TO THE EDITOR:
High rates of alloimmunization have been observed among patients requiring chronic red blood cell (RBC) transfusions, especially those with sickle cell disease (SCD) and thalassemia.1-3 Alloimmunization in SCD increases the risk of hemolytic transfusion reactions, complicates procurement of compatible RBCs, and is associated with worse clinical outcomes.2,3 Beyond the SCD patient population, the economic and clinical impacts of alloimmunization are not as clear.
Previous studies, largely focusing on the SCD population, have reported that transfusion of RBCs that are phenotypically matched to the transfusion recipient may reduce the incidence of alloimmunization.4-10 Current RBC transfusion guidelines recommend Rh (D,C,c,E,e) and K antigen matching for patients with SCD and thalassemia who are regularly transfused.11-13 Expert opinion suggests extended prophylactic antigen matching beyond Rh and K could provide additional protection against alloimmunization, but data to confirm this suggestion are limited.4-10
The present study evaluates the association between alloimmunization and worsened economic and clinical outcomes in hospitalized patients with SCD and thalassemia, as well as anemia from other causes such as bleeding and bone marrow hypoplasia.
This nested case-control study used the Premier chargemaster dataset, an alliance of >2000 hospitals and >520 million patient encounters (20% of US discharges) with standard discharge files including demographics, disease state, billed services, and hospital characteristics (supplemental Table 1). All data are de-identified per Health Insurance Portability and Accountability Act requirements. Data analysis does not require institutional review board review or patient consent.
The study population included all inpatient admissions from January 2015 to June 2019. Because alloimmunization is not a structured diagnosis in this dataset, it was defined by reporting of both the following codes: antiglobulin crossmatch and RBC antibody identification. An accuracy assessment of this approach is included in the supplemental Material.
The alloimmunized population was matched to the non-alloimmunized population by sex, age, month/year of encounter, and diagnosis of SCD, thalassemia, and other anemias (eg, nutritional and enzymatic anemias, bone marrow failure, blood loss; supplemental Table 2) using propensity score matching through a nearest neighbor technique.14
The primary objective was to quantify the correlation between incremental cost per patient and alloimmunization. Secondary objectives were to assess the correlation between increased length of hospital and intensive care unit (ICU) stays, risk of ICU admission, and inpatient mortality with alloimmunization. Outcomes were assessed in the general population and 3 subpopulations (SCD, thalassemia, anemia). Inpatient hospital cost consisted of fixed costs plus variable costs (volume-linked expenses and patient care).
Statistical analyses (SAS v9.4) assumed a 2-tailed significance test and an α-level of 0.05. Demographics, clinical, billing, and hospital characteristics are displayed using counts and percentages for categorical variables and central tendency measures for continuous variables.
Multivariable regression models were conducted to assess the association between outcomes and alloimmunization while adjusting for diagnosis-related groups (DRG) with an incidence ≥1% (supplemental Tables 3-6).
Inpatient costs were modeled using quantile regression of medians; hospital length of stay (LoS) was modeled as a count variable using negative binomial regression; ICU LoS was modeled as a count variable using zero-inflated negative binomial regression; and inpatient mortality risk was modeled as a binary variable using logistic regression.
After matching, the study population resulted in 478 898 cases and 478 898 controls. Mean age was similar for the alloimmunized and non-alloimmunized groups, with more females (∼71%) than males. The population was racially balanced between groups (supplemental Table 7).
Three subpopulations (patients with SCD, thalassemia, and anemia) were identified (supplemental Table 8). Alloimmunized discharges were able to be matched to a similar number of non-alloimmunized controls, except for the thalassemia subpopulation (968 vs 576).
Patients with SCD and thalassemia presented with lower mean ages compared with the general population; as expected, this was in contrast to the anemia subpopulation (supplemental Table 8). The percentage of females was slightly higher than males across all subgroups, and Black patients were more frequently identified within the SCD and thalassemia subpopulations. Conditions leading to hospital admission and proportion of surgical vs medical admissions based on the Agency for Healthcare Research & Quality indicator are described in the supplemental material (text and supplemental Tables 9 and 10). Unadjusted clinical and economic outcomes for alloimmunized and non-alloimmunized patients are described in supplemental Table 11.
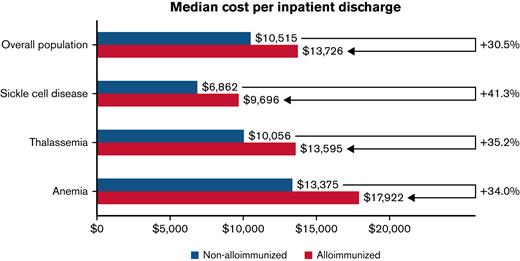
Compared with non-alloimmunized controls, the median cost per discharge was 30.5%, 41.3%, 35.2%, and 34.0% higher for the overall population and patients with SCD, thalassemia, and anemia, respectively (Figure 1). In addition, alloimmunized patients in the overall population had a 42.0% increased overall LoS, a 114.0% increased ICU LoS, a 79% higher likelihood of ICU admission, and a 79% increased inpatient mortality (Table 1).
Median cost per inpatient admission and incremental cost of alloimmunized vs non-alloimmunized groups adjusted by DRG.
Median cost per inpatient admission and incremental cost of alloimmunized vs non-alloimmunized groups adjusted by DRG.
For the alloimmunized SCD, thalassemia and anemia subpopulations, respectively, hospitalizations were 28%, 61%, and 39% longer, and ICU LoS was 59%, 214%, and 80% longer compared with non-alloimmunized controls. In addition, alloimmunized patients had an increased likelihood to require ICU admission (60% increase in SCD, 45% increase in thalassemia, and 57% increase in anemia). Inpatient mortality was significantly higher among alloimmunized patients in the anemia group. All these findings were statistically significant. In contrast, differences in inpatient mortality between alloimmunized and non-alloimmunized patients in the SCD and thalassemia groups did not reach statistical significance (Table 1).
The present study suggests that alloimmunization may be associated with worse economic and clinical outcomes. These findings may indicate that the negative implications of alloimmunization, previously reported among patients with SCD, may also extend to other anemic populations.
Prophylactic matching has been reasonably adopted to prevent alloimmunization in SCD.15 However, this has been questioned on the grounds of cost, limited resources, and lower success rates in patients with RHCE variants.16-23 Previous cost-effectiveness analyses of antigen matching focused on patients with SCD and alloimmunization costs were limited to specific complications.19,20,22 The incremental cost observed herein for the alloimmunized population could be driven by the increased LoS or ICU admission or because of blood procurement and associated testing. However, the longer ICU stays for alloimmunized patients suggests their increased costs were not because of antigen-negative blood procurement alone.
Previous research had investigated the clinical impact of alloimmunization, primarily focused on specific complications of regularly transfused populations.3,24,25 The present study may be the first evaluating the association of alloimmunization with worsened clinical and economic burden for broader patient populations beyond SCD.
However, our work has some limitations. The chargemaster data represent a log of health care diagnostics, cost, and utilization for accounting purposes and may misclassify certain conditions. Data are accumulated and analyzed at discharge level; therefore, a comprehensive history of a patient’s previous treatments or complications could not be obtained, and it is also possible that a single patient may be represented more than once. Alloimmunized admissions could not be identified directly because alloimmunization is not a discrete variable. Additionally, although study groups were matched based on demographic and clinical characteristics and models were adjusted based on the reason for hospitalization, the lack of granular data such as severity of the disease at admission, history of chronic transfusion, comorbidities, or socio-economic status, among others, curtailed our ability to assess whether these factors, rather than alloimmunization, are responsible for the observed differences in economic and clinical outcomes. Thus, additional studies are needed to determine whether our observations are truly caused by alloimmunization vs a confounding factor. Additional study is needed to determine whether provision of prophylactically matched RBCs could feasibly prevent alloimmunization in populations beyond SCD.
Acknowledgments: The authors thank Connie McLaughlin-Miley for support in the initial study design, Jerry Holmberg for support in the literature review, Michael James for support on medical writing activities, and Reuben Howden and Jessica Payne for support on the manuscript review.
Contribution: E.V., E.A.G., C.B., G.M.M., G.N., M.H., and M.C.R. designed the study and interpreted the results; E.V., C.B., G.M.M., and G.N. designed the protocol, developed the statistical analysis plan, and analyzed data; E.A.G., G.M.M., G.N., and M.H. contributed to the initial literature review; and E.V., E.A.G., C.B., G.M.M., G.N., M.H., and M.C.R. reviewed and contributed to the content of the final manuscript. All authors approved the content of the final manuscript.
Conflict-of-interest disclosure: E.V. is an employee of Grifols S.A. E.A.G., and C.B. are consultants to Grifols SSNA. G.M.M., M.H., and M.C.R. are employees of Grifols SSNA. G.N. was employed by Grifols SSNA at the time of project implementation. C.B. is currently an employee of Novo Nordisk Inc.
Correspondence: Eric A. Gehrie, American Red Cross, 431 18th St NW, Washington, DC 20006; e-mail: eric.gehrie@redcross.org.
References
Author notes
Presented in abstract form at the 2021 Conference of the American Society of Pediatric Hematology/Oncology (Virtual, 23 April 2021), at the 2021 Conference of the Association for the Advancement of Blood and Biotherapies (Virtual, 19 October 2021), and at the 2021 American Society of Hematology Annual Meeting (Virtual/Atlanta, 11-14 December 2021).
For original data, please contact: Christopher Blanchette (cblanchette@me.com).
The full-text version of this article contains a data supplement.
final version published online 19 October 2022
E.V. and E.A.G. contributed equally to this study.
E.V. and E.A.G. are joint first authors.