TO THE EDITOR:
Individuals who are immunocompromised, including patients with non-Hodgkin lymphoma and chronic lymphocytic leukemia (NHL/CLL), often mount ineffective antibody responses after SARS-CoV-2 vaccination1-3 and remain at a high risk of severe COVID-19.4 Several monoclonal antibodies against the SARS-CoV-2 spike protein have been developed for prophylaxis or treatment against infection.5 AZD7442 is a combination of 2 such antibodies (tixagevimab and cilgavimab) with a half-life of ∼90 days.6 It received emergency use authorization (EUA) for use as preexposure prophylaxis in patients who are immunocompromised based on the PROVENT trial, which showed a reduced risk of symptomatic infection among patients deemed at risk of inadequate vaccine response or increased viral exposure.7 However, only 7.2% of the participants had cancer, and 3.2% received immunosuppressive therapy. Importantly, PROVENT was conducted before the emergence of the B.1.1.529 (Omicron) variant. Using purified antibodies and/or pseudoviruses, some studies showed that many antibody formulations developed against the original SARS-CoV-2, including AZD7442, lost significant in vitro activity against Omicron variants.8 Additionally, sera from patients who received AZD7442 blocked the binding between the wild-type spike receptor binding domain (RBD) and plates coated with its receptor ACE2 but had minimal efficacy at blocking the binding between Omicron BA.1 RBD and ACE2.9 Reduced efficacy against Omicron variants was observed in patients treated with half-dose AZD7442,10 and ∼10% of AZD7442-treated kidney transplant recipients developed COVID-19 afterwards, with 35.9% of them requiring hospitalization.11 Although these reports raise concerns that AZD7442 has limited efficacy against Omicron variants, the neutralizing activity of full dose AZD7442 against live, contemporary Omicron variants after administration to patients who are immunocompromised remains unknown. We measured the antibody binding and neutralizing activities of plasma from AZD7442-treated patients with NHL/CLL for several live SARS-CoV-2 variants, including Omicron BA.2.75, BA.5, BQ.1.1, and XBB, which are currently in circulation.
Adult patients with NHL/CLL at the Winship Cancer Institute of Emory University who received AZD7442 in accordance with the EUA fact sheet were enrolled in this prospective observational study approved by the institutional review board of Emory University. As such, patients received either a dose of 150 mg of each antibody followed by a repeat dose within 3 months or a single dose of 300 mg of each antibody. Blood was drawn after providing written informed consent, and antibody binding and live virus neutralization activities were measured as described previously1,12 and in the supplemental Material.
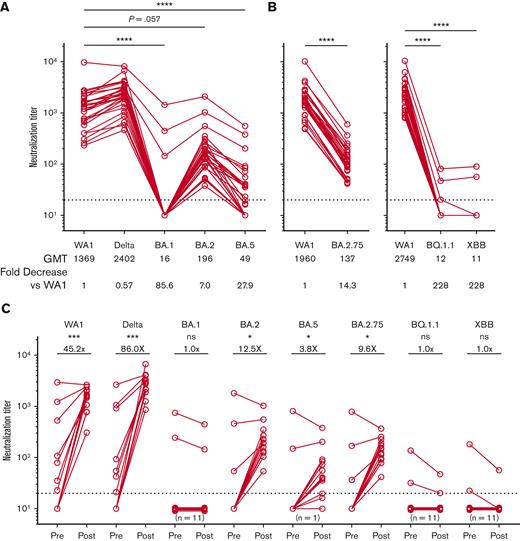
The median age of the 28 patients was 63 years, and 50% of them were female (Table 1). Most patients (93%) had been previously vaccinated, and the median time of assessment after AZD7442 administration was 42 days. At least 4 patients (14%) had a confirmed SARS-CoV-2 infection after AZD7442 administration, although this number is likely an underestimate because patients who were tested at home or at a local health care system were not included. All patients achieved high neutralization titers against the ancestral WA1 strain and B.1.617.2 (Delta) variant after AZD7442 (Figure 1A). In contrast, neutralization titers against Omicron variants were significantly lower, with geometric mean titers that were 7- to 228-fold lower than those against WA1 (P≤.0001, Figure 1A-B). All patients demonstrated lower titers against BA.2 and BA.2.75, whereas titers against BA.5 were further reduced. Most patients did not have detectable neutralization titers against the BQ.1.1, and XBB variants. There were 3 patients with detectable titers against BA.1, 2 of which also showed detectable titers against BQ.1.1 and XBB. These 3 patients were previously vaccinated and received AZD7442 around the time of anti-CD20 therapy initiation. One patient had prior SARS-CoV-2 infection, and 2 had higher titers before AZD7442. Titers against BQ.1.1 and XBB were lower than those against BA.1 in the 2 patients who had detectable titers against these variants. No statistical differences were observed in the postinjection titers based on the number of prior vaccinations.
Reduced efficacy of AZD7442 in neutralizing SARS-CoV-2 Omicron variants. (A-B) Neutralization titers against different SARS-CoV-2 variants of concern after administration of AZD7442 (N = 28). (C) Neutralization titers against WA1 and Delta increased substantially after AZD7442 administration but were less pronounced against Omicron variants. No statistically significant increase in neutralizing titers was observed for BA.1, BQ.1.1, or XBB. Number represents the median fold change after AZD7442. For all graphs, the horizontal dotted line represents the limit of detection. ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001 using Friedman tests and Dunn test or Wilcoxon test to correct for multiple comparisons, as appropriate. GMT, geometric mean antibody titer.
Reduced efficacy of AZD7442 in neutralizing SARS-CoV-2 Omicron variants. (A-B) Neutralization titers against different SARS-CoV-2 variants of concern after administration of AZD7442 (N = 28). (C) Neutralization titers against WA1 and Delta increased substantially after AZD7442 administration but were less pronounced against Omicron variants. No statistically significant increase in neutralizing titers was observed for BA.1, BQ.1.1, or XBB. Number represents the median fold change after AZD7442. For all graphs, the horizontal dotted line represents the limit of detection. ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001 using Friedman tests and Dunn test or Wilcoxon test to correct for multiple comparisons, as appropriate. GMT, geometric mean antibody titer.
Among patients with available pre- and post-AZD7442 samples, neutralization titers against WA1, Delta, BA.2, BA.5, and BA.2.75, increased significantly after AZD7442 administration (Figure 1C). However, the observed fold increase in titers against the Omicron variants was substantially lower than that against WA1 and Delta. Neutralization titers against BA.1, BQ.1.1, and XBB did not increase after AZD7442 (Figure 1C).
Immunoglobulin G–binding titers in AZD7442-containing plasma against the spike RBD of multiple SARS-CoV-2 variants were also measured (supplemental Figure 1). Binding titers against wild-type and earlier variants were high after AZD7442 but significantly reduced against Omicron variants. Importantly, neutralization titers correlated with binding titers (supplemental Figure 2).
In summary, the live virus neutralization activity of plasma from AZD7442-treated patients with NHL/CLL against currently circulating Omicron variants is minimal, highlighting a clinically important limitation of this agent. Peak neutralizing activity against WA1 in these patients who are immunocompromised after AZD7442 was similar to those observed in healthy individuals.6 However, the neutralizing activity of AZD7442 against contemporary variants was significantly reduced, providing a potential rationale on why patients with lymphomas did not experience a statistically significant decrease in SARS-CoV-2 infections after AZD7442, as reported recently.13 Although data correlating neutralizing titers with protection after AZD7442 administration are not available, neutralizing antibody levels have been shown to be a powerful correlate of protection from infection, with lower titers needed to protect against severe disease.14-16 The lack of detectable neutralization titers against BQ.1.1 and XBB is concerning, but neutralization activity was measurable against some Omicron variants, including BA.5, which is still in circulation. Thus, patients may still derive some, albeit low, benefit from AZD7442, particularly against severe infections. Booster vaccination using bivalent vaccines for patients who are immunocompromised and their close contacts may decrease the risk of severe disease because these vaccines elicit better neutralizing antibody responses against all Omicron variants.17 However, as the SARS-CoV-2 virus continues to evolve, novel prophylactic agents with broad neutralizing activity targeting conserved regions of the virus are urgently needed to improve protection for patients with impaired humoral responses because AZD7442 remains the only approved agent for this vulnerable population.
Acknowledgments: This work was partially supported by NCI grants U54CA260563 and P30CA138292 and Winship Cancer Institute institutional funds. A.C. is partially supported by the American Society of Hematology Research Training Award for Fellows. M.S.S. is partially supported by grants from the National Institutes of Health (P51 OD011132, HHSN272201400004C, and U19AI090023), the Emory Executive Vice President for Health Affairs Synergy Fund Award, the Pediatric Research Alliance Center for Childhood Infections and Vaccines and Children’s Healthcare of Atlanta, the Emory-UGA Center of Excellence for Influenza Research and Surveillance as well as, COVID-Catalyst-I3 funds from the Woodruff Health Sciences Center, and Emory School of Medicine and Woodruff Health Sciences Center 2020 COVID-19 CURE Award. M.V.D. is partially supported by grant R35CA197603 and the Leukemia & Lymphoma Society Specialized Center of Research Award.
Contribution: A.C., M.S.S., and R.A. contributed to conception and design; M.V.D., M.S.S., and R.A. provided financial support; A.C., J.L.K., M.S., P.B.A., A.M.K.L., C.B.O., M.C.C., and J.B.C. contributed to the provision of study materials or patients; A.C., S.L.L., L.L., V.M.O.-N., A.M.K.L., M.L.E., B.W., A.M., C.B.O., M.C.C., and M.S.S. collected and assembled the data; A.C., J.L.K., S.L.L., V.M.O.-N., M.V.D., M.S.S., J.B.C., and R.A. analyzed and interpreted the data; and all authors contributed to manuscript writing, gave the final approval, and are accountable for all aspects of the work.
Conflict-of-interest disclosure: J.L.K. has a consulting or advisory role in Janssen/Pharmacyclics, MorphoSys, TG Therapeutics, Gamida Cell and reports research funding from Celgene, Janssen/Pharmacyclics, Oncternal Therapeutics, Viracta Therapeutics, and Atara Biotherapeutics. V.M.O.-N. has a consulting or advisory role for ADC Therapeutics and reports uncompensated relationships with Roche/Genentech. P.B.A. has a consulting or advisory role in ADC Therapeutics, Epizyme, Kyowa Kirin, Daichii Sankyo, and Secura Bio. M.V.D. has a consulting or advisory role in Roche/Genentech, Amgen, Kite, a Gilead company, LAVA therapeutics, Janssen Oncology, and Celgene. M.S.S. reports a consulting or advisory role in Moderna Therapeutics and Ocugen and reports research funding from Moderna Therapeutics (Inst) and Ocugen (Inst). J.B.C. has a consulting or advisory role in AbbVie, Janssen, Loxo, Kite/Gilead, AstraZeneca, Aptitude Medical Systems, Adicet Bio, and Adaptive Biotechnologies and reports research funding from Bristol Myers Squibb (Inst), Janssen (Inst), Novartis (Inst), Takeda (Inst), AI Therapeutics (Inst), Genentech (Inst), ASH (Inst), Lymphoma Research Foundation (Inst), Loxo (Inst), BioInvent (Inst), and AstraZeneca (Inst). R.A. reports research funding from Merck. There are patents on PD-1-directed immunotherapy. R.A. is listed as a coinventor on an Emory University–held patent for SARS-CoV-2 serology assays. The remaining authors declare no competing financial interests.
Correspondence: Rafi Ahmed, Emory Vaccine Center, Department of Microbiology and Immunology, Emory University School of Medicine, 1510 Clifton Rd, Atlanta, GA 30329; e-mail: rahmed@emory.edu.
References
Author notes
Individual participant data, after deidentification, that underlie the results reported in this article and study protocol will be made available beginning 3 months and ending 5 years after the publication of the article to investigators whose proposed use of the data has been approved by an independent review committee (learned intermediary) identified for this purpose.
Data are available on request from the corresponding author, Rafi Ahmed (rahmed@emory.edu).
Data requestors will need to sign a data access agreement.
The full-text version of this article contains a data supplement.