) for CPX-351 and ven/aza, respectively.
OS and subgroup survival. (A) OS according to treatment arm (n = 656). HR and 95% CI are shown. (B) Selected univariate subset analyses for comparison of CPX-351 vs ven/aza.
OS and subgroup survival. (A) OS according to treatment arm (n = 656). HR and 95% CI are shown. (B) Selected univariate subset analyses for comparison of CPX-351 vs ven/aza.
There was no clear subset with improved OS with either CPX-351 or ven/aza (Figure 2B) by univariate analysis. In multivariate analysis controlling for all covariates with univariate P < .20 (sex, insurance, Eastern Cooperative Oncology Group performance status, myelodysplastic-related cytogenetics, ELN risk category, type, HCT-CI score at diagnosis, mutations in ASXL1, DNMT3A, IDH, NF1, NPM1, RUNX1, TET2, TP53, and baseline white blood cell count, hemoglobin, platelets), induction choice did not affect OS (limited to complete cases, n = 132; HR, 0.71; P = .33; supplemental Table 2).
Sensitivity analyses
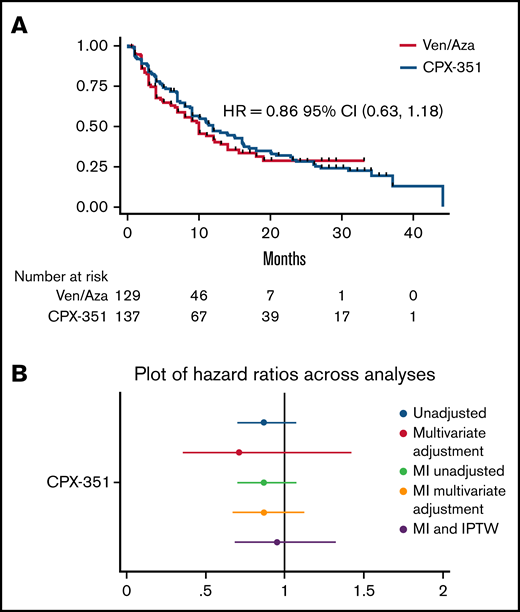
Restricting to only the population eligible for the CPX-351 pivotal trial (63% for CPX-351 and 29% for ven/aza), there was no significant difference in mOS (CPX-351, n = 138, 12 months; ven/aza, n = 129, 10 months; HR, 0.86; P = .33; supplemental Figure 3a). To address missingness and imbalances in baseline covariates, we undertook a separate analysis using multiple imputation and inverse probability of treatment weighting. After multiple imputation, using the entire 656 patients for multivariate analysis, survival was similar (HR, 0.87; P = .31; supplemental Tables 3 and 4). After multiple imputation and inverse probability of treatment weighting, baseline covariates were well balanced, with <10% absolute standardized differences between groups (supplemental Figure 3). Survival remained similar (HR, 0.96; P = .77; 95% CI, 0.69-1.32; Figure 3B).
Survival with restriction to CPX-351 trial-eligible patients and across sensitivity analyses. (A) OS with population restricted to 60 to 75 year olds with a history of a therapy-related myeloid neoplasm, myelodysplasia-related cytogenetics, or history of MDS (n = 267). (B) Plot of HR for OS for CPX-351 vs ven/aza across multiple analyses including unadjusted univariate Cox analysis, multivariate Cox analysis, univariate Cox analysis after multiple imputation, multivariate Cox regression analysis after multiple imputation, and Cox regression analysis after multiple imputation with inverse probability of treatment weighting.
Survival with restriction to CPX-351 trial-eligible patients and across sensitivity analyses. (A) OS with population restricted to 60 to 75 year olds with a history of a therapy-related myeloid neoplasm, myelodysplasia-related cytogenetics, or history of MDS (n = 267). (B) Plot of HR for OS for CPX-351 vs ven/aza across multiple analyses including unadjusted univariate Cox analysis, multivariate Cox analysis, univariate Cox analysis after multiple imputation, multivariate Cox regression analysis after multiple imputation, and Cox regression analysis after multiple imputation with inverse probability of treatment weighting.
Effect of allogeneic hematopoietic cell transplant on OS
Significantly fewer patients treated with ven/aza (10%, 44 of 440) went on to receive allogeneic transplant compared with CPX-351 (28%, 62 of 219, P < .0005). Censoring at time of transplant resulted in median OS of 7 vs 9 months for CPX-351 vs ven/aza (HR, 1.26; 95% CI, 1.05-1.52). Among patients who underwent transplant, patients received a median of 2 cycles of CPX-351 (range, 1-3) and a median of 4 cycles of ven/aza (range, 2-10). Median time to transplant was 171 days in the CPX-351 group and 186 days in the ven/aza group. Among transplanted patients, the median survival time was 37 months (HR of CPX-351 vs ven/aza was 1.27; P = .562; supplemental Figure 2). Examining transplant as a time-varying covariate shows that transplant does improve survival as expected (HR, 0.33; P < .0005), but choice of therapy did not influence survival at any time point while controlling for transplant status alone (HR, 0.97; P = .78).
Secondary outcomes
Response rates
Forty-nine percent of patients undergoing CPX-351 had a CR or CRi after cycle 1 (12% CR, 37% CRi). Of the 46% of patients who were refractory, 9% were reinduced with CPX-351 and 70% had a CR/CRi (total 56% including reinduction). Forty-three percent of patients undergoing ven/aza had a CR or CRi after cycle 1 (17% CR and 26% CRi). Nine percent of patients undergoing CPX-351 did not have a bone marrow biopsy for response assessment compared with 34% of patients undergoing ven/aza (supplemental Table 6). Within the HUP dataset, 48% of patients undergoing CPX-351 were reinduced, and of those patients, 36% received CPX-351 again. Among the 22% of patients undergoing ven/aza who had a bone marrow biopsy assessment after course 1 and did not achieve a CR or CRi, 58% received ven/aza again, and the remainder received high-dose cytarabine, gemtuzumab ozogamicin, or CPX-351. Notably, within the entire cohort, 2 patients in the ven/aza group received CPX-351 after not achieving CR or CRi, and 28 patients received venetoclax and an HMA after CPX-351 induction failure. At the end of 2 cycles of therapy among patients with bone marrow biopsies, 65% (n = 100 of 288) of patients undergoing ven/aza achieved a CR or CRi vs 61% (77 of 197) of patients undergoing CPX-351. Of 439 patients receiving ven/aza, 106 patients (24%) were alive at 60 days and had not had a bone marrow biopsy to assess response compared with only 12 (9%) of the 217 patients undergoing CPX-351. Academic practices were more likely to have obtained a bone marrow biopsy (80% of patients undergoing ven/aza, 97% of patients undergoing CPX-251) compared with community-based practices (68% of patients undergoing ven/aza, P = .01 and 91% of patients undergoing CPX-351, P = .12).
Relapse-free survival
Among patients achieving a CR or CRi, median relapse-free survival was 16 months (95% CI, 13-19 months) for ven/aza and 11 months for CPX-351 (95% CI, 13-23 months; HR, 0.86; supplemental Figure 4a) Median event-free survival was 2 months for CPX-351 and 5 months for ven/aza (HR, 1.28; 95% CI, 1.06-1.55), largely driven by measured refractory disease after cycle 1 (supplemental Figure 4b; supplemental Table 6).
Mortality and infection
Thirty-day all-cause mortality was similar at 5% in each group (n = 11 of 217 and n = 22 of 439; P =.51); 60-day mortality was 10% for CPX-351 and 13% for ven/aza (n = 21 of 217 and n = 58 of 439; P = .10). However, documented diagnosed infections were higher in patients undergoing CPX-351 and, within the HUP cohort where charts could be reviewed, rates of febrile neutropenia and culture positive infection were also higher (Table 2). Length of stay, including any admission before the next cycle of therapy, was more than twice as long for CPX-351 in the HUP cohort (41 vs 15 days; P < .00005). For the HUP cohort, this time reflects readmission, prolonged admission, and the practice of administering CPX-351 inpatient for the HUP cohort.
Discussion
In older adults with AML, initial treatment with CPX-351 or ven/aza resulted in similar OS. This equivalence persists when restricting the analyses to only patients who would have been eligible for the CPX-351 approval trial. This equivalence also persists for the entire population in multivariable regression controlling, as well as in several other analyses including multiple imputation with inverse probability of treatment weighting accounting for missing or imbalanced baseline data. One important finding our study highlights is the critical role of allogenic stem cell transplant in this population. The similar OS for both groups despite more than twice as many patients undergoing CPX-351 undergoing transplant may reflect the role of ongoing therapy with multiple courses of ven/aza or lower rates of serious adverse events over treatment duration. There may be further potential to improve survival with ven/aza if additional patients have early referral for transplant evaluation. Another key finding is that 34% of patients treated with ven/aza did not undergo a bone marrow biopsy after cycle 1. This bone marrow biopsy is essential to allow for a pause in therapy to avoid prolonged myelosuppression in responding patients.
Our study confirms the high unmet need among older adults with AML. OS without transplant is poor and consistent with other studies. Other real-world series have found a median OS with ven/aza ranging from 9.8 to 12.5 months13,28,29 vs the 14.7 months seen in the VIALE-A study.8 Similarly, several groups have reported their real-world experience with CPX-351 including national, multicenter efforts from France,30 Germany,31 and Italy,32 as well as smaller single center studies,33 with median OS ranging from 9 to 21 months, with 28% to 62% of patients going on to allogeneic stem cell transplant. Our national study shows similar results with differences in transplant rates likely accounting for variation in median survivals. Furthermore, our study does not assess clinician adherence to strategies designed to optimize care. These OS results reflect real-world effectiveness today; they may not reflect optimal outcomes for either regimen.
A definitive evidence-based recommendation favoring ven/aza or CPX-351 in older adults will require a randomized trial. Other single center studies have shown similar outcomes as our study including Salhotra et al34 (mOS, 11 vs 10 months for ven/aza vs CPX-351, respectively; P = .76) in 50 patients at City of Hope, and Asghari et al35 (mOS, 14 vs 11 months for ven/aza vs CPX-351, respectively; P = .82) in 199 patients at Moffitt Cancer Center and Memorial Healthcare System. Other efforts to compare venetoclax and HMA with intensive chemotherapy are also emerging and require careful comparison, or adjustment, of baseline characteristics to overcome confounding by indication14 and rate of transplant in both groups. This study was not designed to assess the impact of initial treatment on transplant. To properly assess this, a larger sample size, detailed information regarding conditioning regimen, donor source, graft-versus-host disease prophylaxis, and transplant related outcomes with longer follow-up would be needed, especially considering posttransplant follow-up was shorter for ven/aza than CPX-351.
To inform this daily clinical choice, this large, multicenter, national, well-powered real-world retrospective series shows there is no major difference in OS between CPX-351 and ven/aza. This study was powered to detect a clinically significant difference in OS of the magnitude seen in most AML pivotal trials. Although subject to ascertainment and misclassification bias risks, key adverse events including length of stay, febrile neutropenia, and infections appear to favor treatment with ven/aza. This raises the question of whether intensive chemotherapy should remain the standard recommendation for fit older patients or even younger patients with adverse genetics. Although this study attempted to address missingness and imbalance in baseline covariates, there may still be some unmeasured confounding, especially given the high rate of missing data for some baseline variables like performance status. That said, these methods did allow for a balanced comparison as assessed by the small absolute standardized difference between the groups. Instrumental variable analysis, especially randomization, is needed to fully address this concern. Prospective protocols with consistent inclusion criteria (eg, randomized phase 2 trial like #NCT04801797), predefined disease monitoring, and longitudinal assessment of access to curative intent transplant, as well as quality-of-life and detailed adverse event analyses are needed. Shared decision making regarding risks and benefits beyond OS will be necessary until prospective, randomized data are available.
Acknowledgments
A.H.M. received tuition support for an MS in clinical epidemiology from the Abramson Cancer Center and the Center for Clinical Epidemiology and Biostatistics of the University of Pennsylvania.
Authorship
Contribution: A.H.M. and K.W.P. conceived of the study and reviewed the charts; A.H.M. conducted all analyses with the help of W.-T.H. and D.M.; A.H.M. drafted the manuscript with contributions from K.W.P. and A.E.P.; and all authors read, edited, and approved the final manuscript.
Conflict-of-interest disclosure: A.E.P. received research funding from Fujifilm, Daiichi Sankyo, Astsellas, Arog, and AbbVie and was a consultant for Daiichi Sankyo, Sumitomo Dainippon, Astellas, BMS/Celgene, Genentech, Loxo, Onconova, Syndax, Forma, Actinium, Roche, and AbbVie. S.M.L. received honoraria from Syros, Agios, Daiichi Sankyo, Jazz Pharmaceuticals, Brystol Myers Squibb, Acceleron, Astellas, and Pfizer and research funding from Onconova, Celgene, Biosight, Hoffman LaRoche, and Kura. N.V.F. was a consultant for Sana Biotechnology, Kite Pharma, and Syndax Pharmaceuticals and received research funding from Novartis. S.I.G. has licensed intellectual property with Novartis; received research funding from Novartis, Carisma Therapeutics, and Interius Biotherapeutics; and is a current holder of stock options in Carisma Therapeutics and Interius Biotherapeutics. E.O.H. received research funding from Tmunity Therapeutics and Blueprint Medicines and had membership on an entity's board of directors or advisory committees for Blueprint Medicines and PharmaEssentia. D.L.P. received honoraria from the American Society for Transplantation and Wiley and Sons Publishing; had membership on an entity's board of directors or advisory committees for American Society of Hematology, DeCart, Incyte, Janssen, Kite/Gilead, National Marrow Donor Program, and Novartis; is a current equity holder in Genentech; and has patents and royalties with Unity. E.A.S. has consulted for Oncopeptides, Amgen, BMS Celgene, GSK, Janssen, and AbbVie and received research funding from AbbVie, Maillard, and Regeneron. W.-T.H. received research funding from Novartis. D.M. has consulted for Pfizer, Leo, and Sanofi and has membership on an entity's board of directors or advisory committees for the National Eczema Association. K.W.P. has consulted for Abbvie, Agios, BMS, Astellas, and Novartis; received honoraria from Abbvie, BMS, Astellas, and Cellgene; and received research funding from Abbvie, Astellas, and Millenium. The remaining authors declare no competing financial interests.
Correspondence: Keith W. Pratz, 3400 Civic Center Blvd, PCAM 12-155 Philadelphia PA 19104; e-mail: keith.pratz@pennmedicine.upenn.edu.
References
Author notes
Please contact andrew.matthews@pennmedicine.upenn.edu for original data. Flatiron data can be obtained through agreement; please contact academic-partnerships@flatiron.com.
The full-text version of this article contains a data supplement.