Key Points
CD47 overexpression is common in non–GCB-type intestinal DLBCL and closely associated with 18q21 gain.
The intestinal type CD47-high DLBCL has different clinicopathologic characteristics from the nonintestinal type.
Abstract
The CD47/signal regulatory protein α pathway is an emerging immune checkpoint that is a new therapeutic target. We investigated CD47 expression in diffuse large B-cell lymphoma (DLBCL) of various subtypes and organs. Moreover, the relationship between CD47 expression and genetic alterations was analyzed using panel-based massively parallel sequencing (next-generation sequencing [NGS]). CD8, CD68, and CD47 immunohistochemical staining were performed on 238 patients with DLBCL. CD47 was scored according to intensity on a 5-level scale, and CD8 and CD68 were quantitatively evaluated using QuPath software. Panel-based NGS was performed in 37 patients. In CD8 and CD68 quantitative analyses by organs, intestinal DLBCL showed significantly lower cytotoxic T-cell infiltration than that in others (P < .001). The CD47-high group comprised 24 of 58 (41.4%) patients in the group with DLBCL from intestine and 15 of 180 (8.3%) patients in the group with DLBCL from other organs (P < .001). The 18q21 gain/amplification was found in 10 of 37 patients, and all of them were CD47-high. Intestinal CD47-high DLBCL occurred in terminal ileum to ascending colon and was restricted to nongerminal center B-cell type. In the survival analyses, the prognosis of nonintestinal CD47-high DLBCL was poorer than that of intestinal CD47-high DLBCL (P = .025). CD47-high DLBCL was closely associated with 18q21 gain/amplification and showed a high prevalence in intestine. We propose to classify CD47-high DLBCL into intestinal and nonintestinal types. Further studies are necessary to assess whether the constellation of features seen here is reproducible and sufficient to consider primary intestinal DLBCL as a distinct biological entity.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous disease group. Several specific subtypes have been classified as separate entities, but a significant number of DLBCL cases remain unclassified as not otherwise specified (NOS).1 Although the prognosis of patients with DLBCL improves after the rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone regimen has been used, only approximately 60% of patients may be cured.2 In addition to conventional chemotherapy, immunotherapy has recently been introduced as a new treatment strategy for non-Hodgkin lymphoma, and much progress has been made.3-5 To date, immunotherapy studies on lymphomas have often targeted immune checkpoints related to adaptive immunity represented by cytotoxic T cells and the programmed cell death protein 1/programmed death-ligant 1 pathway.6 However, innate immune checkpoints, such as the CD47/signal regulatory protein α (SIRPα) pathway, are also important components of antitumor immunity, and interest in it has been increasing.2
CD47 is a glycosylated cell surface protein that is expressed in various cell types.7,8 CD47 forms a signaling complex with SIRPα, which is highly expressed on the membrane of myeloid cells, such as macrophages, granulocytes, monocytes, and myeloid dendritic cells.9 When CD47 is highly expressed in tumor cells, it suppresses macrophage phagocytosis around tumor cells by sending a “don’t eat me” signal and consequently contributes to immune escape of tumor cells from host immunity.10 CD47 is overexpressed in various solid tumors, including non-Hodgkin lymphoma, and is associated with poor prognosis of patients.11,12 Given that CD47 in tumor cells downregulates antitumor host immune response, it is possible that therapies that inhibit the CD47/SIRPα signaling pathway can promote phagocytosis of tumor cells by macrophages and improve the prognosis of patients.13
Rituximab kills CD20-positive B-cell lymphoma cells in several ways, and antibody-dependent cell-mediated cytotoxicity is 1 of the mechanisms of rituximab.14 Through this process, tumor cells are killed by macrophage phagocytosis. If CD47 suppresses the activity of macrophages, the effect of rituximab would be diminished. Therefore, inhibition of CD47 may play a more important role in patients with B-cell lymphoma receiving rituximab-containing chemotherapy, and synergistic effects may occur when CD47 inhibitors are used in combination with rituximab.15,16 Recently, a phase 1 clinical trial of a CD47 blockade (TTI-621) in patients with lymphoma demonstrated its activity as monotherapy and combined with rituximab in patients with relapsed/refractory B-cell lymphoma.16
For effective immunotherapy, finding a patient group suitable for treatment and developing a biomarker for recognizing the patient group are very important. To identify patients eligible for a CD47 blockade, it is necessary to investigate which tumors express CD47 strongly. In this study, we determined CD47 expression in various DLBCL subtypes using immunohistochemistry. The molecular mechanism of CD47 overexpression in DLBCL was also investigated using panel-based massively parallel sequencing (next-generation sequencing [NGS]).
Materials and methods
Patient selection
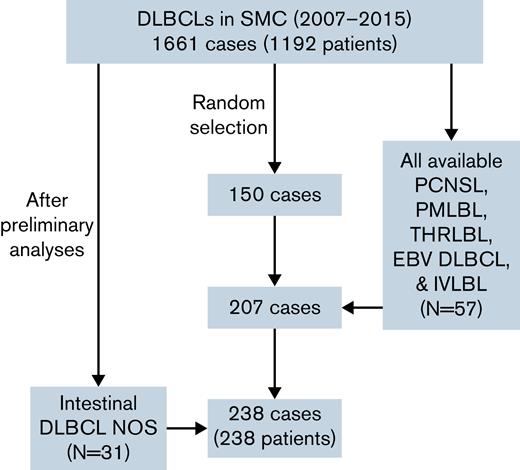
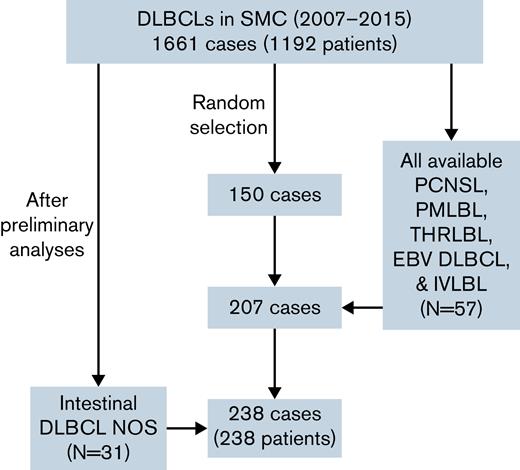
Patients diagnosed with DLBCL at the Samsung Medical Center, Seoul, Korea, between January 2007 and December 2015 were enrolled in the training cohort. During this period, a total of 1661 cases and 1192 patients of DLBCL were diagnosed at the Samsung Medical Center. At first, because of financial reasons, 150 cases in which formalin-fixed paraffin-embedded blocks were available were randomly selected. To investigate the difference of various DLBCL subtypes, primary DLBCL of the central nervous system (PCNSL), primary mediastinal large B-cell lymphoma (PMLBL), T-cell/histiocyte-rich large B-cell lymphoma (THRLBL), Epstein-Barr virus–positive (EBV) DLBCL NOS, and intravascular large B-cell lymphoma (IVLBL) cases diagnosed from 2007 to 2015 were searched and included in the study if tissues were available. In addition, to compare cases of various locations among DLBCL NOS, cases that occurred in intestine, testis, and spleen were additionally searched and included in the study. Eventually, 207 patients with DLBCL were included in the study. Based on the World Health Organization classification,1 DLBCL NOS included 132 cases (63.8%), PCNSL included 44 cases (21.3%), PMLBL included 10 cases (4.8%), EBV DLBCL included 9 cases (4.3%), THRLBL included 6 cases (2.9%), and IVLBL included 6 cases (2.9%). Among DLBCL NOS, the number of lymphomas that occurred in intestine, testis, and spleen was 27, 9, and 8 cases, respectively. Clinicopathologic information including age, sex, location, Ann Arbor stage, and survival data were evaluated by reviewing electronic medical records. Among them, 21 patients were refractory/recurrent patients, and the rest were treatment-naïve patients. As preliminary analyses showed significant results with intestinal lymphoma, a group of 31 additional randomly selected intestinal DLBCL NOS cases was added to the training cohort (Figure 1). To determine the cell of origin (COO) according to the Hans algorithm,17 CD10, Bcl-6, and MUM1 immunohistochemical staining results (positive cell percentage) performed at the time of diagnosis were gathered from the electronic medical records.
Profile of the training cohorts used in this study. A total of 238 cases were included in the cohort, with 57 specific subtype and 31 intestinal lymphoma cases added to 150 patients randomly selected from 1661 DLBCL cases. NOS, not otherwise specified; SMC, Samsung Medical Center.
Profile of the training cohorts used in this study. A total of 238 cases were included in the cohort, with 57 specific subtype and 31 intestinal lymphoma cases added to 150 patients randomly selected from 1661 DLBCL cases. NOS, not otherwise specified; SMC, Samsung Medical Center.
A separate validation cohort included 186 patients with DLBCL diagnosed at the Samsung Medical Center between January 2000 and December 2009. Tissue microarray paraffin blocks were assembled by collecting a 2-mm core tissue from the representative area of each tumor. PCNSL included 12 cases (6.5%), EBV DLBCL included 12 cases (6.5%), PMLBL included 4 cases (2.0%), THRLBL included 4 cases (2.2%) and IVLBL included 1 case (0.5%). Among the remaining 153 DLBCL NOS cases, the number of lymphomas that occurred in intestine, spleen, and testis was 54, 8, and 6 cases, respectively. Clinicopathologic features similar to those listed for the training cohort were evaluated (supplemental Table 1). All methods were carried out in accordance with Helsinki declaration, and all protocols of this study were approved by the institutional review board of the Samsung Medical Center (IRB file number: SMC 2021-01-093-002).
Immunohistochemistry
Immunohistochemical staining for CD8 (clone SP57, Ventana; for cytotoxic T cells), CD68 (clone 514H12, Leica Biosystems; for macrophage), and CD47 (clone HPA044659; Sigma-Aldrich) were performed in whole sections of each representative formalin-fixed paraffin-embedded block in all training cohort cases. Details of the immunohistochemical stains are provided in supplemental Table 2. To measure the degree of infiltration of CD8 and CD68 positive cells in tumors, all slides were scanned using a digital slide scanner Pannoramic 1000 (3DHistech, Budapest, Hungary). INFINITT DPS (INFINITT Healthcare, Seoul, Korea) was used as an image viewing system, and images with an area of 0.37 mm2 were captured in 1 representative area. The number of positive cells and the ratio (%) of positive cells to total cells in captured images were investigated in all cases except IVLBL using QuPath software18 (Figure 2A-E). CD47 was scored on 5 levels according to intensity (0, complete negative; 1, faint; 2, weak; 3, intermediate; 4, strong; Figure 2F-J). CD47 immunostaining was scored by 2 hematopathologists (J.C. and Y.H.K.), and if there were any discrepancies in scores, opinions were adjusted by looking at them together. To set the cutoff value for classifying CD47-high and -low groups, the CD68 ratio for each CD47 score was investigated. In the validation cohort, only CD47 immunostaining was performed in tissue microarray blocks, and CD47 score was evaluated in the same manner described for the training cohort.
Immunohistochemical stains of CD8, CD68, and CD47. (A-B) The degree of CD8-positive cytotoxic T-cell infiltration varied. (C) Image of CD8 staining analyzed by QuPath. CD8-positive cells are highlighted in red. (D-E) The degree of CD68-positive macrophage infiltration varied. (F-J) Representative images of each CD47 score (score 0 [complete negative], score 1 [faint], score 2 [weak], score 3 [intermediate], and score 4 [strong]).
Immunohistochemical stains of CD8, CD68, and CD47. (A-B) The degree of CD8-positive cytotoxic T-cell infiltration varied. (C) Image of CD8 staining analyzed by QuPath. CD8-positive cells are highlighted in red. (D-E) The degree of CD68-positive macrophage infiltration varied. (F-J) Representative images of each CD47 score (score 0 [complete negative], score 1 [faint], score 2 [weak], score 3 [intermediate], and score 4 [strong]).
Panel-based massively parallel sequencing (NGS)
NGS was performed in 37 cases of the training cohort using the HemaSCAN panel (version 1.1), including 425 genes that are related to hematologic malignancies (supplemental Table 3). Based on preliminary immunostaining results, we included a large number of intestinal lymphomas in the NGS cohort. Of the 37 cases, 20 were intestinal DLBCL, and the other 17 were located in other extranodal sites (n = 10) and lymph nodes (n = 7). All 37 cases were DLBCL NOS. Extracted genomic DNA was sheared using a Covaris S220 (Covaris, Woburn, MA). Targeted gene was captured using a SureSelect XT Reagent Kit, HSQ (Agilent Technologies) and a paired-end sequencing library was constructed with a barcode. DNA sequencing was performed on a NextSeq 550 Dx sequencer (Illumina, San Diego, CA). The paired-end reads were aligned to the human reference genome (hg19) using BWA-MEM v0.7.5, Samtools v0.1.18, GATK v3.1-1, and Picard v1.93. We called single nucleotide variants using MuTect version 1.1.4, LoFreq version 0.6.1, and VarDict version 1.06 software with a variant allele frequency ≥ 1% or the number of variant supporting reads > 4. We manually reviewed variants with supporting reads <20 using an Integrative Genomics Viewer browser and filtered out sequencing errors. We identified small insertions and deletions using Pindel version 0.2.5a4 with the number of variant supporting reads >9. We further filtered out variants present with a minor allele frequency ≥1% in the 1000 Genomes Project database (https://www.internationalgenome.org/), the Genome Aggregation Database (gnomAD) (https://gnomad.broadinstitute.org/), the National Heart, Lung, and Blood Institute’s Exome Sequencing Project database (https://esp.gs.washington.edu/drupal/), the Korean Reference Genome Database,19 the Korean Variant Archive (https://www.kobic.re.kr/kova/), and an in-house database from 192 Korean individuals. To measure the number of mutations consistently, only single nucleotide variant/indel results were used, whereas copy number variation and fusion results were discarded. To filter out false-positive results, variants with variant allele frequency less than 5% and total reads less than 100 were excluded.
Statistical analysis
We used the SPSS 27.0 statistical software program (IBM Corporation) for statistical analyses. Pearson’s χ2 test was used for the crossover analysis of the clinicopathologic features. When comparing 2 or more groups of continuous variables, the Student t test for 2 groups or 1-way analysis of variance test for more than 2 groups was performed. A Kaplan-Meier curve (log-rank test) was used for survival analysis. P < .05 was considered statistically significant.
Results
Patient characteristics
In the training cohort, the median age of the 238 patients was 60 years (range, 3-87 years). There were 149 males and 89 females (male:female ratio = 1.67). The mean follow-up period was 42.0 months (range, 0.2-152.8 months). Among 163 DLBCL NOS cases, 56 (34.4%) were germinal center B-cell (GCB) type and 107 (65.6%) were non-GCB type. In Ann Arbor stage, 157 (66.0%) were in stage I to II, and 81 (34.0%) were in stage III to IV. The clinicopathologic characteristics of the training cohort patients are summarized in Table 1.
In the validation cohort, the median age of the 186 patients was 54 years (range, 1-81 years). There were 118 males and 68 females (male:female ratio = 1.74). The mean follow-up period was 95.7 months (range, 0.1-291.6 months). Among 153 DLBCL NOS cases, 48 (31.4%) were GCB type and 105 (68.6%) were non-GCB type.
Quantitative assessment of CD8 and CD68 immunohistochemistry
In CD8 immunostains for evaluation of intratumoral cytotoxic T cells, the average number of CD8-positive cells per 0.37 mm2 was 765.61 (standard deviation [SD], 815.59; range, 7-3840), and the average proportion of CD8-positive cells in total cells was 21.35% (SD, 21.19; range, 0.1-90.4). According to diagnosis, CD8-positive cell number and ratio were significantly higher in THRLBL (mean number, 2116.7; mean ratio, 53.1%), and PMLBL (mean number, 1405.6; mean ratio, 38.4%). CD8-positive cell number and ratio were lowest in PCNSL (mean number, 400.1; mean ratio 13.2%; P < .001 for both number and ratio). When comparing the differences in CD8-positive cell infiltration by clinicopathologic factors, both the number and ratio of CD8-positive cell were significantly higher in Ann Arbor stage III to IV (mean number, 996.6; mean ratio, 26.7%) than that in Ann Arbor stage I to II (mean number, 624.8; mean ratio, 18.1%; P = .003 [number] and 0.009 [ratio]). CD8 stains also showed significant differences according to tumor location. The most CD8-positive cell infiltration was observed in mediastinum (mean number, 1480.1; mean ratio, 41.1%), and the least CD8-positive cell infiltration was observed in intestine (mean number, 376.0; mean ratio, 10.6%; P < .001 for both number and ratio). There was no significant association between sex, age, COO, and CD8-positive T-cell infiltration (Table 1).
In CD68 immunostains for evaluation of intratumoral macrophages, the average number of CD68-positive cells per 0.37 mm2 was 761.66 (SD, 592.09; range, 41-3500), and the average ratio of CD68-positive cells in total cells was 19.93% (SD, 14.07; range, 1.89-68.70). Both the number and ratio of CD68-positive cells were significantly higher in THRLBL (mean number, 1976.2; mean ratio, 41.2%), PMLBL (mean number, 1164.0; mean ratio, 38.6%), and EBV DLBCL (mean number, 1629.0; mean ratio, 36.5%) than those in PCNSL (mean number, 591.3; mean ratio, 18.0%) and DLBCL NOS (mean number, 675.2; mean ratio, 17.9%; P < .001 for both number and ratio). The number of CD68-positive cells was significantly higher in Ann Arbor stage III to IV (mean, 878.2) than that in Ann Arbor stage I to II (mean, 691.5; P = .040), whereas the ratio of CD68-positive cells showed no significant difference by Ann Arbor stage (P = .180). CD68-positive cell infiltration was not associated with sex, age, COO, or tumor location (Table 1).
Scoring of CD47 immunohistochemistry and classification of CD47-high and -low groups
In CD47 staining, scores 0, 1, 2, 3, and 4 were 88 (37.0%), 77 (32.4%), 34 (14.3%), 36 (15.1%), and 3 (1.3%) cases in the training cohort, respectively. To set cutoff score of high- and low-expression groups of CD47, the CD68-positive cell ratio according to the CD47 score was investigated. The CD68-positive cell ratio for each CD47 score was 19.93 (score 0), 22.34 (score 1), 20.45 (score 2), 13.50 (score 3), and 8.40 (score 4), respectively (Table 2). When scores 0 to 2 was classified as CD47-low and scores 3 to 4 as CD47-high, the CD68-positive cell ratio of the CD47-low and CD47-high groups showed a significant difference (P < .001). Among all 238 cases of the training cohort, CD47-low group was 199 cases (83.6%) and CD47-high group was 39 cases (16.4%). Almost all cases of CD47-high group were DLBCL NOS except 1 PCNSL case. The ratio of the CD47-high cases was significantly higher in non-GCB type than that in GCB type by the Hans algorithm in DLBCL NOS (P = .001). In comparison, by tumor location, the CD47-high case ratio was highest in the intestine (41.4%; P < .001; Table 1). When CD47 expression according to the COO was analyzed by tumor location, CD47 expression in the non-GCB type was significantly higher than that in GCB type only in the intestine (P < .001; Table 3). In the validation cohort, the CD47-low group had 165 cases (88.7%) and the CD47-high group had 21 cases (11.3%). All CD47-high cases were DLBCL NOS. The ratio of CD47-high case was significantly higher in the non-GCB type than that in the GCB type by the Hans algorithm in DLBCL NOS (P = .005). By tumor location, the CD47-high case ratio was highest in the intestine (22.2%), although it was not statistically significant (P = .081; supplemental Table 1).
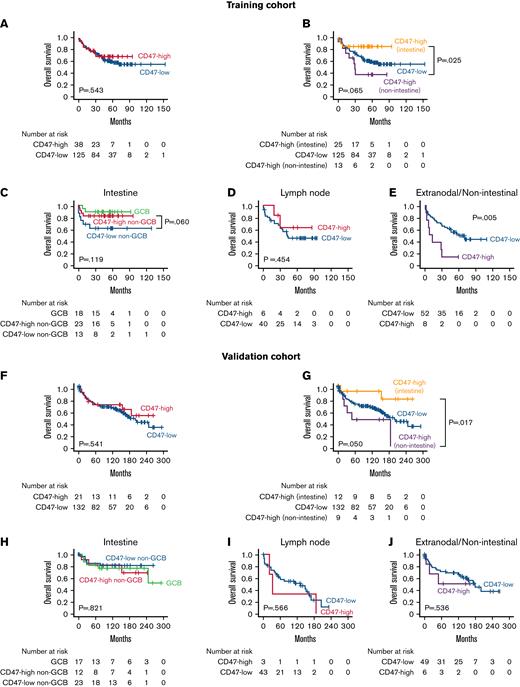
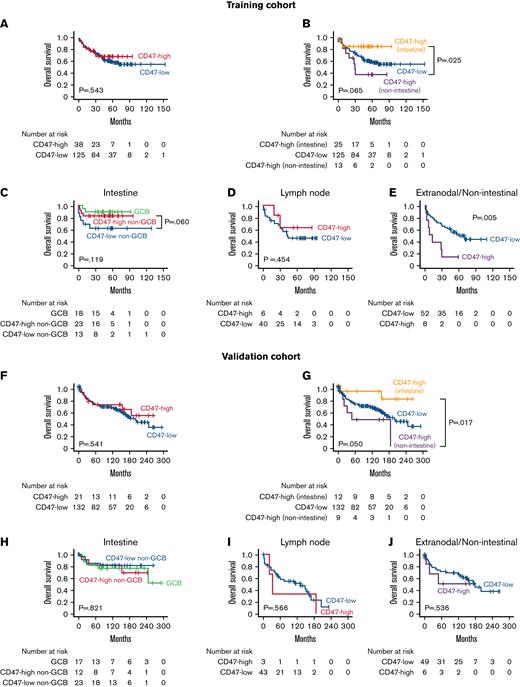
In the intestine, CD47-high non–GCB-type DLBCL showed a significantly lower proportion of advanced Ann Arbor stage (15.2%) than GCB-type (31.5%), and CD47-low non–GCB-type DLBCL (41.4%; P = .035). In survival analyses of DLBCL NOS cases, there was no significant difference in prognosis between the CD47-high and CD47-low groups for all patients (Figure 3A). However, when the CD47-high group was separated into intestinal and other site lymphomas, intestinal CD47-high DLBCL showed significantly better prognosis than CD47-high DLBCL of other sites (P = .025; Figure 3B). In the intestine, the prognosis of CD47-high non–GCB-type DLBCL was not significantly worse than that of CD47-low non–GCB-type DLBCL (P = .060; Figure 3C). In lymph node, there was no significant prognostic difference between the CD47-high and CD47-low group (P = .454; Figure 3D). In contrast, in extranodal and nonintestinal DLBCL, the prognosis of the CD47-high group was significantly worse than that of the CD47-low group (P = .005; Figure 3E). The survival analysis results of the validation cohort were generally consistent with those of the training cohort (Figure 3F-I), except that CD47-high group did not show a statistically significantly poor prognosis in extranodal and nonintestinal DLBCL (P = .536; Figure 3J).
Results of survival analyses. Kaplan-Meier curves of overall survival according to CD47 status and tumor locations of patients with DLBCL NOS in the training (A-E) and validation cohorts (F-J). CD47 expression was not a significant prognostic factor in intestinal diffuse large B-cell lymphoma; however, the prognosis of CD47-high cases was worse than that of CD47-low cases at extranodal/nonintestinal organs in the training cohort. In the validation cohort, CD47-high DLBCL showed a worse prognosis than CD47-low in the extranodal/nonintestinal sites, but it was not statistically significant.
Results of survival analyses. Kaplan-Meier curves of overall survival according to CD47 status and tumor locations of patients with DLBCL NOS in the training (A-E) and validation cohorts (F-J). CD47 expression was not a significant prognostic factor in intestinal diffuse large B-cell lymphoma; however, the prognosis of CD47-high cases was worse than that of CD47-low cases at extranodal/nonintestinal organs in the training cohort. In the validation cohort, CD47-high DLBCL showed a worse prognosis than CD47-low in the extranodal/nonintestinal sites, but it was not statistically significant.
Panel-based deep parallel sequencing and correlation with clinicopathologic features
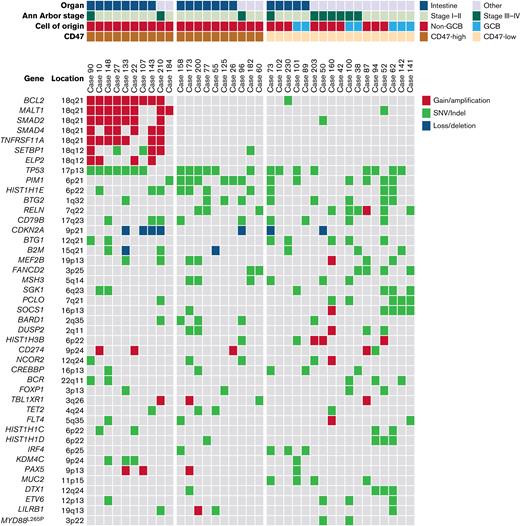
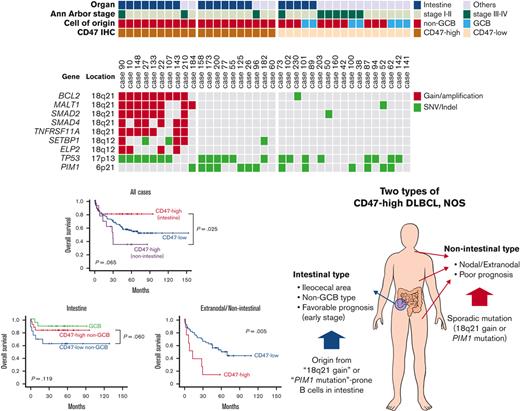
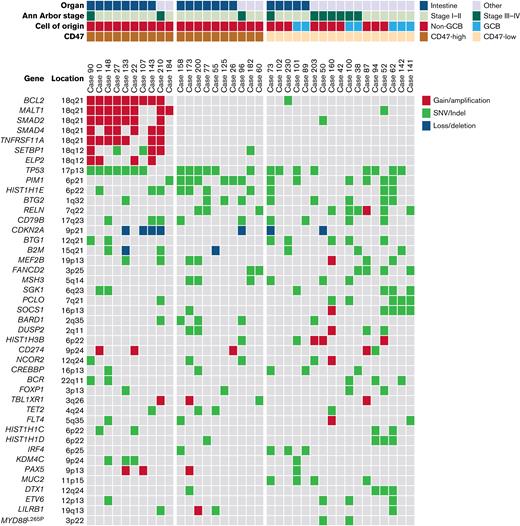
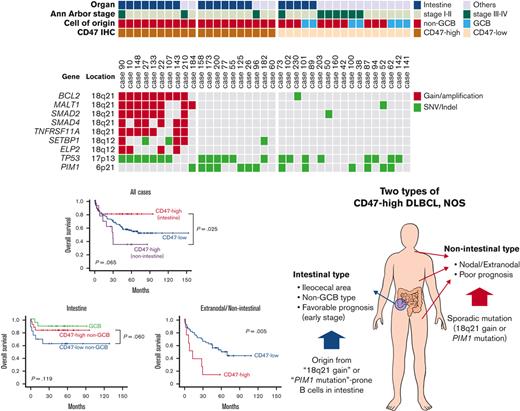
In NGS analysis of 37 cases, the most frequently mutated gene was TP53 (21 of 37). TP53 mutation was observed in 15 of 20 cases of intestinal DLBCL but only in 6 of 17 cases of nonintestinal DLBCL (P = .015). The second most common genetic alteration was the PIM1 mutation, which was identified in 13 cases. There was no difference in PIM1 mutation frequency between intestinal (7 of 20) and nonintestinal (6 of 17) DLBCL (P = .985). The next was gain/amplification of 18q21 locus, including the MALT1 and BCL2 genes. The 18q21 gain/amplification was observed in 10 of 37 cases, of which 8 were in intestinal DLBCL and 2 were in nonintestinal DLBCL (P = .054). The PIM1 mutation (n = 13) and 18q21 gain/amplification (n = 10) were observed simultaneously in only 1 case, showing a tendency not to overlap with each other (P = .051). Comparing NGS and CD47 immunohistochemistry results, all 10 18q21 gain/amplification cases were CD47-high regardless of tumor location. In CD47-high cases without 18q21 gain/amplification, TP53 and PIM1 mutations were observed in 6 of 10 (60%) cases, which was slightly higher than TP53 (47.1%, 8 of 17) and PIM1 mutations (35.3%, 6 of 17) in the CD47-low group. In CD47-high intestinal DLBCL, no PIM1 mutation was found in the 8 cases with 18q21 gain/amplification, whereas 5 of 7 cases without 18q21 gain/amplification showed PIM1 mutation (Figure 4).
Results of panel-based massively parallel sequencing of 37 cases. The information on organ, Ann Arbor stage, cell of origin, and CD47 immunohistochemistry are depicted. All 10 cases with 18q21 gain/amplification were CD47-high cases. SNV, single nucleotide variant.
Results of panel-based massively parallel sequencing of 37 cases. The information on organ, Ann Arbor stage, cell of origin, and CD47 immunohistochemistry are depicted. All 10 cases with 18q21 gain/amplification were CD47-high cases. SNV, single nucleotide variant.
Discussion
As 1 of the immune checkpoints, CD47 plays a role in inhibiting phagocytosis of macrophage via the “don’t eat me” signal. Recently, the results of a phase 1 study of CD47 blocker TTI-621 in non-Hodgkin lymphomas were published.16 In this clinical trial, TTI-621 showed overall response rate in 2 of 7 patients in monotherapy and 5 of 24 patients in combination with rituximab in patients with relapsed/refractory DLBCL. For effective therapeutic utilization of CD47 blocker in lymphomas, it is very important to select patients suitable for anti-CD47 treatment. In this study, we classified CD47-high DLBCL using CD47 immunostain and investigated the clinical, pathologic, and molecular characteristics of the CD47-high group in a cohort of 238 patients with DLBCL including various subtypes and locations of tumor.
To use CD47 immunostaining as a biomarker, we divided all 238 DLBCL cases into 5 scores according to the intensity of staining and quantitatively evaluated the degree of macrophage infiltration in each score. There was no difference in the degree of macrophage infiltration in scores 0, 1, and 2, which correspond to CD47 complete negative to weak positive staining, but the infiltration of macrophage tended to decrease in scores 3 (intermediate) and 4 (strong). When scores 0 to 2 was classified as CD47-low and scores 3 to 4 as CD47-high group, the CD47-low and -high groups showed a significant difference in the degree of macrophage infiltration.
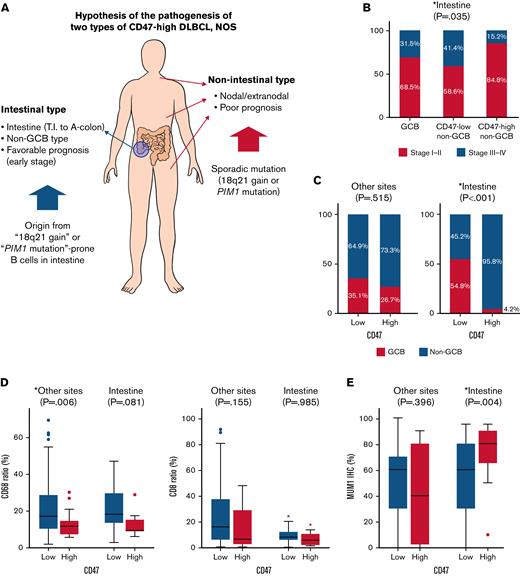
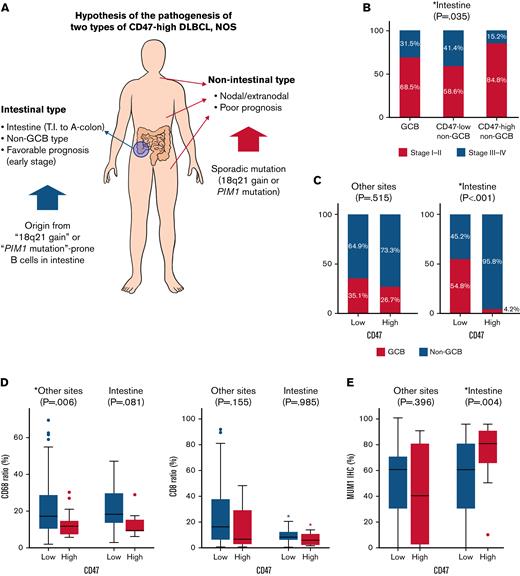
One of the characteristic features of CD47-high DLBCL was that a large number of cases were located in the intestine, especially in the terminal ileum, ileocecal valve, cecum, appendix, and ascending colon, and virtually all of them were non-GCB type in COO. In the validation cohort, the ratio of CD47-high case in intestinal DLBCL was lower than that in the training cohort, but the ratio of intestinal DLBCL in CD47-high DLBCL was not significantly different between the training cohort (61.5%) and the validation cohort (57.1%). The immune activity, evaluated by CD8 and CD68 immunostains of intestinal CD47-high DLBCL was decreased compared with that of the CD47-low group (Figure 5), but the prognosis of the CD47-high group was not poor. CD47-high DLBCL occurring at nonintestinal sites showed significantly decreased macrophage infiltration (Figure 5). Nonintestinal CD47-high DLBCL contained both the non-GCB (73.3%) and GCB (26.7%) type in the training cohort; however, this result was not reproduced in the validation cohort (non-GCB, 88.9%; GCB, 11.1%). The prognosis of patients with nonintestinal and extranodal CD47-high DLBCL was significantly worse than that of the CD47-low group. However, despite clinical and pathologic differences, molecular analysis revealed that intestinal and nonintestinal CD47-high DLBCL shared similar genetic alteration: 18q21 gain/amplification or PIM1 mutation.
Two types of CD47-high diffuse large B-cell lymphoma, not otherwise specified. (A) Schematic illustration of the differences between intestinal and nonintestinal type CD47-high DLBCL. (B) Proportion of advanced Ann Arbor stage according to cell of origin and CD47 status in patients with intestinal DLBCL. (C) Difference in cell of origin of intestinal and nonintestinal DLBCLs according to CD47 status. (D) Differences in CD68 and CD8 ratios according to tumor locations and CD47 status. (E) MUM1 immunostaining results (review of electronic medical records) according to tumor locations and CD47 status. This graph shows high homogeneity of tumor cells of intestinal CD47-high DLBCL that is different from that of other site lymphomas.
Two types of CD47-high diffuse large B-cell lymphoma, not otherwise specified. (A) Schematic illustration of the differences between intestinal and nonintestinal type CD47-high DLBCL. (B) Proportion of advanced Ann Arbor stage according to cell of origin and CD47 status in patients with intestinal DLBCL. (C) Difference in cell of origin of intestinal and nonintestinal DLBCLs according to CD47 status. (D) Differences in CD68 and CD8 ratios according to tumor locations and CD47 status. (E) MUM1 immunostaining results (review of electronic medical records) according to tumor locations and CD47 status. This graph shows high homogeneity of tumor cells of intestinal CD47-high DLBCL that is different from that of other site lymphomas.
Based on these findings, we could make several hypotheses. First, intestinal CD47-high DLBCL arises from specific B cells distributed in specific location (from terminal ileum to ascending colon), and the cells have a potential trait, which is high prevalence of 18q21 gain/amplification in tumorigenesis. Because CD47-high DLBCL occurring at other organs is not limited to specific organ or COO, nonintestinal CD47-high DLBCL is presumed to overexpress CD47 by sporadic 18q21 gain/amplification or PIM1 mutation independent of normal counterpart cell. Second, overexpression of CD47 reduces host immune activity regardless of organs, and nonintestinal CD47-high DLBCL shows a poor prognosis through immune evasion of tumor cells. However, intestinal DLBCL has a characteristic that the immune activity is lower than that of other organs. Theoretically, overexpression of inhibitory immune checkpoint suppresses the inflammatory response existing around tumor cells, which allows the tumor to escape from host immunity and proliferate unhindered. If the inflammatory response around the tumor cells is weak, the effect of the inhibitory immune checkpoint is likely to be weakened as well. Therefore, CD47-induced immune escape does not lead to a prognostic difference in intestinal DLBCL. These hypotheses are schematically summarized in Figure 5.
In our study, 10 DLBCL cases harboring 18q21 gain/amplification were all CD47-high. PIM1 mutation tends to be mutually exclusive with 18q21 gain/amplification and showed a high prevalence in CD47-high DLBCL without 18q21 gain/amplification cases. These molecular characteristics correspond to cluster 5 (C5) of the paper published in Nature Medicine in 2018,20 and the MCD group of the paper published in Cancer Cell in 2020.21 Both C5 and MCD are consistent with our results in that most cases are non-GCB type. However, MYD88L265P, 1 of the most characteristic mutations in C5 and MCD, was found in only 2 cases of CD47-low DLBCL in our study. In addition, the frequency of the CD79B mutation did not show significant differences according to 18q21 or CD47 status in our cases. If so, how do 18q21 gain/amplification and the PIM1 mutation related to CD47 overexpression? The 18q21 locus contains several genes such as BCL2 and MALT1. MALT1 is an oncogene known to cause extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue through translocation,22,23 resulting in nuclear factor-κB (NF-κB) activation.24,25 In addition to translocation of MALT1, amplification of MALT1 can be also observed in non-Hodgkin B-cell lymphomas.26,MALT1 overexpression can also induce NF-κB activation through CD40 signaling.27 The NF-κB pathway is known as 1 of the factors that upregulate CD47.28,29,PIM1 is an oncogene that plays a critical role in the control of cell proliferation, survival, homing, and migration30,31 and also regulates the NF-κB pathway.32 In 2008, Dierlamm et al33 published a paper on 18q21 gain in DLBCL, wherein 43 of 44 cases with MALT1 gain were accompanied by BCL2 gain. The 18q21 gain was more common in the non-GCB type and was associated with poor prognosis regardless of COO. DLBCL with the 18q21 gain showed upregulation of NF-κB target genes; however, it was not independent of COO. Despite theories that could correlate CD47 upregulation with NF-κB activation, the low incidence of CD47-high cases in total non–GCB-type DLBCL, which is known to be closely related to NF-κB activation,34,35 does not fully explain their correlation. Further studies on the relationship between MALT1 (18q21) gain, PIM1 mutation, NF-κB activation, and CD47 upregulation are needed.
In our study, a tendency to decrease cytotoxic T-cell infiltration and macrophage infiltration was observed in CD47-high DLBCL both in intestinal and nonintestinal types, although the difference was not statistically significant. SIRPα, a ligand for CD47, is present not only in macrophages but also in dendritic cells.36,37 Dendritic cells play a role in antigen presentation of tumor cell antigens to CD4-positive and CD8-positive T cells. Therefore, the expression of CD47 can play a role in suppressing not only macrophages but also cytotoxic T cells, and CD47 blockade can promote both phagocytosis of macrophage and antitumor T-cell activity.38,39
In localized intestinal DLBCL, surgical resection followed by chemotherapy has been considered the optimal therapeutic strategy, whereas the first treatment option of other most DLBCL is chemotherapy only.40 This is contrary to the common idea that malignant lymphoma is a systemic disease, although it is still at the early stage. This different therapeutic approach suggests that primary intestinal DLBCL may have different pathologic and molecular characteristics from other DLBCL NOS and could be classified into a specific subtype. In our study, intestinal DLBCL showed several characteristics different from other DLBCL NOS. First, the frequency of 18q21 gain/amplification, TP53 mutation, and CD47 overexpression was higher than that of DLBCL from other organs. Second, the degree of infiltration of immune cells including cytotoxic T cells was low. Given that the reason why PMLBL and PCNSL have been classified separately from DLBCL NOS, (ie, distinct normal counterpart cell; characteristic clinical, pathologic, and molecular features), our findings suggest the possibility that “primary intestinal DLBCL” could be separated from DLBCL NOS as a new subtype.
Our study has several limitations. First, selection bias could not be excluded because it was not a cohort of consecutive patients. Second, it is difficult to say that the entire copy number variation was sufficiently reflected because it was evaluated through panel-based NGS rather than array comparative genomic hybridization (CGH). Third, scoring CD47 immunostaining according to the intensity (0-4) can be subjective, and may induce interobserver discrepancy, especially in the distinction between scores 2 and 3. Therefore, it is necessary to validate the results of this study through mRNA analysis, whole exome sequencing, and array CGH analysis in a larger cohort of consecutive patients with DLBCL.
In conclusion, CD47-high and CD47-low DLBCL could be determined using CD47 immunohistochemistry. CD47-high DLBCL in intestine and other organs showed different clinicopathological characteristics despite similar patterns of molecular alterations and effects on tumor immune microenvironment. The CD47-high DLBCL in the intestine is localized to a specific location and restricted to the non-GCB type and does not show significant prognostic effect, whereas other CD47-high DLBCL is not limited to a specific organ and has a worse prognosis than CD47-low DLBCL. Accordingly, the authors suggest that the location of the tumor should be considered as an important factor when evaluating CD47 expression in DLBCL. It is necessary to study how CD47-high and CD47-low DLBCL respond to CD47 blockade treatment, especially in nonintestinal type CD47-high DLBCL. Furthermore, additional studies with a sufficient number of cases are necessary to determine whether the characteristics of intestinal type CD47-high DLBCL observed in this study are reproducible and may represent a distinct biological entity.
Authorship
Contribution: J.C. contributed to study design, data interpretation, data analysis, created the figures, and led the write-up; S.E.Y. contributed to data interpretation and data analysis; S.J.K. contributed to the model evaluation and led the statistical trends analysis; Y.H.K. contributed to the study design, data interpretation, data analysis, and model evaluation; and W.S.K. oversaw all aspects of study design, model evaluation, data interpretation, and the write-up.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Won Seog Kim, Division of Hematology and Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, #81, Irwon-ro, Gangnam-Gu, Seoul 06351, Korea; e-mail: wskimsmc@skku.edu; and Young Hyeh Ko, Department of Pathology, Korea University Guro Hospital, #148, Gurodong-ro, Guro-gu, Seoul 08308, Korea; e-mail: yhko310@skku.edu.
Supplemental Material
References
Author notes
NGS data to NCBI SRA database (accession number PRJNA848986).
Requests for data sharing may be submitted to Junhun Cho (jununius@naver.com).
The full-text version of this article contains a data supplement.
Y.H.K. and W.S.K. contributed equally to this study.

![Immunohistochemical stains of CD8, CD68, and CD47. (A-B) The degree of CD8-positive cytotoxic T-cell infiltration varied. (C) Image of CD8 staining analyzed by QuPath. CD8-positive cells are highlighted in red. (D-E) The degree of CD68-positive macrophage infiltration varied. (F-J) Representative images of each CD47 score (score 0 [complete negative], score 1 [faint], score 2 [weak], score 3 [intermediate], and score 4 [strong]).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/6/24/10.1182_bloodadvances.2021006305/5/m_blooda_adv-2021-006305-gr2.jpeg?Expires=1763475932&Signature=mLMf8yn0HwoLJv5T2ZlLA2v3uU6vniSTze6HtXG8vrmrh2lxPnCeV33udLxCen2New7AxRebnGdO0z~4xvv8qKZJoblJS0EO-LlENfu3Qkxci3cWdGWzF26I6euCpzOEW0TGh6G5d~Mh9q9QbQ938TvO4q4PV5HfDW9ULtccw6UFRhnnIhpCpJAq3LjIFt1mND7N-wwBKQKJ3RUsl5WTVbFWkGqzdcz4pEtTmF1Rthet6qb28LkAYhVxZsbsPxQywTrfUzAKBdwLBYky84cF4o2X1Qs6hrKXf05HCRb03dm9BUWRXhYdi9w-ry2pAv5JllOwrnId0zQoOxDZjC4zkw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Immunohistochemical stains of CD8, CD68, and CD47. (A-B) The degree of CD8-positive cytotoxic T-cell infiltration varied. (C) Image of CD8 staining analyzed by QuPath. CD8-positive cells are highlighted in red. (D-E) The degree of CD68-positive macrophage infiltration varied. (F-J) Representative images of each CD47 score (score 0 [complete negative], score 1 [faint], score 2 [weak], score 3 [intermediate], and score 4 [strong]).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/6/24/10.1182_bloodadvances.2021006305/5/m_blooda_adv-2021-006305-gr2.jpeg?Expires=1763475933&Signature=qhFOwIENdfzRr-UiCgZeBPsfl8zdcmKSXHC1b~DC8pjNMgXpWjxHqloQ3fu66kGULMflT4oVbNTUhzUhPN7pk-xRkqS6roJ897-JuBtWZ~D6HNn9TEyp6YXCfmu62V8YMa~ciH1fi2lJzKqpyi02Gchu6y55~M8w1iteExkScQYWpoc5QY8gYaobvAN~b-9OXL3sGEkIVYlALIMRcU1SmUewviOS7XyJESSiS7dH8gyRhZyhFguhv5c2itd58LXHz3NABv-QkBOkRadSvY39GjcSUDCXZcq38pa1a4LySTqRdmyh0JogbuEw34SQ0HLdJ8JNbEoR5S50bfL1Eh5NQA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)