Key Points
Belumosudil is associated with clinical responses in BOS, particularly in less advanced disease.
No significant correlation was identified between FEV1 evaluations and measures of symptoms in predominantly mild or moderate disease.
Abstract
Chronic graft-versus-host disease (cGVHD) of the lung, or bronchiolitis obliterans syndrome (BOS), is a high-risk disease manifestation associated with poor outcomes. Currently available treatments have demonstrated limited clinical efficacy in this setting. Belumosudil is a novel oral selective rho-associated coiled-coil–containing protein kinase-2 inhibitor that was recently approved by the US Food and Drug Administration in the treatment of cGVHD. We identified 59 subjects with BOS who were enrolled and treated in 2 prospective clinical trials of belumosudil. Patients with BOS had a percentage predicted forced expiratory volume in 1 second (FEV1) of ≤79% at enrollment and clinician attribution of lung disease owing to cGVHD. The National Institutes of Health (NIH) cGVHD lung scores at enrollment were 1 (n = 30, 59%), 2 (n = 23, 39%), or 3 (n = 6, 10%). According to NIH response criteria, the best overall response rate (ORR) for lung cGVHD was 32% (partial response: 17%; complete response: 15%). Response rates were inversely proportional to baseline NIH GVHD lung score at enrollment (lung score 1: ORR 50%; lung score 2: ORR 17%, lung score 3: ORR 0%) (P = .006). In multivariable analysis, male sex, lower baseline NIH cGVHD lung score, and partial response to previous line of cGVHD therapy before enrollment were associated with higher rates of lung-specific response. No significant correlation was identified between pulmonary function evaluations and measures of patient symptoms (NIH lung symptom score or Lee Symptom Scale score for lung). In conclusion, belumosudil treatment was associated with lung-specific clinical responses for subjects with BOS, which were more commonly observed in less advanced disease. Optimization of treatment response evaluations remains a challenge in patients with BOS.
Introduction
Chronic graft-versus-host disease (cGVHD) is an immune-mediated inflammatory and fibrotic disorder, which is a major late complication after allogeneic hematopoietic cell transplant (HCT).1-3 Among the numerous manifestations of cGVHD, lung involvement is associated with the poorest prognosis.4,5 Pulmonary cGVHD or bronchiolitis obliterans syndrome (BOS) results from an immunological attack of the small airways, leading to fibrotic narrowing of the respiratory bronchioles and subsequent obliteration.6 Historically, treatment options for BOS have consisted of standard cGVHD therapies, including systemic corticosteroids and immunosuppressive agents, with the hope of intervening the disease before irreversible damage has occurred. In a small randomized, double-blind study, inhaled budesonide/formoterol led to a significant improvement in forced expiratory volume in 1 second (FEV1) compared with placebo in patients with mild/severe BOS.7 Similarly, a phase II trial evaluating the combination of inhaled fluticasone, azithromycin, and montelukast and a brief steroid pulse suggested that this therapeutic approach may limit pulmonary decline in new-onset BOS.8 Other immunosuppressive or immunomodulatory therapeutics can halt disease progression but rarely improve either pulmonary function or symptoms.9,10 Therefore, novel therapeutic approaches for BOS remain an unmet need.
Belumosudil is an oral selective rho-associated coiled-coil–containing protein kinase-2 (ROCK2) inhibitor. ROCK2 inhibition acts on the dysregulated adaptive immune system and the fibrosis that occurs because of aberrant tissue repair.11,12 Treatment of cGVHD with belumosudil was first evaluated in a phase 2a dose-finding study (KD025-208) that demonstrated a pooled overall response rate (ORR) of 65% and improvements in quality of life in subjects with cGVHD after failure of 1 to 3 prior systemic lines of therapy (LOTs).13 These results were confirmed in a randomized phase 2 study (KD025-213), in which the best ORR for belumosudil 200 mg daily and 200 mg twice daily was 74% and 77%, respectively, with high response rates observed in all subgroups.14 Based on these results, the US Food and Drug Administration approved belumosudil for the treatment of cGVHD after failure of at least 2 prior systemic LOTs.
In these studies of belumosudil, all affected organs demonstrated responses, including the lungs. However, a more in-depth analysis of lung-specific responses is lacking. In this analysis, we sought to evaluate the treatment effect of belumosudil in subjects with BOS, given the unique mechanism of ROCK2 inhibition to potentially address both the inflammatory and fibrotic physiology of BOS.15 Furthermore, we sought to analyze multiple lung-specific metrics to better characterize longitudinal changes in pulmonary function and patient symptoms.
Methods
Subjects and study design
Subjects enrolled on the previous clinical studies of belumosudil (KD025-208 and KD025-213) served as the population for this combined analysis. Detailed descriptions of these studies have been previously published.13,14 In brief, KD025-208 (NCT02841995) was a multicenter phase 2a, dose-finding, open-label study that enrolled adult allogeneic HCT recipients with persistent cGVHD manifestations after having received 1 to 3 prior systemic LOTs. Subjects were enrolled into 3 sequential cohorts investigating belumosudil dosing: 200 mg once daily, 200 mg twice daily, and 400 mg once daily. Belumosudil was subsequently investigated in KD025-213 (NCT03640481), a phase 2 randomized multicenter study which enrolled allogeneic HCT recipients aged ≥12 years with persistent cGVHD manifestations after receiving 2 to 5 prior systemic LOTs. Treatment consisted of belumosudil 200 mg daily or 200 mg twice daily. In both studies, belumosudil was administered continuously until cGVHD progression or unacceptable toxicity. The study received institutional review board approval and was conducted according to the Declaration of Helsinki.
Patients with BOS were identified as: (1) % predicted forced expiratory volume in 1 second (%FEV1) of ≤79% at enrollment and (2) clinician attribution of lung disease owing to cGVHD. Subjects were excluded from KD025-213 if they had a %FEV1 of <40% or a National Institutes of Health (NIH) lung symptom score of 3. In KD025-208, lung function assessments were conducted at baseline and on day 1 of each cycle for patients with suspected known lung involvement. In KD025-213, lung function assessments were conducted at baseline and at the time of response assessments on day 1 of cycles 2 to 5 and then on day 1 of every other cycle thereafter.
The severity of cGVHD was graded according to the 2014 NIH Consensus Criteria.16 Treatment response was defined using an organ-specific cGVHD response assessment, as defined by the 2014 NIH Consensus Criteria.17 According to these criteria, a complete response (CR) in the lung is defined as normal %FEV1 after previous involvement or in the absence of pulmonary function tests (PFTs), a NIH lung symptom score of 0 after previous involvement. Partial response (PR) in the lung is defined as an increase by 10% predicted absolute value of %FEV1 or in the absence of PFTs, a decrease in NIH lung symptom score by 1 or more points. Progression of lung disease is defined as a decrease by 10% predicted absolute value of %FEV1 or in the absence of PFTs, an increase in NIH lung symptom score by 1 or more points, except 0 to 1.
Statistical analysis
Baseline characteristics were reported descriptively. Univariable and multivariable logistic regression analysis was performed to investigate clinical factors (listed in Table 1) that were associated with NIH lung response. Correlation analysis was performed to assess the correlation among lung response metrics. All testing was 2-sided at a significance level of 0.05. All analyses were performed using SAS 9.3 (SAS Institute Inc, Cary, NC), and R v3.5.2 (the CRAN project).
Baseline demographics and clinical characteristics
| Characteristic . | Value . |
|---|---|
| Number of subjects | 59 |
| Age, median (range), y | 42.5 (26-77) |
| Sex, n (%) | |
| Male | 33 (56) |
| Female | 26 (44) |
| Female donor to male recipient, n (%) | 14 (24) |
| HLA matching of donor/recipient, n (%) | |
| Matched | 53 (90) |
| Partially matched | 6 (10) |
| Conditioning intensity, n (%) | |
| Myeloablative | 44 (75) |
| Nonmyeloablative | 13 (22) |
| Unknown | 2 (3) |
| Stem cell source, n (%) | |
| Peripheral blood | 54 (92) |
| Bone marrow | 4 (7) |
| Unknown | 1 (1) |
| GVHD prophylaxis, n (%) | |
| CNI/MTX ± Other | 33 (56) |
| CNI/MMF | 11 (19) |
| CNI/sirolimus | 9 (15) |
| PTCy-based | 3 (5) |
| Other | 3 (5) |
| Median time from cGVHD to enrollment (range), m | 22 (1-161) |
| Clinical trial enrollment, n (%) | |
| KD025-208 | 17 (29) |
| KD025-213 | 42 (71) |
| Belumosudil dose, n (%) | |
| 200 mg daily | 27 (46) |
| 200 mg twice daily | 23 (39) |
| 400 mg daily | 9 (15) |
| NIH cGVHD global severity, n (%) | |
| Moderate | 11 (19) |
| Severe | 48 (81) |
| Number of organs involved, n (%) | |
| <4 | 20 (34) |
| ≥4 | 39 (66) |
| Median lines of prior therapy (range), n | 3 (1-6) |
| cGVHD response to last systemic therapy before enrollment, n (%) | |
| PR | 12 (20) |
| SD | 25 (42) |
| PD | 12 (20) |
| Unknown | 10 (17) |
| Median number of cycles of belumosudil therapy (range), n | 14 (1-57) |
| NIH lung score at baseline, n (%) | |
| 1 (FEV1 60%-79%) | 30 (51) |
| 2 (FEV1 40%-59%) | 23 (39) |
| 3 (FEV1 <40%) | 6 (10) |
| Characteristic . | Value . |
|---|---|
| Number of subjects | 59 |
| Age, median (range), y | 42.5 (26-77) |
| Sex, n (%) | |
| Male | 33 (56) |
| Female | 26 (44) |
| Female donor to male recipient, n (%) | 14 (24) |
| HLA matching of donor/recipient, n (%) | |
| Matched | 53 (90) |
| Partially matched | 6 (10) |
| Conditioning intensity, n (%) | |
| Myeloablative | 44 (75) |
| Nonmyeloablative | 13 (22) |
| Unknown | 2 (3) |
| Stem cell source, n (%) | |
| Peripheral blood | 54 (92) |
| Bone marrow | 4 (7) |
| Unknown | 1 (1) |
| GVHD prophylaxis, n (%) | |
| CNI/MTX ± Other | 33 (56) |
| CNI/MMF | 11 (19) |
| CNI/sirolimus | 9 (15) |
| PTCy-based | 3 (5) |
| Other | 3 (5) |
| Median time from cGVHD to enrollment (range), m | 22 (1-161) |
| Clinical trial enrollment, n (%) | |
| KD025-208 | 17 (29) |
| KD025-213 | 42 (71) |
| Belumosudil dose, n (%) | |
| 200 mg daily | 27 (46) |
| 200 mg twice daily | 23 (39) |
| 400 mg daily | 9 (15) |
| NIH cGVHD global severity, n (%) | |
| Moderate | 11 (19) |
| Severe | 48 (81) |
| Number of organs involved, n (%) | |
| <4 | 20 (34) |
| ≥4 | 39 (66) |
| Median lines of prior therapy (range), n | 3 (1-6) |
| cGVHD response to last systemic therapy before enrollment, n (%) | |
| PR | 12 (20) |
| SD | 25 (42) |
| PD | 12 (20) |
| Unknown | 10 (17) |
| Median number of cycles of belumosudil therapy (range), n | 14 (1-57) |
| NIH lung score at baseline, n (%) | |
| 1 (FEV1 60%-79%) | 30 (51) |
| 2 (FEV1 40%-59%) | 23 (39) |
| 3 (FEV1 <40%) | 6 (10) |
CNI, calcineurin inhibitor; m, months; MMF, mycophenolate mofetil; MTX, methotrexate; n, number; PD, progressive disease; PTCy, post-transplantation cyclophosphamide; SD, stable disease.
Results
Subject characteristics
A total of 66 subjects with BOS were identified from the 2 clinical trials. Six subjects were excluded from this analysis for not having a subsequent PFT evaluation beyond baseline and 1 subject was reclassified as not having BOS. Of the 59 evaluable subjects, 17 (29%) were enrolled on KD025-208 and 42 (71%) were enrolled on KD025-213. The dose of belumosudil was either 200 mg daily (n = 27, 46%), 200 mg twice daily (n = 23, 39%), or 400 mg daily (n = 9, 15%).
The baseline demographics and clinical characteristics are shown in Table 1. The median age at enrollment was 42.5 years (range, 26-77). Allogeneic HCT was predominantly performed with myeloablative conditioning (n = 44, 75%) and peripheral blood stem cells (n = 54, 92%) from an HLA-matched donor (n = 53, 90%). The median time from cGVHD diagnosis to enrollment was 22 months (range, 1-161). NIH cGVHD global severity score at enrollment was moderate (n = 11, 19%) or severe (n = 48, 81%). Most subjects (n = 39, 66%) had at least 4 organs involved at enrollment. The median number of prior lines of systemic therapy was 3 (range, 1-6). Overall cGVHD response to the line of systemic therapy before enrollment was PR (n = 12, 20%), stable disease (n = 25, 42%), progressive disease (n = 12, 20%), or unknown (n = 10, 17%).
Treatment duration, infectious complications, and survival
The median number of cycles of belumosudil therapy received on trial was 14 (range, 1-57). At the time of analysis, 16 subjects remained on therapy. The reasons for belumosudil discontinuation included: progressive cGVHD (n = 18), adverse events (n = 8), underlying disease relapse (n = 6), subject withdrawal (n = 4), physician discretion (n = 4), noncompliance (n = 2), and death (n = 1). A total of 31 subjects (53%) experienced at least 1 respiratory infection (any grade) while on treatment, with 11 subjects experiencing ≥2 episodes. These infections were categorized as upper respiratory infections (n = 33) or pneumonia (n = 13). Eleven subjects experienced ≥3 grade respiratory infection for which belumosudil treatment was interrupted or discontinued in 6 cases. With a median follow-up among survivors of 27 months (range, 1.8-55), the 2-year overall survival rate was 82% (95% confidence interval [CI]: 70-90).
BOS responses according to NIH criteria
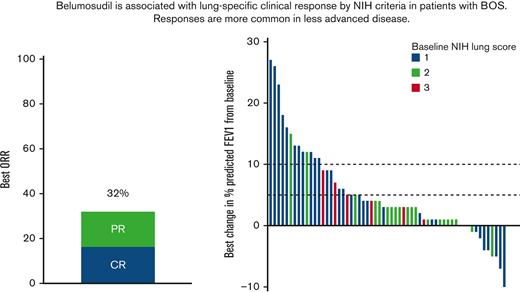
The NIH cGVHD lung score at enrollment was 1 (n = 30, 59%), 2 (n = 23, 39%), or 3 (n = 6, 10%). According to NIH response criteria, the best ORR for pulmonary cGVHD was 32% (PR 17%, CR 15%). The median time to first NIH lung response was 5 cycles (range, 3-39), whereas the median time to best NIH lung response was 7 cycles (range, 3-41). In 3 of the 19 responders, subjects met NIH criteria for lung progression (median 4 cycles; range, 3-7) before later meeting criteria for response (median 11 cycles; range, 4-21). The KD025-213 trial was designed to allow subjects to continue therapy until clinically meaningful progression occurred, thus permitting continued administration of belumosudil.
The NIH response criteria are based on %FEV1 or, in the absence of PFTs, a NIH lung symptom score (provided in Methods). The NIH lung response was defined by the measurement of %FEV1 alone for 12 subjects (63%). In the absence of %FEV1, the NIH symptom score was used to define NIH response in 5 subjects (26%). In 2 subjects, the NIH response was upgraded from a PR (according to %FEV1) to a CR based on the NIH symptom score. When evaluating NIH response with PFTs alone, the best ORR for lung cGVHD was 24% (PR 14%, CR 10%).
BOS responses according to evaluation of FEV1
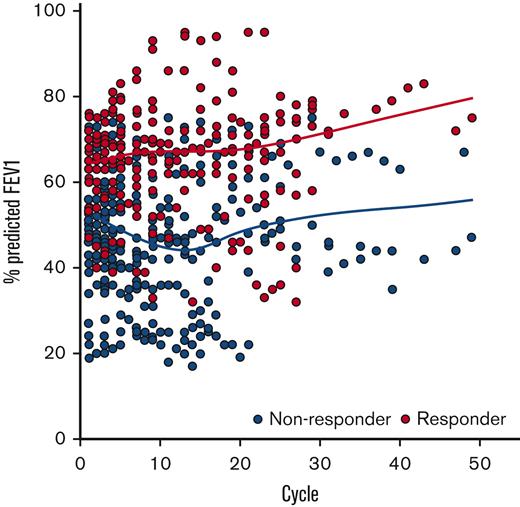
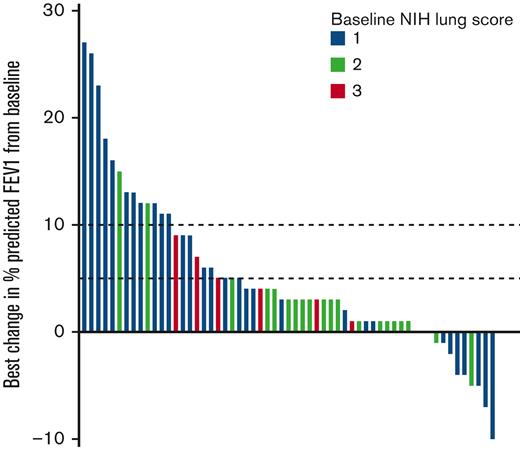
The trajectory of all %FEV1 evaluations collected on the study are shown in Figure 1. The best change in %FEV1 from baseline is shown in Figure 2. Overall, 23 subjects (39%) experienced ≥5% absolute improvement in %FEV1 and 13 subjects (22%) experienced ≥10% absolute improvement in %FEV1 from baseline while on treatment with belumosudil. A best absolute improvement in %FEV1 of ≥5% from baseline was observed in subjects regardless of baseline NIH lung score (Score 1 [n = 17, 57%]; Score 2 [n = 3, 13%]; Score 3 [n = 3, 50%]). An absolute improvement in %FEV1 of ≥5% from baseline was observed in 84% of responders by NIH criteria and 18% of nonresponders. All 13 subjects with ≥10% absolute improvement in %FEV1 were responders by NIH criteria (per definition). For subjects with the best improvement in %FEV1 by ≥5%, 14 of the 23 responses were maintained over 2 consecutive FEV1 evaluations. For subjects with the best improvement in %FEV1 by ≥10%, 9 of the 13 responses were maintained over 2 consecutive FEV1 evaluations.
Trajectoryof all%FEV1 measurements collected while on belumosudil therapy. Graphical representation demonstrates the %FEV1 measurements for subjects who were responders (PR or CR by NIH criteria, n = 19) in red and nonresponders (n = 40) in gray. Fitted lines for responders and nonresponders generated using locally weighted smoothing (LOESS technique) to visually present the relationship between %FEV1 and response over time.
Trajectoryof all%FEV1 measurements collected while on belumosudil therapy. Graphical representation demonstrates the %FEV1 measurements for subjects who were responders (PR or CR by NIH criteria, n = 19) in red and nonresponders (n = 40) in gray. Fitted lines for responders and nonresponders generated using locally weighted smoothing (LOESS technique) to visually present the relationship between %FEV1 and response over time.
Best change in %FEV1 from baseline while on belumosudil therapy. Dotted lines mark absolute improvement by 5% and 10%, respectively. Baseline NIH lung score are indicated by color. Each bar represents an individual subject.
Best change in %FEV1 from baseline while on belumosudil therapy. Dotted lines mark absolute improvement by 5% and 10%, respectively. Baseline NIH lung score are indicated by color. Each bar represents an individual subject.
Lee Symptom Scale (LSS) scores for lung
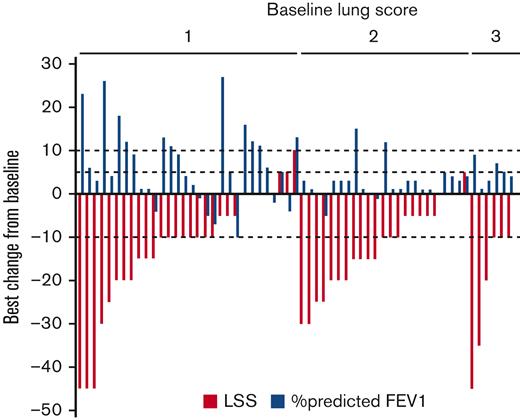
The best change in the LSS scores for the lung from baseline is shown in Figure 3. Following the methodology used for the LSS,18,19 we identified a 10-point difference (half a standard deviation from baseline scores) as being a clinically meaningful organ-specific change in this data set. Forty subjects (68%) experienced a clinically meaningful improvement in the LSS lung score. Clinically meaningful improvements were observed in subjects regardless of baseline NIH lung score (Score 1 [n = 20, 66%]; Score 2 [n = 14, 61%]; Score 3 [n = 6, 100%]). A clinically meaningful improvement was observed in 68% of responders by NIH criteria and 68% of nonresponders.
Best change in LSS score for lung from baseline while on belumosudil therapy. LSS lung scores (red) are grouped according to baseline NIH lung score. A 10-point change (half a standard deviation from baseline scores) was considered clinically meaningful. The corresponding best change in %FEV1 from baseline for the individual subject is shown in blue.
Best change in LSS score for lung from baseline while on belumosudil therapy. LSS lung scores (red) are grouped according to baseline NIH lung score. A 10-point change (half a standard deviation from baseline scores) was considered clinically meaningful. The corresponding best change in %FEV1 from baseline for the individual subject is shown in blue.
Analysis of subgroups and predictors of response
There was an obvious correlation between the response and the baseline NIH lung score. The best ORR was 50% (PR 23%, CR 27%) for baseline NIH lung score 1, 17% (PR 13%, CR 4%) for baseline NIH lung score 2%, and 0% for NIH lung score 3 (Table 2). Logistic regression analysis was performed to identify clinical factors that were associated with lung-specific responses according to NIH criteria (Table 3). Univariable analysis identified male sex, lower baseline NIH cGVHD lung score, and overall cGVHD PR to last treatment before belumosudil as predictors of response. These variables remained significant in multivariable analysis (male sex, OR 14.07, P = .0037; NIH lung score 1, OR 5.65, P = .028; PR to prior line of therapy, OR 7.89, P = .024).
Lung-specific NIH response according to lung score at baseline
| NIH lung score at baseline . | Number of subjects . | PR rate . | CR rate . | Best ORR . |
|---|---|---|---|---|
| 1 | 30 | 23% (7/30) | 27% (8/30) | 50% (15/30) |
| 2 | 23 | 13% (3/23) | 4% (1/23) | 17% (4/23) |
| 3 | 6 | 0% (0/6) | 0% (0/6) | 0% (0/6) |
| Total | 17% (10/59) | 15% (9/59) | 32% (19/59) | |
| NIH lung score at baseline . | Number of subjects . | PR rate . | CR rate . | Best ORR . |
|---|---|---|---|---|
| 1 | 30 | 23% (7/30) | 27% (8/30) | 50% (15/30) |
| 2 | 23 | 13% (3/23) | 4% (1/23) | 17% (4/23) |
| 3 | 6 | 0% (0/6) | 0% (0/6) | 0% (0/6) |
| Total | 17% (10/59) | 15% (9/59) | 32% (19/59) | |
Logistic regression analysis for predictors of NIH lung response
| Clinical variable . | . | Univariable . | Multivariable . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR . | 95% CI . | P value . | OR . | 95% CI . | P value . | ||||
| Belumosudil dose | 200 mg QD vs 200 mg BID | 1.67 | 0.49 | 5.62 | .41 | ||||
| 400 mg QD vs 200 mg BID | 1.42 | 0.27 | 7.52 | .68 | |||||
| Age | <50 vs ≥50 | 0.90 | 0.30 | 2.69 | .85 | ||||
| Sex | Male vs female | 7.22 | 1.81 | 28.8 | .005 | 14.07 | 2.36 | 83.70 | .0037 |
| HLA donor match | Partially matched vs matched | 2.31 | 0.42 | 12.7 | .34 | ||||
| Response to last systemic therapy | PR vs no PR | 4.36 | 1.11 | 17.2 | .036 | 7.89 | 1.31 | 47.71 | .024 |
| Conditioning intensity | MAC vs NMA | 3.14 | 0.62 | 16.0 | .17 | ||||
| NIH severity at enrollment | Moderate vs severe | 3.23 | 0.84 | 12.40 | .088 | 2.80 | 0.50 | 15.79 | .24 |
| Number of organs involved at enrollment | ≥4 vs <4 | 1.68 | 0.50 | 5.61 | .4 | ||||
| NIH lung score at enrollment | 1 vs (2 or 3) | 5.67 | 1.63 | 19.70 | .006 | 5.65 | 1.35 | 29.60 | .028 |
| Time from cGVHD to enrollment in months | <24 vs ≥24 | 0.73 | 0.24 | 2.19 | .57 | ||||
| <36 vs ≥36 | 1.35 | 0.44 | 4.13 | .6 | |||||
| Clinical variable . | . | Univariable . | Multivariable . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR . | 95% CI . | P value . | OR . | 95% CI . | P value . | ||||
| Belumosudil dose | 200 mg QD vs 200 mg BID | 1.67 | 0.49 | 5.62 | .41 | ||||
| 400 mg QD vs 200 mg BID | 1.42 | 0.27 | 7.52 | .68 | |||||
| Age | <50 vs ≥50 | 0.90 | 0.30 | 2.69 | .85 | ||||
| Sex | Male vs female | 7.22 | 1.81 | 28.8 | .005 | 14.07 | 2.36 | 83.70 | .0037 |
| HLA donor match | Partially matched vs matched | 2.31 | 0.42 | 12.7 | .34 | ||||
| Response to last systemic therapy | PR vs no PR | 4.36 | 1.11 | 17.2 | .036 | 7.89 | 1.31 | 47.71 | .024 |
| Conditioning intensity | MAC vs NMA | 3.14 | 0.62 | 16.0 | .17 | ||||
| NIH severity at enrollment | Moderate vs severe | 3.23 | 0.84 | 12.40 | .088 | 2.80 | 0.50 | 15.79 | .24 |
| Number of organs involved at enrollment | ≥4 vs <4 | 1.68 | 0.50 | 5.61 | .4 | ||||
| NIH lung score at enrollment | 1 vs (2 or 3) | 5.67 | 1.63 | 19.70 | .006 | 5.65 | 1.35 | 29.60 | .028 |
| Time from cGVHD to enrollment in months | <24 vs ≥24 | 0.73 | 0.24 | 2.19 | .57 | ||||
| <36 vs ≥36 | 1.35 | 0.44 | 4.13 | .6 | |||||
BID, twice daily; MAC, myeloablative conditioning; NMA, nonmyeloablative; OR, odds ratio; QD, daily
Correlation between metrics for lung-specific response
Correlations between multiple metrics for BOS response were investigated (definitions provided in supplemental Table 1). A heatmap of demonstrating best changes in lung response metrics in 59 patients is shown in Figure 4. When examining the association between NIH lung score (score 0-3) and NIH lung symptom score (score 0-3), 24 pairs (41%) were concordant and 35 pairs (59%) were discordant. The NIH lung symptom score was lower than the NIH lung score in 32 of 35 discordant pairs. In addition, LSS lung scores were not correlated with other metrics. For best lung response by NIH criteria, 12 of 19 (63.2%) responders and 27 of 40 (67.5%) nonresponders (P = .77) had at least 10-point reduction in LSS lung score. For best improvement in %FEV1, 14 of 23 (61%) with ≥5% and 25 of 36 (69%) with <5% %FEV1 showed clinically meaningful improvement in LSS lung scores (P = .58) (Figure 3).
Heatmap of best response metrics of disease and symptoms in BOS. Baseline characteristics for all 59 subjects and best improvement in multiple metrics of lung response are shown. Detailed definitions of metrics provided in supplemental Table 1. F, female; M, male; mod, moderate; NR, no response; Sev, severe; Unk, unknown.
Heatmap of best response metrics of disease and symptoms in BOS. Baseline characteristics for all 59 subjects and best improvement in multiple metrics of lung response are shown. Detailed definitions of metrics provided in supplemental Table 1. F, female; M, male; mod, moderate; NR, no response; Sev, severe; Unk, unknown.
To further assess correlation among metrics, we aggregated measurements from all time points captured while on treatment (supplemental Figure). The discordance observed between the NIH lung score (based on %FEV1) and the NIH symptom score suggests that the symptom score largely overestimates the PFT response in subjects with predominantly less advanced disease. In addition, the LSS lung score was largely dissociated with the NIH lung score ≥1 and the NIH lung symptom score in general.
Discussion
In this combined analysis of 2 prospective studies, belumosudil was associated with a best ORR of 32% for BOS, according to 2014 NIH response criteria, in a population with predominantly less advanced disease. Response rates were inversely proportional to the NIH cGVHD lung score, with the highest response rates for subjects with a baseline lung score of 1. No responses were observed for the limited number of subjects with a baseline lung score of 3. Furthermore, both lower baseline NIH cGVHD lung scores and overall cGVHD PR to previous line of systemic therapy before enrollment were associated with higher rates of organ-specific response in multivariable analysis. This highlights the importance of initiating treatment for patients with early stages of the disease because an advanced disease may have irreversible fibrotic change and lung destruction. In subjects with responses, the median time to best response was 7 cycles, suggesting that BOS compared with inflammatory-like manifestations of cGVHD may require longer periods of treatment to achieve responses. While on treatment, the trajectory of FEV1 measurements is rarely linear. As noted in this analysis, only 60% to 70% of 5% or 10% improvements in absolute %FEV1 are achieved on consecutive evaluations. Nonetheless, the responses in FEV1 suggest a clinically meaningful improvement in lung function for a subset of subjects with earlier forms of BOS.
The NIH response criteria were established by the 2014 NIH cGVHD consensus conference to bring standardization to overall and organ-specific responses and have laid the groundwork for subsequent clinical trials and US Food and Drug Administration approvals. However, every scale of evaluation with thresholds for response has inherent limitations which may overestimate or underestimate the overall impact of therapy. The threshold of meaningful treatment response for BOS was questioned in this analysis. Our analyses did not identify a significant correlation between measurements of FEV1 (%FEV1 or FEV1 in L) and symptom measures (NIH symptom score or LSS lung score). The lack of correlation raises questions about how such metrics can best be integrated when evaluating treatment responses; although, a comparative analysis of patient-reported symptoms could not be performed because the 2 trials lacked control arms. Symptoms are usually a late sign of BOS and may not manifest until a more significant decrease in pulmonary function has occurred. Thus, the NIH response criteria use of the NIH symptom score in the absence of FEV1 measurement may lead to an overestimation of response, especially in patients with less advanced disease. In this data set, the NIH symptom score was used to define or upgrade clinical response in 7 of the 19 subjects (37%) who achieved a NIH response. Moreover, it may be that the clinical meaning of symptoms scores is dependent on the clinical setting. For example, in BOS, symptom measures may carry more weight in patients with more advanced disease, in which a significant FEV1 response is less likely. Given the limited size and lack of standardized follow-up in many cGVHD data sets, it remains unknown how individual metrics (PFT, symptoms) and other measurements (functional assessments, biological markers) can be integrated to refine response criteria for BOS.
BOS remains one of the most morbid manifestations of cGVHD.20 In a recent analysis from the cGVHD Consortium, lung involvement was the clinical factor most strongly associated with an increased risk for nonrelapse mortality (mild disease, hazard ratio, 1.68; moderate or severe disease, hazard ratio, 2.25; P = .002).5 Currently, many patients with BOS receive fluticasone, azithromycin, and montelukast as part of their treatment. However, there is no consensus regarding a standard approach to systemic therapy in these patients. Although the treatment landscape is expanding with the US Food and Drug Administration approval of multiple agents for the treatment of cGVHD in the last 5 years, little is known at this time regarding the impact of these newer agents on BOS. Data about lung-specific responses to ibrutinib are lacking. In the phase 3 randomized clinical trial of ruxolitinib, REACH3, 74 subjects receiving ruxolitinib were identified as having lung involvement at baseline, including those with NIH cGVHD lung scores of 2 (n = 24) and 3 (n = 14). At week 24, the organ response rate was 8.6% (6 out of 70). An ongoing phase II multicenter trial is investigating the use of ruxolitinib in subjects with BOS after allo-HCT (NCT03674047). Although we report an encouraging response with belumosudil, further investigation of novel agents and approaches to BOS are needed. It will be important to better characterize early responses if these responses can be maintained over long periods of time and if clinical responses can reduce the mortality rate of the disease. Prospective clinical trials enrolling subjects with BOS will be important to better understand the biology of this organ manifestation and its responsiveness to individual therapeutic agents.21,22
Additional limitations to the current analysis exist. First, this exploratory analysis was performed in subjects identified as having lung involvement of cGVHD by the treating physician and a reduced %FEV1. As more rigorous evaluations of pulmonary disease (including complete PFTs and computerized tomography imaging) were not available to refine criteria for BOS, the presence of alternative lung pathology cannot be excluded. Ongoing and future prospective clinical trials in BOS are adopting detailed standardized eligibility criteria, such as the NIH Consensus Criteria.16 Second, the evaluation of subjects with NIH cGVHD lung score of 3 was limited because this was an exclusion criterion in KD025-213, owing to the established poor survival for this cohort and the lack of organ-specific response in KD025-208. Although an inverse relationship was noted between GVHD lung score and best ORR, a larger evaluation of subjects with more advanced BOS would provide a wider context for these early findings. Third, neither of the clinical studies were specifically tailored to the treatment of BOS, nor did they include a randomized control arm. Consequently, treatment decisions for subjects on study were highly influenced by other cGVHD manifestations, which could limit the ability to fully evaluate pulmonary cGVHD treatment effects. Finally, there were missing PFT evaluations, which was in part because of the limitations in testing availability during the COVID-19 pandemic.
In conclusion, belumosudil was associated with lung-specific clinical responses for subjects with mild-to-moderate BOS. Identification and treatment of patients with early-stage disease may enhance clinical response. Optimization of treatment response evaluations remains a challenge in patients with high-risk cGVHD manifestations. Future prospective studies should be conducted focusing on subjects with BOS, to investigate multiple metrics of response to best characterize treatment effects in this difficult-to-treat population.
Acknowledgments
The authors acknowledge support from the Goodman Family Fund, National Institutes of Health P01 AI056299 and R37 AI34495.
Authorship
Contribution: Z.D., H.T.K., and C.C. designed the study and performed the analyses; Z.D. wrote the manuscript; H.T.K. edited the manuscript; and all authors approved the final version of the manuscript and its submission.
Conflict-of-interest disclosure: Z.D. has received research support from Incyte Corporation, REGiMMUNE Corporation, and Taiho Oncology, Inc. and has received consulting fees from Syndax Pharmaceuticals Inc., Kadmon Corporation, Omeros Corporation, Incyte Corporation, and MorphoSys AG. S.J.L. has received research funding from Amgen, AstraZeneca, Incyte Corporation, Kadmon Corporation, Novartis, Pfizer, Syndax Pharmaceuticals, and Takeda, has consulted for Kadmon Corporation, Mallinckrodt, and Equillium, and has served on the steering committee for Incyte Corporation. S.Z.P. received research support from the Center for Cancer Research at the National Cancer Institute through the National Institutes of Health Intramural Research Program, which includes Clinical Research Development Agreements with Celgene, Actelion, Eli Lilly and Company, Pharmacyclics, and Kadmon Corporation. B.R.B. receives remuneration as an advisor to Magenta Therapeutics and BlueRock Therapeutics; receives research funding from BlueRock Therapeutics, Rheos Medicines, and Carisma Therapeutics, Inc., and is a cofounder of Tmunity Therapeutics, Inc. C.C. has consulted for and received honoraria from Incyte Corporation, Jazz, CareDx, Mesoblast, Syndax Pharmaceuticals, Omeros Corporation, and Pfizer.
Correspondence: Zachariah DeFilipp, Massachusetts General Hospital, 55 Fruit St, Boston, MA 02114; e-mail: zdefilipp@mgh.harvard.edu.
References
Author notes
Data are available on request from the corresponding author, Zachariah DeFilipp (zdefilipp@mgh.harvard.edu).
The full-text version of this article contains a data supplement.