Key Points
Letermovir is a highly effective and well-tolerated CMV prophylaxis in adult CBT recipients.
Extended CMV prophylaxis for at least 6 months is beneficial in adult CBT recipients, but optimal prophylaxis duration remains unknown.
Abstract
Cord blood transplantation (CBT) can be complicated by a high incidence of clinically significant cytomegalovirus infection (csCMVi). We have investigated the efficacy of extended letermovir prophylaxis in seropositive adult CBT recipients. The aim was to continue prophylaxis for ≥6 months (insurance permitting). By day 100, the incidence of csCMVi was 0% in 28 patients who received letermovir prophylaxis. Moreover, of 24 patients alive at day 100, none had csCMVi by day 180, having continued prophylaxis for all (n = 20) or part (n = 4) of that period. Overall, 20 patients stopped letermovir at a median of 354 days (range, 119-455 days) posttransplant, with only 5 requiring 1 (n = 4) or 2 (n = 1) courses of valganciclovir (median total duration, 58 days; range, 12-67 days) for postprophylaxis viremia, with no subsequent csCMVi. There were no toxicities attributable to letermovir. Of the 62 historic control subjects who received acyclovir only, 51 developed csCMVi (median onset, 34 days; range, 5-74 days), for a day 100 incidence of 82% (95% confidence interval, 73-92). Seven patients developed proven/probable CMV disease, and 6 died before day 100 (3 with proven/probable CMV pneumonia). Forty-five patients required extended therapy during the first 6 months for 1 (n = 10), 2 (n = 14), or 3/persistent (n = 21) csCMVi, with 43 (84%) of 51 developing significant treatment toxicities. Letermovir is a highly effective, well-tolerated prophylaxis that mitigates CMV infection, CMV-related mortality, and antiviral therapy toxicities in CBT recipients. Our data support prophylaxis duration of at least 6 months after CBT.
Introduction
Cord blood (CB) is a standard alternative stem cell source for patients in need of allogeneic transplantation without suitable adult donors. Cytomegalovirus (CMV) infection is common after CB transplantation (CBT) and contributes to morbidity and mortality in CMV-seropositive CBT recipients.1-8 Frequent viral load monitoring and preemptive antiviral therapy for CMV viremia have decreased the risk of CMV disease.7-9 However, the high incidence of CMV infection, commonly requiring prolonged antiviral therapy, is associated with significant hematologic or renal toxicity in CMV-seropositive CBT recipients. Therefore, routine use of an effective nontoxic prophylaxis to mitigate CMV infection is warranted.
Letermovir is a novel antiviral that prevents CMV replication by binding at the virus terminase complex.10 In the pivotal phase 3 trial, letermovir prophylaxis for 14 weeks posttransplant reduced the risk of clinically significant CMV infection (csCMVi), defined as viremia leading to preemptive treatment or CMV end-organ disease, in CMV-seropositive hematopoietic stem cell transplant (HSCT) recipients.11 Several studies have since corroborated that letermovir continued through day 100 posttransplant is safe and effective CMV prophylaxis in adult donor HSCT12-20 or CBT15,21,22 recipients. However, a high incidence of delayed-onset csCMVi after letermovir discontinuation has been observed when prophylaxis is limited to the first 14 weeks posttransplant.11 Moreover, in a recent analysis of CMV-seropositive CBT recipients receiving letermovir prophylaxis through day 98, Hill et al22 observed that the early benefit of letermovir prophylaxis was lost by 6 months post-CBT due to the high incidence of delayed-onset csCMVi, with an overall day 180 incidence of csCMVi similar to that of a control group of CBT recipients who only received valacyclovir.
Based on these observations, the potential benefit of extended letermovir prophylaxis after HSCT is being investigated in an ongoing randomized phase 3 trial (clinicaltrials.gov #NCT03930615). We have hypothesized, however, that extended prophylaxis in all CMV-seropositive CBT recipients would be safe and effective in preventing csCMVi in the first 6 months post-CBT and would not prohibit the subsequent development of CMV-specific immunity. Herein, we report our experience of extended prophylaxis in a retrospective analysis of CMV-seropositive adult CBT recipients including a comparison with historic CBT control patients.
Methods
Patients and transplant procedures
Recipients of letermovir prophylaxis
Recipients of letermovir prophylaxis were CMV-seropositive adults with hematologic malignancies and underwent double-unit CBT between March 2018 and June 2020. All consecutive adults who received intermediate-intensity conditioning (cyclophosphamide 50 mg/kg, fludarabine 30 mg/m2 × 5 doses, thiotepa 5 mg/kg × 1-2 doses, and total body irradiation 200 cGy × 2 doses)23 and graft-versus-host disease (GVHD) prophylaxis with cyclosporin A (CSA) and mycophenolate mofetil (MMF) plus tocilizumab (8 mg/kg) on day −1 (clinicaltrials.gov #NCT03434730) were included in the analysis. Grafts were selected using standard cell dose, HLA match, and unit quality selection criteria.23,24
Historic control patients
Consecutive adult CMV-seropositive historic control patients underwent double-unit CBT between March 2013 and December 2017. These patients received identical conditioning but GVHD prophylaxis was with CSA and MMF only. The double-unit CB grafts were selected using identical criteria. In a subset of these patients, the CB grafts were supplemented with haplo-identical CD34+ cells (clinicaltrials.gov #NCT01682226), as previously described.25
This study was performed with the approval of the Memorial Sloan Kettering Cancer Center Institutional Review Board and was conducted in accordance with the Declaration of Helsinki.
Antiviral prophylaxis and monitoring
Letermovir was dosed at 240 mg daily, starting day 7 posttransplant along with standard-dose acyclovir (250 mg/m2 IV every 8 hours or 400 mg every 12 hours orally) from admission (day −7). The aim was to continue prophylaxis until at least 6 months posttransplant (assuming continued insurance approval). Prescription coverage screening was conducted at the preadmission visit, and the bone marrow transplant pharmacists obtained prior authorizations, wrote appeal letters, and/or applied for manufacturer patient assistance as needed. The subsequent discontinuation of letermovir was ultimately at the treating physician’s discretion and patient preference given the lack of data to guide optimal prophylaxis duration beyond day 100. The letermovir dose was increased to 480 mg daily11 if CSA was discontinued before letermovir. Historic control patients received ganciclovir prophylaxis (5 mg/kg per day) from day −7 until day −2 and then standard-dose acyclovir as of day −1.
In all patients, plasma viral load was monitored by quantitative polymerase chain reaction (COBAS AmpliPrep/COBAS TaqMan CMV Test, Roche Diagnostics; quantification limit, 137 IU/mL) twice weekly until day 60, at least weekly from day 60 to 100, and less frequently beyond day 100 at the physician’s discretion based on presence of GVHD and immunosuppression burden. In recipients of letermovir prophylaxis, viral load monitoring was started on day 5, before drug commencement on day 7; in historic control CBT recipients, monitoring was started on day 14. After discontinuation of letermovir prophylaxis, CMV viral load monitoring was continued approximately every 1 to 2 weeks for a minimum of 2 months.
In letermovir recipients, initiation of preemptive CMV therapy was considered for a viral load >300 IU/mL in 2 consecutive measurements. In contrast, only 1 positive viral load measurement at any level was required to initiate preemptive therapy in historic control patients. Management of CMV viremia after letermovir discontinuation was at the treating physician’s discretion.
Definitions of CMV infection
CMV infection was defined as detection of any CMV DNAemia in the plasma by polymerase chain reaction, or other body fluid or tissue specimen.26 csCMVi was defined as CMV DNAemia requiring preemptive treatment or development of end-organ disease.11 CMV clearance was defined as 3 consecutive polymerase chain reaction tests with undetectable DNAemia and no evidence of disease. CMV pneumonia was defined by respiratory symptoms with compatible imaging combined with CMV in bronchoalveolar lavage fluid or biopsy samples.26 Gastrointestinal disease was defined by symptoms with or without macroscopic mucosal lesions combined with evidence of CMV infection in a tissue sample by culture, immunohistochemical analysis, or in situ hybridization.26 Clinically significant neutropenia was defined as an absolute neutrophil count of <2000/μL requiring granulocyte colony-stimulating factor support.
Biostatistics
The cumulative incidence of engraftment, acute GVHD, and CMV infection were calculated using the competing risk framework considering death as a competing risk. Fine and Gray regression was used to compare the incidences of CMV infection between patient cohorts. Overall survival (OS) and progression-free survival (PFS) were estimated by using Kaplan-Meier methods. A swimmer plot was used to represent episodes of CMV viremia during the first 6 months posttransplant in patients who received letermovir prophylaxis. Two-sided P values <.05 were considered significant. Biostatistical analysis was performed in R version 3.5.3 (The R Foundation for Statistical Computing).
Results
Demographic characteristics and transplant outcomes of recipients of letermovir prophylaxis
The demographic and graft characteristics of the 28 letermovir recipients are shown in Table 1. Twenty-six (93%) patients engrafted, whereas 1 had graft failure and 1 died early with incomplete count recovery. The day 180 cumulative incidence of grade 2 to 4 acute GVHD was 64% (95% confidence interval [CI], 47-82) with a median onset of 42 days (range, 16-72 days). With a median follow-up among survivors of 29 months (range, 17-43 months), the 1-year OS and PFS estimates were 79% (95% CI, 65-95) and 75% (95% CI, 61-93), respectively.
CMV infections in patients receiving letermovir prophylaxis: the first 100 days
Of the 28 letermovir prophylaxis recipients, 8 (29%) had detectable viremia (all <137 IU/mL) at baseline before initiation of letermovir on day 7 (Figure 1). Twenty-four of the 28 patients received letermovir prophylaxis throughout the first 100 days. Of the remaining 4 patients, 1 remained on letermovir until initiating foscarnet on day 36 for human herpesvirus 6 pneumonia and died 72 days posttransplant. The other 3 remained on letermovir until their deaths caused by multiorgan failure (n = 1), acute GVHD (n = 1), and disseminated adenovirus (n = 1) on days 26, 71, and 71, respectively. The patient with adenovirus infection also received cidofovir from day 23.
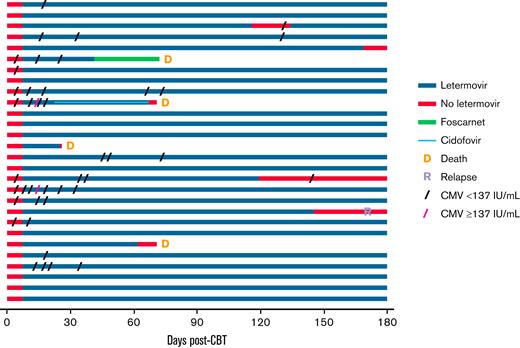
Pattern of CMV viremia in the first 180 days post-CBT in recipients of letermovir prophylaxis. Patients are listed in chronological order. One patient discontinued letermovir upon initiation of foscarnet on day 36 for human herpesvirus 6 pneumonia. One patient with adenovirus infection also received cidofovir from day 23.
Pattern of CMV viremia in the first 180 days post-CBT in recipients of letermovir prophylaxis. Patients are listed in chronological order. One patient discontinued letermovir upon initiation of foscarnet on day 36 for human herpesvirus 6 pneumonia. One patient with adenovirus infection also received cidofovir from day 23.
Twelve patients (43%) had transient low-level viremia by day 100 posttransplant while receiving letermovir prophylaxis (median peak viral load, <137 IU/mL; range, <137-206 IU/mL), including 7 (88%) of 8 patients with detectable viremia at baseline and 5 (25%) of 20 patients without baseline viremia (Figure 1). In all patients, low-level CMV viremia resolved spontaneously without therapy. Importantly, no letermovir-treated patients developed csCMVi in the first 100 days (Figures 1 and 2). No toxicities were attributed to letermovir prophylaxis, and no patient discontinued letermovir due to drug toxicity through day 100 post-CBT.
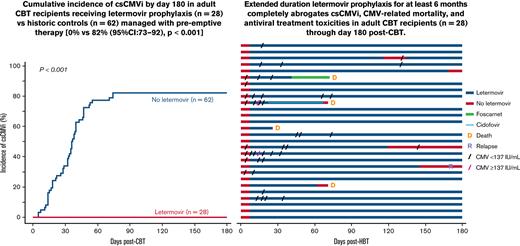
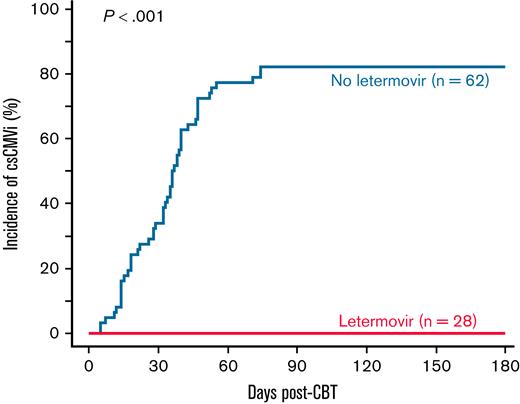
Cumulative incidence of csCMVi by day 180 in adult CBT recipients receiving letermovir prophylaxis or historic control patients managed with preemptive therapy. No recipient of extended-duration letermovir prophylaxis (n = 28) developed csCMVi in the first 180 days post-CBT. The day 180 cumulative incidence of csCMVi in historic control patients (n = 62) was 82% (95% CI, 73-92).
Cumulative incidence of csCMVi by day 180 in adult CBT recipients receiving letermovir prophylaxis or historic control patients managed with preemptive therapy. No recipient of extended-duration letermovir prophylaxis (n = 28) developed csCMVi in the first 180 days post-CBT. The day 180 cumulative incidence of csCMVi in historic control patients (n = 62) was 82% (95% CI, 73-92).
CMV infections in patients receiving letermovir prophylaxis: 100 to 180 days
Twenty-four patients alive at day 100 continued letermovir prophylaxis (Figure 1). Of these, 20 patients received continuous prophylaxis through day 180 and had no csCMVi, with a single patient having an isolated episode of detectable viremia. One additional patient had an 18-day interruption in prophylaxis (days 116-134) due to temporary insurance denial. This was complicated by a single episode of detected viremia <137 IU/mL with no subsequent viremia by day 180.
The remaining 3 patients (patients 1-3) (Table 2) discontinued prophylaxis before day 180. Patients 1 and 2 discontinued due to insurance denial at 119 and 145 days, respectively. Patient 1 had a single episode of detected viremia <137 IU/mL (onset 26 days after letermovir discontinuation) that spontaneously resolved. Patient 2, who relapsed 172 days posttransplant, had no CMV viremia through day 180. Patient 3 discontinued letermovir at 169 days due to physician’s preference and had no viremia through day 180. Thus, none of the 24 patients had csCMVi between days 100 and 180 (Figure 2). There were no toxicities requiring discontinuation of letermovir in this period.
CMV infections in patients receiving letermovir prophylaxis: beyond day 180
Of the 24 patients alive at day 180, 3 remained on letermovir until they died at 282, 300, and 432 days due to infection/organ failure (n = 2) and COVID-19 infection (n = 1). None of these patients had CMV infection before their death. One additional patient with ongoing GVHD therapy continues letermovir prophylaxis at last follow-up 506 days’ posttransplant without CMV infection.
The remaining 20 patients discontinued letermovir at a median of 354 days (range, 119-455 days) posttransplant (patients 1-20) (Table 2). At the time of letermovir discontinuation, 4 patients were off all immunosuppressive agents, whereas the remaining 16 were still receiving systemic immunosuppression (most commonly subtherapeutic doses of CSA or low-dose prednisone).
Of the 3 patients (patients 1-3) (Table 2) who stopped letermovir and had no csCMVi before day 180, 2 (patients 1 and 3) also had no subsequent CMV viremia beyond day 180 through last follow-up at days 817 and 1194, respectively. The third patient with leukemia relapse (patient 2) developed low-level viremia at day 186 (41 days after discontinuation), resumed letermovir on day 193 with subsequent viremia resolution, and continued prophylaxis thereafter until death 437 days’ posttransplant.
The other 17 patients completed the intended minimum prophylaxis of 180 days (patients 4-20) (Table 2). They discontinued letermovir at a median of 372 days’ (range, 211-455 days) posttransplant and have a median follow-up of 569 days (range, 126-1030 days) since discontinuation. Of these 17 patients, 4 have had no CMV viremia (patients 4-7), 8 had transient low-level viremia (peak viral load, <137 IU/mL [n = 7], 324 IU/mL [n = 1]) with a median onset of 61 days (range, 21-140 days) after stopping letermovir that spontaneously resolved (patients 8-15), and 5 had csCMVi (median peak viral load, 877 IU/mL; range, 347-1806 IU/mL) with a median onset of 18 days (range, 7-30 days) after discontinuation (patients 16-20). These 5 csCMVi patients received preemptive valganciclovir that was initiated at a median of 33 days (range, 25-44 days) after letermovir discontinuation, for a median total duration of 58 days (range, 12-67 days). One (patient 16) received secondary letermovir prophylaxis, which was discontinued at 399 days with subsequent transient low-level viremia (peak viral load, <137 IU/mL) but no csCMVi. Three patients had no subsequent viremia after completing valganciclovir (patients 17, 18, and 20). The fifth patient (patient 19) had a second episode of csCMViagain treated with short-course valganciclovir with prompt clearance and no subsequent viremia. None of the 5 patients developed CMV end-organ disease. There were no toxicities requiring discontinuation of letermovir in any of the patients beyond day 180.
Demographic characteristics and transplant outcomes of historic control patients
Historic control patients (n = 62) had characteristics similar to those who received letermovir (Table 1). However, their CB grafts had a lower infused CD34+ cell dose. Sixty-one (98%) patients engrafted with CB, whereas 1 patient had graft failure and underwent successful re-transplant with a CB unit. The day 180 cumulative incidence of grade 2 to 4 acute GVHD was 89% (95% CI, 81-97), with a median onset of 37 days (range, 15-155 days). The 1-year OS and PFS estimates were 84% (95% CI, 75-94) and 77% (95% CI, 68-89), respectively.
CMV infections in historic control patients
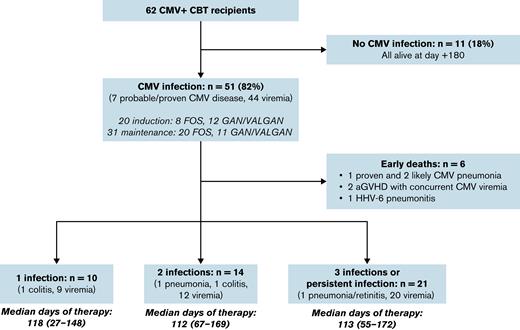
Of the 62 control patients, 51 developed csCMVi by day 100, for a cumulative incidence of 82% (95% CI, 73-92) (Figure 2). The median onset of CMV infection was 34 days (range, 5-74 days) with a median viral load at first detection of <137 IU/mL (range, <137-245 IU/mL). The median peak viremia in the first 100 days was 376 IU/mL (range, <137-146 304 IU/mL). Seven patients developed probable or proven CMV disease (5 cases of CMV pneumonia that required supplemental oxygen or intubation; 2 cases of gastrointestinal disease).
Of the 51 patients with csCMVi, 8 patients were treated with foscarnet induction, 20 foscarnet maintenance, 12 ganciclovir/valganciclovir induction, and 11 ganciclovir/valganciclovir maintenance dose. Overall, 6 infected patients died before day 100 (range, 36-76 days’ posttransplant), including 3 with CMV pneumonia (Figure 3). The remaining 45 patients required extended therapy during the first 6 months posttransplant for 1 (n = 10), 2 (n = 14), or 3/persistent (n = 21) infections (median duration of therapy, >100 days in each group). A short duration of initial therapy was associated with a high risk of persistent viremia or rapid second reactivation.
Summary of CMV infections in the first 180 days post-CBT in CBT recipients who did not receive letermovir prophylaxis. aGVHD, acute GVHD; FOS, foscarnet; GAN, ganciclovir; HHV-6, human herpesvirus 6; VALGAN, valganciclovir.
Summary of CMV infections in the first 180 days post-CBT in CBT recipients who did not receive letermovir prophylaxis. aGVHD, acute GVHD; FOS, foscarnet; GAN, ganciclovir; HHV-6, human herpesvirus 6; VALGAN, valganciclovir.
Toxicities of CMV antiviral therapy are summarized in Table 3. Of 51 historic control patients who received therapy within the first 100 days, 4 received only foscarnet, 16 received only ganciclovir or valganciclovir, and 31 sequentially received both agents. Therapy switches were due to toxicity, inadequate response, or the convenience of oral valganciclovir. Of the 35 patients who received either induction foscarnet or maintenance foscarnet between days 1 and 100, a total of 14 patients (40%) developed nephrotoxicity with a median serum creatinine increase of 1.8-fold (range, 1.2-4) over baseline. Of the 47 patients who received ganciclovir/valganciclovir between days 1 and 100, a total of 32 (68%) developed neutropenia requiring granulocyte colony-stimulation factor support (median, 5 doses; range, 1-22).
During days 101 to 180, 1 patient died of GVHD with CMV viremia, and the majority of the remaining patients either continued on therapy or had subsequent csCMVi after treatment withdrawal. Similar serious therapy toxicities were seen in these patients during this period (Table 3).
Discussion
The efficacy of letermovir prophylaxis in reducing the risk of csCMVi after HSCT is well established.12-22,27 However, high rates of late csCMVi have been observed when letermovir prophylaxis is limited to the first 100 days’ posttransplant, especially in high-risk patient groups such as HLA-disparate donor recipients.11,22 A single-center analysis has shown that extending prophylaxis duration beyond 100 days is highly effective in preventing csCMVi in patients with GVHD, another high-risk patient group.28 Although the results of the 100-day vs 200-day prophylaxis, randomized phase 3 trial (#NCT03930615) are awaited, we hypothesized that prophylaxis for at least 180 days’ posttransplant in adult CBT recipients would be beneficial.
Herein, we report our experience with extended letermovir prophylaxis in adult CMV-seropositive CBT recipients. Most patients were able to continue letermovir through day 180 as intended. We show that extended letermovir prophylaxis mitigated csCMVi with complete abrogation of the need for preemptive therapy, CMV end-organ disease, and CMV-related mortality in the first 180 days’ posttransplant. These results compare very favorably to a historic control cohort of patients managed with preemptive therapy who had a high CMV infection burden with the associated serious toxicities of antiviral therapy. Importantly, letermovir was well tolerated, with no adverse events requiring medication discontinuation.
Interestingly, approximately one-third of our patients had detectable low-level viremia before letermovir initiation. These patients derived the same benefit from prophylaxis as those without viremia at baseline with no cases of csCMVi through day 180 in either group. Patients with baseline viremia, however, had a higher incidence of low-level viremia after letermovir initiation, suggesting that prophylaxis may have prevented impending csCMVi in these patients. This may have been due to the universal early initiation of prophylaxis on day 7. These findings are in contrast to those of the pivotal phase 3 trial in which letermovir-treated patients with detectable baseline viremia had higher csCMVi rates compared with those without.11,29 In that trial, letermovir was started at any time before day 28 (at a median of 9 days) posttransplant.
Similar to previous reports,15,21,22,30 we also observed that low-level viremia while receiving letermovir prophylaxis was common in our CBT patient cohort and did not require CMV-directed therapy. This observation supports the concept that detectable low-level viremia in letermovir recipients may reflect abortive infection rather than replicating virus.31 Therefore, such episodes are of limited clinical significance and can resolve spontaneously.30,31 This is in contrast to our CBT historic control patients in whom low-level viremia commonly progressed to csCMVi, mandating early initiation of preemptive therapy at any level of viral load detection.
Our observation that letermovir prevented any csCMVi in the first 100 days is consistent with 1 prior study in CBT recipients21 but in contrast with 2 other series of prophylaxis that reported a day 100 csCMVi incidence of 5% and 19%, respectively.15,22 It is also in contrast to the findings of most analyses of letermovir prophylaxis in adult donor HSCT recipients that have reported higher incidence of breakthrough infections by day 100 posttransplant.11-15,18,19 Differences in patient characteristics, timing of prophylaxis initiation, type of assay used for viral load monitoring, and threshold to initiate antiviral agents for csCMVi may account for the differences between these studies. It should be acknowledged that the practice of extended letermovir prophylaxis at our center coincided with the addition of tocilizumab to standard CSA/MMF for GVHD prevention in adult CBT recipients (clinicaltrials.gov #NCT03434730). Notably, the randomized phase 3 trial of tocilizumab-based GVHD prophylaxis in adult donor HSCT recipients found that the addition of tocilizumab was associated with a lower incidence of CMV infections,32 likely independent of its effect on mitigating GVHD severity.33,34 Thus, the use of tocilizumab may have reduced the risk of CMV infection in our CBT recipients receiving letermovir. Nevertheless, our findings provide strong support for the high efficacy of letermovir prophylaxis in the first 100 days’ posttransplant in adult CBT recipients when started by day 7 even when using a threshold of 300 copies/mL for csCMVi.
Moreover, we found that continued prophylaxis beyond day 100 remained highly efficacious, with no csCMVi observed between 100 and 180 days’ posttransplant when letermovir was used through at least day 180. This benefit was observed despite letermovir interruptions or early discontinuation in a small number of patients. Thus, we show that extension of prophylaxis abrogates the increased incidence of postprophylaxis csCMVi between days 100 and180 that has been observed when letermovir is discontinued at 100 days’ posttransplant.11,15,21,22
One strength of our study is the detailed analysis of CMV infections after letermovir discontinuation in long-term survivors. Detectable viremia after letermovir discontinuation was common in CBT recipients receiving extended prophylaxis. However, only a minority of patients developed csCMVi that responded promptly to preemptive oral valganciclovir, and there were no cases of CMV disease. It is also possible that some of the patients who developed csCMVi after letermovir discontinuation could have been observed expectantly, as the viral load threshold that warrants preemptive antiviral therapy is not established. Notably, all patients who were deemed to have csCMVi had viremia onset within 30 days of, and initiated preemptive treatment within 45 days of, letermovir discontinuation. This finding is consistent with prior studies reporting that the risk of late csCMVi occurs within a few weeks after prophylaxis discontinuation.11,18,21,22,29,30 Accordingly, as suggested by others,30 and regardless of prophylaxis duration, viremia monitoring is warranted for approximately the first 8 weeks after discontinuing letermovir; intensive monitoring for an extended period after cessation is likely not necessary.
Finally, studies have suggested that letermovir may delay emergence of CMV-specific immunity due to suppression of CMV antigen exposure.35,36 Although we did not assess CMV-specific immune responses in our patients, we observed a low incidence of postprophylaxis csCMVi with lack of recurrent CMV infections after permanent cessation of CMV-active agents. It is likely that extended letermovir prophylaxis protects against CMV infection until the development of prompt CMV-specific immune responses is possible, at a time when T-cell reconstitution has improved and the burden of immunosuppression is reduced. In addition, it is known that CMV reactivation has a strong impact on quantitative and qualitative T-cell reconstitution after HSCT,37-40 may impair thymopoiesis,37 and has been associated with inferior CD4+ T-cell recovery in CBT recipients.38 It is therefore possible that extended prophylaxis may also promote CD4+ T-cell recovery by suppressing early CMV infections. A detailed analysis of immune reconstitution patterns in CMV-seropositive patients receiving letermovir prophylaxis vs patients managed preemptively is needed.
As with other HSC sources, the optimal duration of letermovir prophylaxis in CBT recipients remains unknown. The minimum acceptable letermovir prophylaxis duration in CBT recipients has been considered to be 100 days’ posttransplant, with recommended consideration to extended prophylaxis in patients with GVHD or delayed immune reconstitution.41 However, given the high burden of csCMVi between 100 and 180 days in our historic control patients, as well as the high efficacy and tolerability of letermovir, our findings support extended letermovir prophylaxis until at least day 180 as a new standard of care in adult CBT recipients. Moreover, we currently continue letermovir prophylaxis beyond 6 months in patients receiving ongoing immunosuppression for GVHD therapy or have delayed immune reconstitution. This approach greatly simplifies early posttransplant care given the substantial toxicities of preemptive CMV therapy, and also mitigates the risk of late csCMVi. It is also consistent with the recommendations of Hill et al22 for extended letermovir prophylaxis in CBT recipients. Improved understanding of the biology of CMV-specific T-cell immunity in letermovir recipients and elucidation of the immune milestones required for protection against csCMVi are needed to guide the optimal timing of letermovir cessation, especially in high-risk seropositive HSCT recipients.
Acknowledgments
This research was supported in part by the National Institutes of Health award P01 CA23766, the National Institutes of Health, National Cancer Institute Cancer Center Support grant P30 CA008748, as well as an unrestricted educational grant from Merck.
Authorship
Contribution: I.P., C.L., and J.N.B. designed the study, assembled and analyzed the data, and wrote the manuscript; S.M.D. performed the statistical analysis; I.P., C.L., and S.Q. maintained the patient database and procured data for the study; I.P., C.L., A.L., M.-A.P., G.L.S., S.K.S., G.A.P., and J.N.B. interpreted the data and reviewed and edited the manuscript; and all authors have approved the final version of the manuscript.
Conflict-of-interest disclosure: I.P. has received research funding from Merck; and serves on a Data and Safety Monitoring Board for ExCellThera. A.L. has received consulting fees from Incyte Corporation, EUSA Pharma, and Medexus; and speaker honoraria from Pharmacy Times Continuing Education and Clinical Education Alliance. M.-A.P. has received honoraria from AbbVie, Astellas, Bristol Myers Squibb, Celgene, Equillium, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Novartis, Nektar Therapeutics, Omeros, Orca Bio, Takeda, VectivBio AG, and Vor Biopharma; serves on Data and Safety Monitoring Boards for Cidara Therapeutics, Medigene, Sellas Life Sciences, and Servier; serves on the scientific advisory board of NexImmune; has ownership interests in NexImmune and Omeros; and has received research support for clinical trials from Incyte, Kite/Gilead, Miltenyi Biotec, and Novartis. G.L.S. has received research funding from Janssen, Amgen, and Beyond Spring. S.K.S. has received research funding from Merck. G.A.P. serves as investigator and has received research funding from Merck and Takeda; and consulting and other fees from Merck, Takeda, AlloVir, and SymBio. J.N.B. has received consultancy payments from Gamida Cell and the New York Blood Center; and an unrestricted research graft from Merck. The remaining authors declare no competing financial interests.
Correspondence: Ioannis Politikos, Adult Bone Marrow Transplant Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, 530 E. 74th St, New York, NY 10021; e-mail: politiki@mskcc.org.
References
Author notes
∗I.P. and C.L. contributed equally to this study.
Requests for original data may be submitted to the corresponding author (Ioannis Politikos; e-mail: politiki@mskcc.org).