TO THE EDITOR:
Venetoclax is an effective therapy for chronic lymphocytic leukemia (CLL) with the potential to induce deep remissions.1-5 Furthermore, venetoclax in combination with an anti-CD20 antibody is now approved on fixed-duration treatment schedules (front-line: 12 months with obinutuzumab3 ; relapsed/refractory: 24 months with rituximab).2 As fixed-duration venetoclax-based therapies are increasingly used, more patients will require CLL therapy after venetoclax.
Whether patients who had initial responses to venetoclax-based therapies may be effectively re-treated with venetoclax in a later line of therapy is an important question. The current literature describes venetoclax re-treatment in only a small number of patients. Four patients from the phase 1b study of venetoclax and rituximab in CLL have been re-treated with venetoclax (3 evaluable patients, 100% with partial responses [PR]; duration of responses 18.7-40.3 months).6 Additionally, preliminary data reported a 72.2% overall response rate (ORR) for patients included in the MURANO study who have been re-treated with venetoclax (18 evaluable patients; median treatment duration 11.4 months; range, 0.7-27.6 months).7
We conducted a multicenter, international retrospective study investigating outcomes and safety data for patients with CLL treated with a venetoclax-based regimen (Ven1) in any line of therapy and then re-treated with a second venetoclax-based regimen (Ven2) in a later line of therapy. Data were collected from 3 sources: 15 medical centers (n = 30), CLL Collaborative Study of Real-World Evidence database (n = 5), and patients from the MURANO trial dataset (n = 11). This study was approved by the institutional review board and conducted in accordance with the Declaration of Helsinki. Collected data included patient demographics, prognostic disease characteristics at Ven1 start, tumor lysis syndrome (TLS) incidence, clinical response, and reasons for treatment discontinuation (Ven1 and Ven2). Patients were excluded if best response to the initial venetoclax regimen was progression of disease (PD). The primary study endpoint was investigator-assessed ORR for Ven2 (complete response [CR], PR, stable disease [SD], PD, International Workshop on CLL [iwCLL] 2018).8 The Kaplan-Meier method was used to estimate progression-free survival (PFS). All other analyses were descriptive. Data were analyzed using Stata/SE 17.0.9
We identified 46 patients with CLL who were re-treated with venetoclax. Patient characteristics prior to initial venetoclax regimen (Ven1) are summarized in Table 1. In most cases (91.3%), Ven1 was administered in the relapsed and/or refractory setting. The median number of prior therapies was 2 (0-10), and 40.0% of patients had received a Bruton’s tyrosine kinase inhibitor (BTKi) prior to Ven1 (unknown prior BTKi status, n = 1). Ven1 was commonly administered in combination with anti-CD20 antibody therapy (rituximab 47.8%; obinutuzumab 4.3%) or as monotherapy (37.0%).
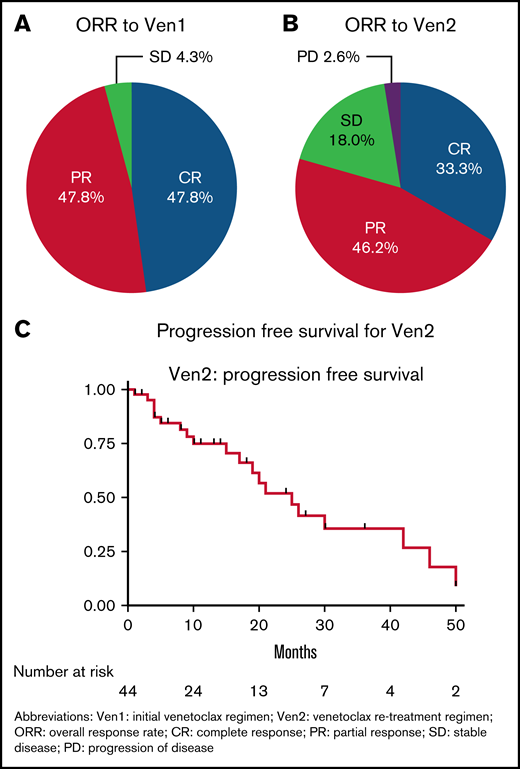
For Ven1, 85.3% of patients received a maximal dose of venetoclax of 400 mg daily. Clinical or laboratory TLS was consistent with prior studies (n = 2 of 33 patients with available data; 1 laboratory, 1 clinical TLS). With a median exposure to Ven1 of 18 months (76.1% ≥ 12 months), the ORR to Ven1 was 95.7% (CR 47.8% [n = 22], PR 47.8% [n = 22], SD 4.3% [n = 2]; Figure 1A). Eighteen of 28 patients (64.2%) with available minimal residual disease (MRD) data for Ven1 had undetectable MRD by flow cytometry (threshold 10−4 sensitivity).
Patient outcomes with venetoclax re-treatment. (A) ORR for Ven1. (B) ORR for Ven2. (C) PFS for Ven2.
Patient outcomes with venetoclax re-treatment. (A) ORR for Ven1. (B) ORR for Ven2. (C) PFS for Ven2.
The reasons for Ven1 discontinuation included completion of planned therapy, 39.1% (n = 18); toxicity, 21.7% (n = 10); MD/patient preference, 19.6% (n = 9); other, 8.7% (n = 4); progression of CLL on therapy, 4.3% (n = 2); stem cell transplantation, 4.3% (n = 2); and cost, 2.1% (n = 1). Toxicities leading to discontinuation included neutropenia (n = 2), dermatologic toxicity (n = 2), diarrhea/colitis (n = 2), viral infection (n = 2), bacterial infection (n = 1), and other (n = 1).
There was a median of 16 months between the completion of Ven1 and the start of Ven2 (range 3-52 months; 84.8% of patients with ≥ 6-month interval; 58.7% ≥ 12-month interval). Most patients (84.8%) did not have another line of therapy between Ven1 and Ven2. Reasons for initiation of Ven2 were iwCLL PD (93.0% of 43 cases with available data) and MRD-positive relapse without clinical progression (7.0%). Ven 2 was administered as monotherapy (45.7%) or in combination with rituximab (28.2%), obinutuzumab (10.9%), ibrutinib (4.4%), or other (10.9%).
The 5-week dose escalation ramp-up was used for Ven2 in 86.7% of cases (n = 26 of 30 patients with available data). Alternative methods used for introduction of venetoclax included a ramp-up schedule to a maximum dose of 100 mg daily (n = 2), a more rapid ramp-up (n = 1), and 1 patient who resumed at 400 mg daily without ramp-up. TLS occurred with Ven2 in 3 patients (2 clinical, 1 unknown; 1 patient used a modified dose escalation of 20 mg venetoclax daily for 2 weeks, 50 mg daily for 2 weeks, and then 100 mg daily; 2 patients with unknown strategies).
The ORR to Ven2 was 79.5% (n = 39 patients with available response data; CR 33.3%, n = 13; PR 46.2%, n = 18; SD 18.0%, n = 7; PD 2.6%, n = 1; Figure 1B). Twelve patients had flow cytometry MRD assessments during Ven2 therapy, with 41.7% (n = 5) of these patients having undetectable MRD; 3 CR, 1 PR, 1 missing response assessment). At a median follow-up of 10 months (range 1-50 months), the median Ven2 PFS for the overall cohort was 25 months (95% CI, 17-42 months; Figure 1C). The median time on Ven2 was 10 months. Of the 26 patients who have discontinued Ven2, reasons for discontinuation of Ven2 included progression of CLL (n = 12), death not due to CLL progression (n = 3), completion of planned therapy (n = 4), MD/patient preference (n = 2), other or unknown (n = 2), toxicity (n = 1), sudden death on therapy (n = 1), and Richter Transformation (n = 1). For the subgroup of patients with BTKi exposure prior to Ven1 (n = 18), the ORR to Ven2 was 56.3% (n = 16 patients with available response assessments) and the median PFS was 15 months (median follow-up 8 months, n = 18 patients).
Here we present the largest reported series of patients with CLL who have been re-treated with venetoclax. We observed a high ORR (79.5%) in a heavily pretreated patient population. Furthermore, our data support the safety of venetoclax re-treatment, with 3 patients experiencing TLS.
Importantly, 40% of patients had been treated with a BTKi prior to Ven1. Currently, there are limited treatment options for patients with CLL who require additional therapy after treatment with both a BTKi and venetoclax (ie, “double exposed” patients).10,11 Currently approved therapies include phosphatidylinositol-3-kinase inhibitors, which have limited efficacy after venetoclax and BTKi (median PFS 5 months),12,13 and chemo+/−immunotherapy combinations, which have poor outcomes after targeted agents.13 The observed responses in the patients exposed to BTKi patients in our cohort (ORR 56.3% to Ven2) support the role of venetoclax re-treatment filling an unmet need for select “double exposed” patients with CLL. Future studies should examine venetoclax re-treatment prospectively in a larger population of patients exposed to BTKi.
The efficacy of re-treatment in a heavily relapsed/refractory population also supports further evaluation of venetoclax re-treatment after disease progression following time-limited treatment in the front-line setting. There are currently no data on venetoclax re-treatment after first-line venetoclax. Our study included 4 patients in whom Ven1 was the initial CLL therapy, with 4 of 4 evaluable patients responding to venetoclax re-treatment. Several ongoing prospective studies are examining venetoclax re-treatment following front-line venetoclax-based therapy (NCT04523428, NCT04447768, NCT04419519, and NCT04895436).
We acknowledge limitations of our study design. Data were collected retrospectively from investigators at individual sites; therefore, variation in chart review may exist. Responses were assessed with iwCLL criteria provided as a guide; however, central confirmation of responses was not performed. To address inconsistencies in chart review or missing information, the data provided were reviewed carefully by the lead investigators (A.R.M. and M.C.T.). If required, specific details of cases were clarified with site investigators. Longer follow-up will be required to determine the durability of responses. Additionally, this study included both front-line and relapsed and/or refractory patients who were re-treated with monotherapy as well as venetoclax-based combination therapies. Future dedicated prospective studies with more standardized re-treatment regimens will be important to validate these results. Furthermore, in this study, the median time between Ven1 and Ven2 was relatively short at 16 months, perhaps owing to the heavily pretreated patient population included in this study. The ongoing prospective studies examining venetoclax re-treatment after front-line venetoclax-based therapy will be important in providing re-treatment response and survival estimates for patients with longer remissions to the initial venetoclax-based regimen and fewer prior therapies.
We report the largest experience of the venetoclax re-treatment strategy in patients with CLL. The results of this study have substantial clinical relevance as more patients require treatment of CLL following time-limited venetoclax-based therapy.14 The high ORR and durability of observed remissions support the clinical practice of venetoclax re-treatment and the development of future studies to prospectively validate venetoclax re-treatment in patients with CLL.
Acknowledgments: This research was supported in part by The Lymphoma Foundation (M.C.T.). T.A.E. recognizes support from the Oxford NIHR Biomedical Research Centre.
Contribution: M.C.T. and A.R.M. designed the study, collected, coordinated, analyzed, and interpreted data, and wrote the manuscript; R.A.H. and J.F.S. interpreted data and wrote and edited the manuscript; B.M. and D.L. collected data; and C.C.C., L.E.R., J.J.P., M.Y.C., P.M.B., J.N.A., M.Š., L.L., J.R., E.A.C., M.K., A.S., F.L., K.S., M.J.S.D., H.S.W., M.L., M.T.-M., M.B., J.B., K.S., B.S.M., R.F., K.S.B., A.G., T.F., S.J.S., J.P., L.P., A.Z., T.A.E., and A.P.K. collected and interpreted data and edited the manuscript.
Conflict-of-interest disclosure: M.C.T. has received honoraria from MJH Life Sciences, Curio Science, VJHemOnc, Brazilian Association of Hematology and Hemotherapy (ABHH) and Massachusetts Medical Society. C.C.C. has served as a consultant for AbbVie, served on steering committees for AbbVie and Loxo Oncology, served on independent review committees for AbbVie and Octapharma, received honoraria from AbbVie, AstraZeneca, BeiGene, Genentech, LOXO Oncology, MEI Pharma, Novartis, and TG Therapeutics, and received research funding (paid to the institution) from H3 Biomedicine, Incyte, and Loxo Oncology. L.E.R. has served as a consultant for AbbVie, AstraZeneca, BeiGene, Janssen, Loxo Oncology, Pharmacyclics, Pfizer, TG Therapeutics, and Vaniam group, holds minority ownership interest in Abbott Laboratories, and has received research funding (paid to the institution) from Pfizer, Loxo Oncology, and Aptose Biosciences. M.Y.C. has research support from AbbVie, TG Therapeutics, Oncternal Therapeutics, Merck, Pharmacyclics, and Geron and has received consulting fees from Genentech and AbbVie. P.M.B. has served as consultant for Genentech, AbbVie, PCYC, TG, Seattle Genetics, Janssen, AstraZeneca, BMS, GSK, and BeiGene. J.N.A. has served as a consultant for AbbVie, ADC Therapeutics, AstraZeneca, BeiGene, Epizyme, Genentech, Janssen, Pharmacyclics, and TG Therapeutics and has received research funding from AstraZeneca, BeiGene, Genentech, Janssen, TG Therapeutics. M.Š. has received consultancy fees, advisory board participation fees, travel grants, and honoraria from Janssen, AstraZeneca, and AbbVie. L.L. has served as advisory board or panel participation, speaker bureau and/or consultant for AbbVie, AstraZeneca, BeiGene, Celgene/Bristol Myers Squibb, Epizyme, Karyopharm, Janssen, Kite/Gilead Sciences, Merck, Pharmacyclics, Seagen, and TG Therapeutics. J.R. has received consulting fees from AstraZeneca, BeiGene, PCYC, Genentech, AbbVie, TG Therapeutics and Verastem. E.A.C. has received consultancy fees from Novartis, Bristol-Myers Squibb, KITE, BeiGene, and Tessa Therapeutics. M.K. has served as consultant for AbbVie, AstraZeneca, Celgene/Bristol-Myers Squibb, Adaptive Biotechnologies, ADC Therapeutics, and BeiGene, was on a speaker’s bureau for SeaGen, DMC for Celgene, and Genentech, and received research support/funding from TG Therapeutics, Genentech, and Novartis. A.S. has received honoraria for consultancy and/or speakers bureau from AstraZeneca, AbbVie, ADC Therapeutics, BeiGene, Celgene, Genentech, GenMab, Jazz Pharmaceuticals, Janssen, Kite Pharma, MorphoSys, Novartis, Pharmacyclics, Bristol Myers Squibb, and TG Therapeutics. F.L. has served as steering committee member and consultant for Bristol Myers Squibb. K.S. has received honoraria from Abbvie. M.L. has employment and shares with F. Hoffman-La Roche Ltd. M.T.-M. has employment and shares with F. Hoffman-La Roche Ltd. M.B. has employment and shares with F. Hoffman-La Roche Ltd. J.B. has employment and shares with Genentech, Inc. K.S. is an employee of AbbVie and may own stock or stock options. B.S.M. is an employee of AbbVie and may own stock or stock options. R.F. serves on an advisory board for AbbVie. A.G. has received honoraria for consulting from Celgene, Novartis, Hoffman la Roche, Novartis, Celgene, Kite, Pharmacyclics/AbbVie, and Janssen, honoraria from Elsevier’s PracticeUpdate Oncology, Clinical Advances in Hematology/Oncology, Medscape, Physicians Education Resource LLC, Rosewell Park, OncLive Peer Exchange, Michael J Hennessey Associates, INCH, and Bristol Meyers Squibb, payment for expert testimony from Xcenda, support for attending meetings and/or travel from Physician Education Resources, fees for participation on Data Safety Mentoring Board or Advisory Board from Vincerx Pharma, Janssen, Kite Pharma, Elsevier’s Practice Update Oncology, Gilead, AbbVie/Pharmacyclics, Bristol Meyers Squibb, AstraZeneca, Alloplex, Janssen, and BMS, is on the Board of Directors for COTA, Genomic Testing Cooperative LCA, and Resilience, has stock or stock options with COTA, Genomic Testing Cooperative, Alloplex, and Resilience, is on the steering committee for MorphoSys and Incyte, AstraZeneca, Pharmacyclics LLC, AbbVie, and Janssen, has received services for other than consulting from Hoffman La Roche, and received research funding to institution from Acerta, Celgene, Constellation, Genentech, Hoffman la Roche, Infinity Pharmaceuticals, MorphoSys, Karyopharm, Kite, Janssen, Pharmacyclics, AstraZeneca, Verastem, Seattle Genetics, and Bristol Myers Squibb. T.F. has received honoraria from AbbVie, BMS, Celgene, Janssen, Pharmacyclics, SeattleGenetics, Takeda, ADC Therapeutics, AstraZeneca, Daiichi Sakyo, Karyopharm, MorphoSys, and KITE, and participated on advisory board or DSMB for AbbVie, ADC Therapeutics, AstraZeneca, Daiichi Sakyo, Karyopharm, KITE, and MorphoSys. S.J.S. has served as a consultant for AbbVie, AstraZeneca, BeiGene, Celgene, Genentech, Incyte, Janssen, Legend Biotech, Loxo Oncology, Novartis, and Regeneron Pharmaceuticals and has received research funding from Genentech and Merck. J.P. has a consultancy with Amgen, Autolus, Novartis, BMS, Kite Pharma, Kura Oncology, Servier, Innate Pharma, Minerva, PrecisionBio, Pfizer, Artiva, and Curocel, is on the Data Safety Monitoring Board for Affyimmune, Intellia, and Brightpharma, and is on the Scientific Advisory Board for Allogene. L.P. has received honoraria for advisory boards from Novartis, Synthekine, BeiGene, Kite, and MustangBio, has an immediate family member who received sponsored research support from Seres, has an immediate family member with honoraria from Seres, Vor Biopharma, Rheos, Frazier Healthcare Partners, Nektar Therapeutics, Notch Therapeutics, Ceramedix, Lygenesis, Pluto Therapeutics, GSK, Da Volterra, THymofox, and Garuda, and has an immediate family member with stock options in Seres, Notch Therapeutics, and Pluto Therapeutics. A.Z. has served as a consultant for Genentech/Roche, Gilead, BMS, Celgene, JUNO, Janssen, Novartis, Adaptive Biotechnology, MorphoSys, AbbVie, AstraZeneca, MEI Pharma, and BeiGene, has received research funding from MEI Pharmaceuticals, Genentech/Roche, and BeiGene, and has receive other from BMS, Celgene, Juno–DMC Member. T.A.E. has received honoraria and/or advisory board honoraria from Roche, Gilead, KITE, Janssen, AbbVie, AstraZeneca, LOXO Oncology, BeiGene, Incyte, Takeda, and Secura Bio, has received research support from Gilead, BeiGene, and AstraZeneca, has received travel support Gilead, Takeda, and AbbVie, and served on a trial steering committee for LOXO Oncology. A.P.K. received research funding from Genentech, AbbVie, Janssen, and BMS and received consultancy fees from Genentech, AbbVie, Janssen, BMS, and LAVA. J.F.S. has served on advisory boards for AbbVie, AstraZeneca, BeiGene, GMS, Genentech, Genor Bio, Gilead, Janssen, Roache, Sunesis, and TG Therapeutics, has served on Speakers’ Bureaus for AbbVie, and Roche, has received research funding from AbbVie, BMS, Janssen, and Roche, and has provided expert testimony for TG Therapeutics and Roche. A.R.M. has served on a consultant advisory board/steering committee for Abbvie, AstraZeneca, BeiGene, Bristol Myers Squibb, Celgene, Genentech, Laboratorios Pfizer Ltda., LOXO Oncology, Octopharma, Pharmacyclics LLC, NURIX, GENMAB, TG Therapeutics; DSMB for Celegene, TG Therapeutics, member of Medical Advisory Board for CLL Society, CME speaker for Curio, consultant for DAVA, Medscape, and an ad hoc scientific advisor for the Lymphoma Research Foundation. Employees of AbbVie and Genentech participated in the review of this manuscript. Venetoclax is being developed in collaboration with AbbVie and Genentech/Roche. The MURANO trial was funded by AbbVie and Genentech/Roche. The remaining authors declare no competing financial interests.
Correspondence: Anthony R. Mato, Department of Medicine, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: matoa@mskcc.org.
References
Author notes
M.C.T. and R.A.H. are joint first authors.
J.F.S. and A.R.M. are joint senior authors.
Data will be shared upon e-mail request to the corresponding author: matoa@mskcc.org.