Key Points
Darinaparsin demonstrated clinically meaningful efficacy (ORR: 19.3%) in patients with relapsed or refractory PTCL.
Darinaparsin was well tolerated, and safety was clinically acceptable and manageable.
Abstract
Darinaparsin is a novel organic arsenical compound of dimethylated arsenic conjugated to glutathione, with antitumor activity and a mechanism of action markedly different from other available agents. This phase 2, nonrandomized, single-arm, open-label study evaluated the efficacy and safety of intravenous darinaparsin (300 mg/m2 over 1 hour, once daily for 5 consecutive days, per 21-day cycle) and its pharmacokinetics at multiple doses in 65 Asian patients with relapsed or refractory peripheral T-cell lymphoma (PTCL). The primary end point was the overall response rate (ORR). The ORR based on central assessment was 19.3% (90% confidence interval, 11.2-29.9), which was significantly higher than the predefined threshold of 10% (P = .024). The ORR was 16.2% in patients with PTCL–not otherwise specified and 29.4% in patients with angioimmunoblastic T-cell lymphoma. Tumor size decreased in 62.3% of patients. Treatment-emergent adverse events (TEAEs) were observed in 98.5% of patients. Grade ≥3 TEAEs with an incidence rate of ≥5% included anemia (15.4%), thrombocytopenia (13.8%), neutropenia (12.3%), leukopenia (9.2%), lymphopenia (9.2%), and hypertension (6.2%). Darinaparsin is effective and well tolerated, with TEAEs that were clinically acceptable and manageable with symptomatic treatment and dose reductions. This trial was registered at www.clinicaltrials.gov as #NCT02653976.
Introduction
Peripheral T-cell lymphoma (PTCL) is an aggressive, heterogeneous, and relatively rare type of non-Hodgkin lymphoma (NHL); it is rapidly progressive and associated with poor outcomes, with 5-year overall survival (OS) rates of between 20% and 30%.1-4 In Asia, PTCL accounts for between 15% and 20% of all lymphomas,4 with extranodal natural killer (NK)/T-cell lymphoma (28.6%) being the most prevalent subtype, according to the International Cooperative Non-Hodgkin T-cell Lymphoma Prospective Registry.5 Other predominant subtypes in Asian populations include angioimmunoblastic T-cell lymphoma (AITL, 24.7%), PTCL–not otherwise specified (PTCL-NOS, 20.8%), anaplastic lymphoma kinase (ALK)-positive anaplastic large cell lymphoma (ALCL, 6.9%), and ALK-negative ALCL (6.2%),5 which are all categorized as nodal PTCLs.1
Current first-line treatment strategies for T-cell lymphomas like anthracycline-containing combination chemotherapy are considered to have insufficient efficacy.1,3 Furthermore, because of a lack of effective salvage therapy for relapsed or refractory disease, the prognosis remains very poor.1 A retrospective review of clinical outcomes in patients with relapsed or refractory PTCL found no difference between single-agent or multi-agent salvage regimens.6 Moreover, patients with relapsed or refractory PTCL receiving combination chemotherapy (who had previously received 1 systemic therapy) were more likely to experience grade 3 or 4 adverse events (AEs) than patients receiving single-agent chemotherapy as the first retreatment.7 Therefore, there is a growing need to develop a novel therapeutic agent with good safety and tolerability as a new therapeutic option for patients with relapsed or refractory PTCL.
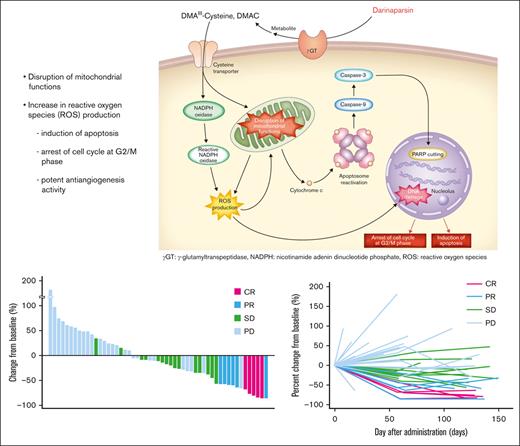
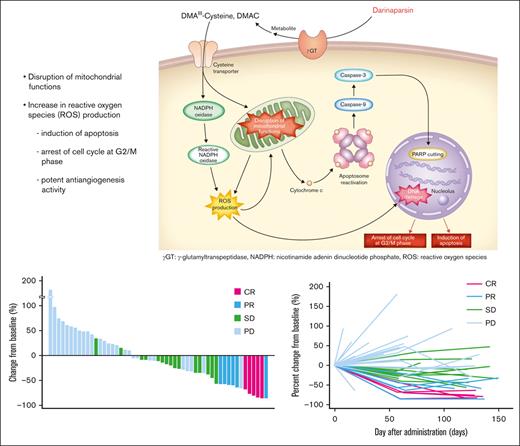
Darinaparsin is a novel organic arsenical compound consisting of dimethylated arsenic conjugated to glutathione that exhibits antitumor activity and a unique mechanism of action.8 It is processed at the tumor cell surface by g-glutamyltranspeptidase, leading to formation of dimethylarsinocysteine, which is imported via importing systems such as the cystine/cysteine transporter, which is highly expressed on tumor cell surface membranes. These mechanisms partially explain the selective toxicity of darinaparsin to cancer cells.9 Darinaparsin has also been shown to have intracellular effects on cell cycle arrest at the G2/M phase and apoptosis by disrupting mitochondrial functions in tumor cells by lowering membrane potential and increasing reactive oxygen species production.8,9
This study aimed to evaluate the efficacy and safety of darinaparsin monotherapy and its pharmacokinetics (PK) at multiple dosages, in patients with relapsed or refractory PTCL (www.clinicaltrials.gov as #NCT02653976).
Methods
Study design
This was a phase 2, multinational, nonrandomized, single-arm, open-label trial conducted in Asian countries (Japan, Korea, Taiwan, and Hong Kong) to evaluate the efficacy and safety of darinaparsin monotherapy in patients with relapsed or refractory PTCL. It was conducted at 25 investigational sites (13 in Japan, 6 in Korea, 5 in Taiwan, and 1 in Hong Kong). All patients signed and dated a written informed consent form at the first screening visit. This study was approved by an ethics committee, and adhered to the principles of the Helsinki Declaration for Good Clinical Practice and its subsequent amendments.
Patient inclusion and exclusion criteria
Only patients with nodal PTCLs were included, whereas those with extranodal PTCLs were excluded. This is because nodal PTCLs are a relatively homogeneous group of PTCLs, but little is known about extranodal PTCLs. Furthermore, the biological and clinical behaviors of nodal PTCLs differ significantly from extranodal types, which have a more cytotoxic T-cell phenotype.10,11
The study included all male and female patients aged ≥20 years with ethnic backgrounds from each participating country; a histopathologically confirmed diagnosis of PTCL-NOS, AITL, or ALCL (ALK positive/negative); a treatment history of ≥1 regimen of antitumor agents; and an enlarged lymph node or extranodal mass lesion measurable on computed tomography (CT).
PTCLs were histopathologically diagnosed by the central pathological review committee according to the 2008 World Health Organization classification. Patients were excluded if they had major organ dysfunction, baseline QT prolongation (QTcF ≥ 450 ms), or previous treatments with chemotherapy and radiation within 3 weeks before study enrolment, antibody therapy, autologous hematopoietic stem cell transplantation within 12 weeks before study enrolment, or allogeneic hematopoietic stem cell transplantation. Patients with concurrent, or a history of, central nervous system disease, cerebrovascular disorder, or psychiatric disorder were excluded.
Treatment
Patients were administered darinaparsin 300 mg/m2 intravenously over 1 hour once daily for 5 consecutive days (days 1-5) per 21-day cycle (3-week cycle), which consisted of 5-day therapy followed by a 16-day rest. This dose was selected based on the phase 1 studies conducted in Japan and Korea.12 Administration through the central vein was recommended. The treatment was given for 6 cycles, but it was continued beyond the treatment period upon the patient’s request and if the investigators judged that continuing treatment was possible and necessary.
Throughout the study period, drugs given for other medical reasons were continued, except for other anticancer chemotherapies or immunotherapies, continuous oral administration or injection of corticosteroids (prednisolone) of >10 mg per day, radiotherapy, hematopoietic stem cell transplants, gene therapy, or major surgery. Antiemetic, antimicrobial, antiviral, or antifungal drugs and gargles for opportunistic infections were also allowed.
Efficacy and safety assessment
The primary end point was the overall response rate (ORR) (complete response [CR] plus partial response [PR]) during 6 cycles of treatment, as assessed centrally by the Efficacy and Safety Review Committee. Based on CT and fluorodeoxyglucose-positron emission tomography (FDG-PET) findings, tumor response was assessed according to the Revised Response Criteria for Malignant Lymphoma developed in 2007.13 Before study initiation, the FDG-PET/CT imaging devices that would be used throughout the study were specified. An individual patient had a battery of imaging examinations using the same device. For the specified FDG-PET/CT imaging devices, phantom tests were conducted in compliance with the Quantitative Imaging Biomarkers Alliance profile at all investigational sites for standardization among FDG-PET/CT scanners in advance.14 The tumor response by local assessment at each investigational site over the treatment period was determined as the second evaluation result. The disease control rate (DCR), defined as the proportion of patients who achieved CR, PR, or stable disease (SD), was also evaluated both centrally and locally. Other secondary efficacy end points included progression-free survival (PFS), time to response (TTR), duration of response (DOR) based on local assessment, and OS up to 2 years from the day darinaparsin was first administered.
Safety assessments included physical examinations, vital signs, electrocardiograms (ECGs), laboratory parameters, and treatment-emergent AE (TEAE) monitoring. TEAE severity was evaluated according to the National Cancer Institute–Common Terminology Criteria for Adverse Events (NCI-CTCAE), version 4.0.
PK assessments
Blood samples were collected for PK analysis at 0, 1, 2, 4, 6, and 8 hours on day 1; 0 hours on days 2, 3, and 4; 0, 1, 2, 4, 6, and 8 hours on day 5; and 0 hours on days 6, 8, and 15 of cycle 1. Urine samples were collected for PK analysis between 0 and 4 hour and between 4 and 24 hours on days 1 and 5 of cycle 1. The arsenic concentrations in plasma and urine were measured as surrogate markers for darinaparsin using the inductively coupled plasma mass spectrometry (ICP/MS) method. Liquid chromatography inductively coupled plasma mass spectrometry (LC-ICP/MS) and liquid chromatography tandem mass spectrometry (LC-MS/MS) were used to detect and identify darinaparsin and its metabolites in plasma at 1, 2, and 4 hours and in urine between 0 and 4 hours and between 4 and 24 hours from the start of infusion on day 5.
Statistical analysis
With an expected ORR of 25%, 55 evaluable patients would have 90% statistical power at a 1-sided alpha error of 0.05 to demonstrate a threshold ORR of 10%. The planned sample size was 65 patients, taking into account exclusions based on central pathological diagnosis. The efficacy analysis set consisted of enrolled patients who met the eligibility criteria and had at least 1 tumor response assessment after darinaparsin administration. Time-to-event end points were calculated using the Kaplan-Meier method. The safety analysis set consisted of patients who received ≥1 doses of darinaparsin. The incidence of TEAEs was summarized by system organ class, preferred term, and severity according to the Medical Dictionary for Regulatory Activities (MedDRA), version 23.0. Psychiatric disorders, neurological disorders, abnormal liver function, and abnormal ECGs were identified as AEs of special interest. The PK analysis set consisted of all patients who received ≥1 doses of darinaparsin and had ≥1 data points of plasma or urine drug concentration.
Results
Patients
In total, 65 patients (37, 19, 8, and 1 patient from Japan, Korea, Taiwan, and Hong Kong, respectively) were treated with darinaparsin. The demographic and baseline characteristics of these patients are shown in Table 1. PTCL-NOS (66.2%, n = 43) was the most prevalent PTCL subtype, followed by AITL (26.2%, n = 17) and ALCL-ALK-negative (4.6%, n = 3), with no ALCL-ALK-positive subtype in the study patient population. According to the Ann Arbor staging classification, 46.2% (n = 30) of patients were in stage III and 43.1% (n = 28) were in stage 4. The median number of prior therapy regimens was 2.0 (range: 1-11), with no evidence of response to the most recent therapy in 16 patients.
Of the 65 patients, 58.5% (n = 38) received ≥3 cycles, 27.7% (n = 18) received ≥6 cycles of treatment with darinaparsin, and 13.8% (n = 9) continued treatment after cycle 6. The median number of cycles was 3.0 (range, 1-39), and the mean ± standard deviation was 5.0 ± 7.4.
Efficacy outcomes
The efficacy analysis set consisted of 57 patients. Moreover, 8 of the 65 patients treated with darinaparsin were excluded from the efficacy analysis set, with 2 patients having a central pathological diagnosis other than PTCL, 5 not having any postbaseline tumor response assessment, and 1 patient not meeting the inclusion criteria. The ORR based on central assessment was 19.3% (90% confidence interval [CI], 11.2-29.9; n = 11), which was significantly higher than the predefined ORR threshold of 10% (P = .024, binomial test). Furthermore, 5 of the 11 responders achieved a CR (8.8%), and 6 had a PR (10.5%). SD was observed in 26.3% of patients (n = 15), and the DCR was 45.6% (90% CI, 34.3-57.3; n = 26) (Table 2).
In 37 patients with PTCL-NOS, the ORR was 16.2% (90% CI, 7.3-29.5; n = 6), whereas it was 29.4% (90% CI, 12.4-52.2; n = 5) in 17 patients with AITL. There was no specific difference in patients’ baseline characteristics that may have accounted for this encouraging tumor response in patients with AITL. The response rate was 18.9% (90% CI, 9.2-32.6; n = 7) in 37 patients who responded to the most recent therapy (CR or PR) and 25.0% (90% CI, 9.0-48.4; n = 4) in the 16 patients who did not (SD or progressive disease). There was no clear correlation between number of previous therapies and tumor response.
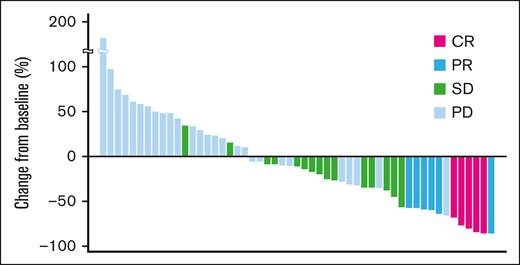
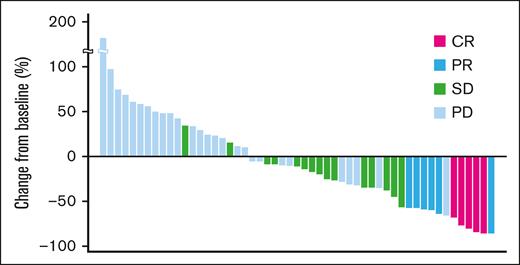
A waterfall plot showing the maximum target lesion shrinkage is shown in Figure 1. Furthermore, 62.3% (33/53) of patients had reduced tumor size.
Maximum change from baseline tumor size (waterfall plot). A waterfall plot showing the maximum target lesion shrinkage demonstrated that the tumor size decreased in 62.3% (33/53) of patients.
Maximum change from baseline tumor size (waterfall plot). A waterfall plot showing the maximum target lesion shrinkage demonstrated that the tumor size decreased in 62.3% (33/53) of patients.
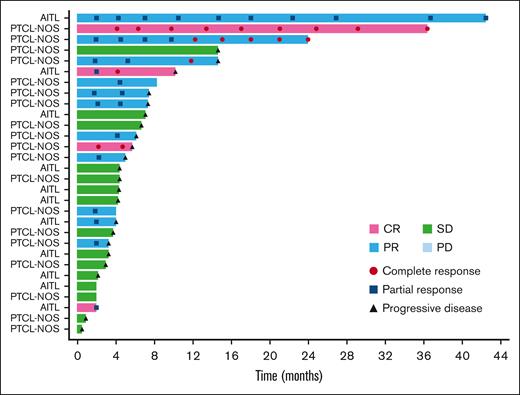
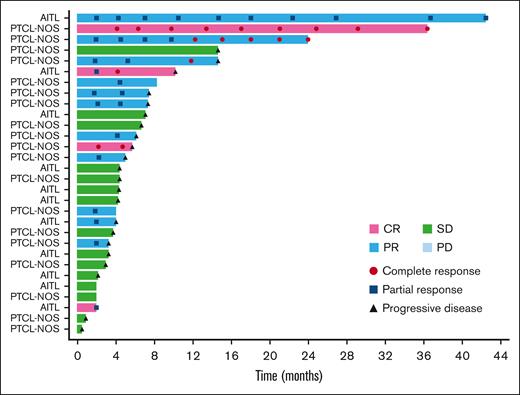
The ORR based on the local assessment was 26.3% (90% CI, 17.0-37.6; n = 15), and 4 of the 15 responders achieved CR (7.0%), whereas 11 had PR (19.3%). SD was observed in 28.1% (n = 16) of patients, and the DCR was 54.4% (90% CI, 42.7-65.7; n = 31). Based on local assessment, the median TTR was 2.0 months (90% CI, 1.9-2.2); 12 of the 15 responders showed response by cycle 3. The median DOR was 5.2 months (90% CI, 2.7-12.6). Overall, 13.8% (n = 9) of patients continued the treatment after cycle 6, with 4 patients opting to continue treatment for >10 cycles. The patient who had the longest treatment course received 39 cycles over the course of 41 months with a continuing response. A swimmer plot of long-term response is shown in Figure 2.
Long-term response (swimmer plot). The swimmer plot demonstrates the long-term response after darinaparsin administration.
Long-term response (swimmer plot). The swimmer plot demonstrates the long-term response after darinaparsin administration.
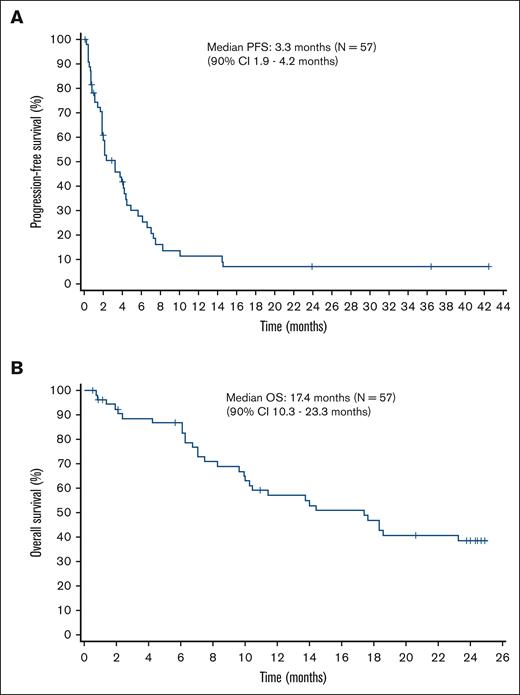
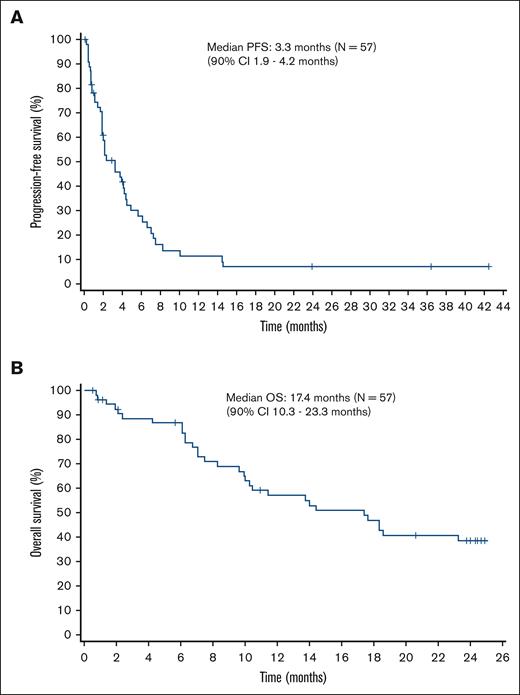
After a median follow-up of 14.1 months, the median PFS based on local assessment was 3.3 months (90% CI, 1.9-4.2). In patients with PTCL-NOS, the median PFS was 3.3 months (90% CI, 1.9-5.7), whereas it was 4.0 months (90% CI, 1.9-4.2) in patients with AITL. The median OS was 17.4 months (90% CI, 10.3-23.3). In patients with PTCL-NOS, the median OS was 18.3 months (90% CI lower limit, 9.6), whereas it was 13.7 months (90% CI, 7.1-17.6) in patients with AITL. The Kaplan-Meier curves of PFS and OS are shown in Figure 3A and Figure 3B, respectively.
Kaplan-Meier curves of PFS and OS. (A) The median PFS is 3.3 months (90% CI, 1.9-4.2). (B) The median OS is 17.4 months (90% CI, 10.3-23.3).
Kaplan-Meier curves of PFS and OS. (A) The median PFS is 3.3 months (90% CI, 1.9-4.2). (B) The median OS is 17.4 months (90% CI, 10.3-23.3).
Safety outcomes
The safety analysis set consisted of 65 patients who had received ≥1 doses of darinaparsin. TEAEs were observed in 98.5% (n = 64) of the patients. Drug-related TEAEs, which are defined as TEAEs with at least a reasonable causal relationship to darinaparsin, were reported in 69.2% (n = 45) of patients.
The TEAEs with an incidence rate of ≥5% are shown in Table 3.
The grade ≥3 hematological TEAEs included anemia (15.4%, n = 10), thrombocytopenia (13.8%, n = 9), neutropenia (12.3%, n = 8), leukopenia (9.2%, n = 6), and lymphopenia (9.2%, n = 6). The sole nonhematological TEAE of grade ≥3 with an incidence rate of ≥5% was hypertension (6.2%, n = 4).
The AEs of special interest were psychiatric and neurological disorders, abnormal hepatic function, and QT prolongation. The incidence rate of psychiatric and neurological disorders was 21.5% (n = 14) and 40.0% (n = 26), respectively. Psychiatric disorders with an incidence ≥5% included delirium (12.3%, n = 8), and insomnia (6.2%, n = 4). Neurological disorders with an incidence ≥5% included dizziness (7.7%, n = 5), headache (7.7%, n = 5), peripheral sensory neuropathy (7.7%, n = 5), and dysgeusia (6.2%, n = 4). Abnormal liver function with an incidence ≥5% included increased aspartate aminotransferase (18.5%, n = 12) and increased alanine aminotransferase (15.4%, n = 10). The incidence rate of QT prolongation was 3.2% (n = 2).
The drug-related TEAEs that led to study discontinuation included peripheral neuropathy (grade 2, n = 1), cerebral infarction (grade 2, n = 1), leukopenia (grade 3, n = 1), vertigo (grade 3, n = 1), rash (grade 3, n = 1), pyrexia (grade 3, n = 1), and myocarditis (grade 1, n = 1).
The incidence rate of serious AEs (SAEs) was 46.2% (n = 30), whereas that of drug-related SAEs was 10.8% (n = 7). The drug-related SAEs were pyrexia (grade 1, n = 1; grade 3, n = 1), hypothermia (grade 5, n = 1), pneumonia (grade 3, n = 1), septic shock (grade 3, n = 1), sinusitis (grade 3, n = 1), dehydration (grade 3, n = 1), confusion (grade 3, n = 1), vertigo (grade 3, n = 1), fatigue (grade 3, n = 1), herpes zoster (grade 2, n = 1), Enterobacter infection (grade 2, n = 1), cerebral infarction (grade 2, n = 1), and abdominal pain (grade 2, n = 1). Fatal SAEs were reported in 9.2% (n = 6) of patients; they were owing to disease progression (n = 2), hemophagocytic lymphohistiocytosis (n = 1), tumor lysis syndrome (n = 1), hypothermia (n = 1), and multiple organ dysfunction syndrome (n = 1). However, the sole incident linked to the investigational drug was hypothermia. Hypothermia was also reported in a patient with PTCL-NOS diagnosed with sepsis on day 5 in cycle 1. The blood culture from the implantable subcutaneous infusion port showed Candida albicans. Fifteen days after starting darinaparsin, the patient developed bradycardia followed by cardiac arrest. Cardiopulmonary resuscitation was not initiated because the patient had signed a do-not-resuscitate form.
PK
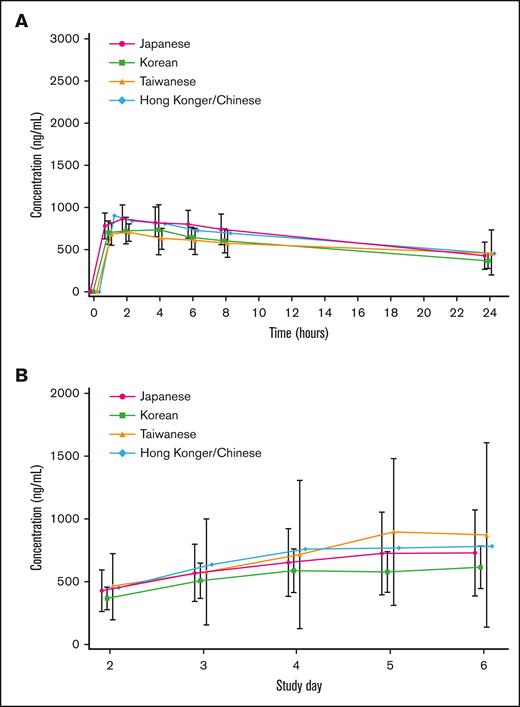
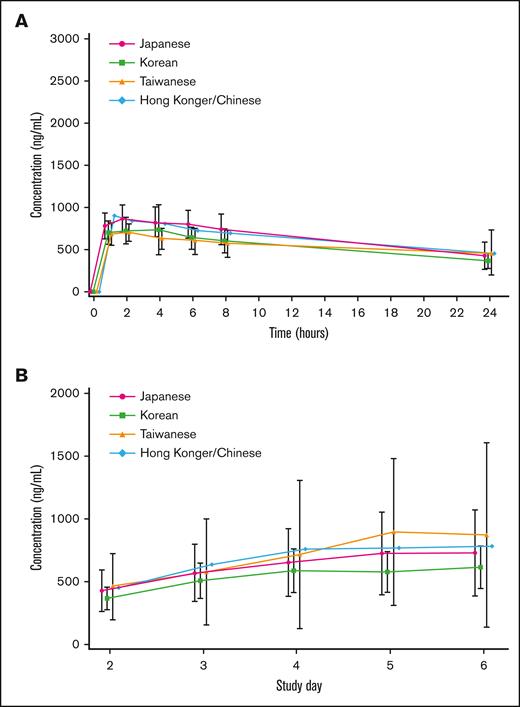
PK was analyzed in 44 patients (18 Japanese, 17 Koreans, 8 Taiwanese, and 1 Hong Konger/Chinese). On days 1 and 5, plasma arsenic concentrations in many patients reached their maximum, 1 to 2 hours after the start of the infusion, and then decreased per the first-order kinetics. The trough concentration after repeated administration increased gradually with the number of doses, and reached a steady state from days 4 to 5. The mean plasma arsenic concentration-time profiles on day 1 by ethnic background are summarized in Figure 4A. The mean plasma trough arsenic concentration-time profiles on days 2 to 6 by ethnic background are shown in Figure 4B. The systemic exposure to darinaparsin was noted to be similar across all ethnic backgrounds.
Mean plasma arsenic and mean plasma trough arsenic concentration-time profiles. (A) Mean plasma arsenic concentration-time profiles on day 1 by ethnic background. Bars represent means ± standard deviation. The systemic exposure of darinaparsin is similar for all ethnic backgrounds. (B) Mean plasma trough arsenic concentration-time profiles on days 2 to 6 by ethnic background. Bars represent means ± standard deviation. The systemic exposure of darinaparsin is similar for all ethnic backgrounds.
Mean plasma arsenic and mean plasma trough arsenic concentration-time profiles. (A) Mean plasma arsenic concentration-time profiles on day 1 by ethnic background. Bars represent means ± standard deviation. The systemic exposure of darinaparsin is similar for all ethnic backgrounds. (B) Mean plasma trough arsenic concentration-time profiles on days 2 to 6 by ethnic background. Bars represent means ± standard deviation. The systemic exposure of darinaparsin is similar for all ethnic backgrounds.
Overlaps of error bars of Cmax and AUC0₋24 in a high number of patients suggested that there was no clear difference in efficacy by exposure to darinaparsin and there was no clear relationship between adverse drug reactions and darinaparsin exposure. Slight accumulation after 5 consecutive days of administration was suggested by slightly higher Cmax and AUC0–24 on day 5 than on day 1. However, on day 15 (240 hours after the final dose), plasma arsenic concentrations had dropped below the lower limit of quantification (50.0 ng/mL) in ∼80% of patients, which only indicates that the accumulation had no effect on the following cycle. Arsenic in darinaparsin was excreted into urine between 0 and 24 hours in 40.75% and 67.73% of patients on days 1 and 5, respectively, suggesting that urine is the primary excretory medium.
Dimethylarsinic acid (DMAv) was the main metabolite in plasma, accounting for ∼90% of the total peak area regardless of blood collection time. Because unchanged darinaparsin was not detectable in plasma, it appears to be metabolized rapidly after administration, and all of the arsenic contained in darinaparsin was present as metabolites in plasma. In addition, unchanged darinaparsin could not be detected in urine, indicating that the arsenic excreted into urine was derived from metabolites.
Discussion
In Western countries, the effects of darinaparsin in patients with relapsed or refractory PTCL have already been suggested in phase 2 studies in patients with relapsed or refractory Hodgkin lymphoma and NHL.15 This current phase 2 study was conducted in Japan, Korea, Taiwan, and Hong Kong to assess the efficacy, safety, and PK of darinaparsin monotherapy in patients with relapsed or refractory PTCL. This is the first phase 2 trial to assess the effects of darinaparsin specifically in Asian patients with PTCL.
In the present study, the ORR based on central assessment was 19.3%. In a phase 2 study conducted in the United States, the ORR of 7 patients with relapsed or refractory PTCL who received intravenous darinaparsin 300 mg/m2 per day for 5 consecutive days every 4 weeks was 28.6%.15 Similarly, in this study, although the ORR was 16.2% in patients with PTCL-NOS, an encouraging tumor response of 29.4% was demonstrated in those with AITL.
Although several new medications have been approved in recent years for the treatment of PTCL, no prolongation of survival has been observed after treatment with these drugs. Therefore, there remains an unmet medical need to develop new PTCL therapeutic options.
Although the ORR in this study was moderate, the DCR based on central assessment was 45.6%, and approximately half of the patients achieved tumor shrinkage, implying that darinaparsin slowed or stabilized disease progression in approximately half of the patients. The median PFS and OS times were 3.3 and 17.4 months, respectively. The median PFS times in patients with relapsed or refractory PTCL treated with pralatrexate,16 romidepsin,17 and belinostat18 were 3.5, 4.0, and 1.6 months, respectively, whereas OS times for patients treated with pralatrexate16 and belinostat18 were 14.5 and 7.9 months, respectively. The PFS and OS of darinaparsin were found to be comparable to the existing single-agent chemotherapy approved by the United States Food and Drug Administration. In this study, the median number of previous therapy regimens was 2.0 (range, 1-11), and an ORR of 25.0% was achieved in patients who did not respond to the most recent previous therapy (SD or progressive disease), that is, refractory cases.
The relatively higher incidence rate of PTCL in the Asian region than in Western countries was 1 reason why this study was performed only in Asian regions. In this study, there was no particular difference in the safety profiles of darinaparsin from that reported in prior clinical trials conducted in Western countries and there was no evidence of any new Asian-specific toxicity.
The incidence of grade ≥3 TEAEs ranged from 1.5% to 15.4%, and darinaparsin toxicity could be managed with dose modifications as well as symptomatic treatment, as appropriate. Four patients had darinaparsin treatment for >10 cycles, with the patient who had the longest treatment course receiving 39 cycles over ∼41 months. Based on the incidence of TEAEs in these patients, no change in the safety profile was observed with long-term darinaparsin treatment, implying that it can be administered for a longer duration; however, the sample size was small. The good safety profile suggests that darinaparsin has the potential to be used in combination with other medications in the future.
Because vascular pain at the injection site was noted in 2 of the 4 patients who received transiently infused darinaparsin via a peripheral line during this study, administration through the central vein was maintained. Psychiatric and neurological disorders were characteristic dose-limiting toxicities reported at the higher dose levels in patients with solid tumors in a phase 1 study in the United States.19 In this study, the incidence rate of grade 3 drug-related TEAEs in the system organ class of psychiatric or neurological disorders was 7.7% (5 patients) including delirium (3.1%), confusion (1.5%), disorientation (1.5%), hallucinations (1.5%), peripheral neuropathy (1.5%), and cognitive disorder (1.5%). The median and mean ages of these 5 patients were 76.0 and 71.6 years, respectively, exceeding those of the general population. Except for one incidence of delirium that occurred shortly before death from another TEAE (hypothermia), all psychiatric or neurological events were resolved by skipping or reducing the darinaparsin dosage.
QT prolongation has frequently been reported after treatment with arsenic trioxide, which is an inorganic arsenic compound.20 However, abnormalities in ECG measurements were not frequently reported in this phase 2 study, which is consistent with the findings of clinical pharmacological ECG evaluation in 2 phase 1 trials in Japanese and Korean patients with PTCL.12
Darinaparsin has a novel mechanism that is distinct from other available agents. Its import route including the cystine/cysteine transporter, which is highly expressed on tumor cell surface membranes,8,9 is thought to promote both the antitumor efficacy and a good safety profile, as shown in this study.
Darinaparsin’s primary target of action is the mitochondria, and it is likely to induce apoptosis and inhibit tumor cell growth via mitochondrial dysfunction and intracellular reactive oxygen species generation. In vivo studies in darinaparsin-treated severe combined immunodeficient mice showed a significant inhibition of tumor growth, followed by improved survival. It has been reported to decrease inhibitory Src homology 2-containing protein tyrosine phosphatase (SHP1), resulting in phosphorylation of extracellular signal-regulated kinase and its downstream substrate. Hence, its activity is primarily MAPK dependent.21 Because it does not target any specific antigen or molecule, the effect of this drug is not limited to any specific tissue.
PTCL is an extremely heterogeneous disease with diverse pathological conditions and patient backgrounds, and there is a high medical need for expanded treatment options. Under the recent circumstances in which several drugs have become available for the treatment of relapsed/refractory PTCL, it is important to select the most appropriate treatment based on the characteristics of each drug, in accordance with the condition of each individual patient. Because of its new mechanism of action and characteristic efficacy and safety profile, darinaparsin can contribute as a new treatment option for patients with relapsed/refractory PTCL, which currently has no established standard therapy.
The limitations of this study stem from its single-arm design with no control group and its open-label design, which makes comparing the results of this study to those of other studies impossible.
In conclusion, darinaparsin showed antitumor activity for patients with relapsed or refractory PTCL and is well tolerated, with a safety profile that was clinically acceptable and manageable with symptomatic treatment and dose reductions.
Acknowledgments
The authors thank all the patients who participated in this study and their families, as well as all of the investigators, physicians, nurses, and clinical research coordinators who helped with this study. The authors thank Kenichi Ishizawa (Yamagata University, Japan), Noriko Usui (The Jikei University, Japan), and Kunihiro Tsukasaki (Saitama Medical University International Medical Center, Japan) for their contributions as members of the Efficacy and Safety Evaluation Committee; Shigeo Nakamura (Nagoya University, Japan), Yoshihiro Matsuno (Hokkaido University, Japan), and Koichi Ohshima (Kurume University, Japan) for their contributions to central pathology review; and Ukihide Tateishi (Tokyo Medical and Dental University, Japan) for his contributions to the independent radiological review. The authors express their gratitude to Yuji Kumagai (Kitasato University, Japan) and Masataka Katashima for their contributions as clinical pharmacology advisers, as well as Yukikazu Hayashi (A2 Healthcare Corporation, Japan) as the sponsor’s biostatistician.
This study was funded and supported by Solasia Pharma (Tokyo, Japan).
Authorship
Contribution: All authors performed and/or designed the clinical study, analyzed the data, contributed equally to the development and revision of the manuscript, and approved the final version for submission.
Conflict-of-interest disclosure: W.S.K. has received research funding from Celltrion, Dong-A, Eisai, Johnson & Johnson, Kyowa Kirin, Roche, Sanofi, and IGM Biosciences. N.F. has received honoraria and research funding from Chugai Pharmaceutical; honoraria from HUYA Bioscience International and SymBio Pharmaceuticals; and research funding from Bayer, Celgene, Genmab, and Incyte. K.Y. has acted as a consultant for, and received honoraria from, Meiji Seika Pharma; received honoraria from AbbVie, Chugai Pharmaceutical, Eisai, Micron, and Takeda Pharmaceutical; and received research funding from SymBio Pharmaceuticals and Yakult Honsha. K.I. has received honoraria and research funding from Ono Pharmaceutical and Janssen Pharmaceutical, and research funding from AbbVie, AstraZeneca, Bayer, Bristol Myers Squibb, BeiGene, Celgene, Chugai Pharmaceutical, Daiichi Sankyo, Genmab, Incyte, Kyowa Kirin, LOXO Oncology, Novartis, Pfizer, and Yakult Honsha. Y.T. has served on the speakers bureau at AbbVie, Celgene, Chugai Pharmaceutical, Esai, Janssen Pharmaceutical, MSD, Ono Pharmaceutical, and Takeda Pharmaceutical. H. Nanajima has received honoraria and research funding from Chugai Pharmaceutical and Daiichi Sankyo; received honoraria from Novartis; and received research funding from Asahi Kasei Pharma, Astellas Pharma, Eisai, Pfizer, and Takeda Pharmaceutical. K.A. received research funding from Astellas Pharma, Celgene, Chugai Pharmaceutical, Kyowa Kirin, Novartis, Solasia Pharma, and Takeda Pharmaceutical. Y. Suehiro received research funding from Amgen, Chugai Pharmaceutical, Genmab, Incyte, Kyowa Kirin, Ono Pharmaceutical, and Otsuka Pharmaceutical. H. Nagai has received honoraria and research funding from Celgene and Chugai Pharmaceutical; honoraria from Ono Pharmaceutical; and research funding from AbbVie, Daiichi Sankyo, Janssen Pharmaceutical, Kyowa Kirin, Mundipharma, Nippon Shinyaku, SymBio Pharmaceuticals, and Takeda Pharmaceutical. H.F.T. has received honoraria and research funding from Celgene, and honoraria from AbbVie and Novartis. F.N. and Y. Sonehara are employees of Solasia Pharma. K.T. received honoraria from Daiichi Sankyo, HUYA Bioscience, SymBio Pharmaceuticals, Yakult Honsha, and Zenyaku Kogyo. The remaining authors declare no competing financial interests.
Correspondence: Won-Seog Kim, Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University, 81 Irwon-ro, Gangnam-gu, Seoul, 06351, Korea; e-mail: wskimsmc@skku.edu.
References
Author notes
Data are available on request from the corresponding author, Won-Seog Kim (wskimsmc@skku.edu).
Individual participant data will not be shared.