Key Points
KPd was found to be safe and effective using carfilzomib 27 mg/m2 twice weekly and pomalidomide 4 mg days 1 to 21 of a 28-day cycle.
Dara-KPd induced deeper and more durable responses than KPd in a predominantly high-risk, lenalidomide-refractory population.
Abstract
We conducted a phase 1/2 study of carfilzomib, pomalidomide, and dexamethasone (KPd) and KPd with daratumumab (Dara-KPd) in relapsed/refractory multiple myeloma. The primary end points were identification of a maximum tolerated dose (MTD) of KPd for phase 1, and rates of overall response (ORR) and near complete response (nCR) after 4 cycles of KPd and Dara-KPd, respectively, for phase 2. The MTD for KPd was carfilzomib 20/27 mg/m2 on days 1, 2, 8, 9, 15, and 16 (cycles 1-8) and days 1, 2, 15, and 16 for cycles 9 and beyond; oral pomalidomide 4 mg on days 1 to 21; and oral dexamethasone 40 mg weekly in 28-day cycles. Sixty-six patients received KPd, including 34 at the MTD. The ORR after 4 cycles of KPd at the MTD was 27/34 (79%; 95% confidence interval [CI], 62%-91%), meeting the statistical threshold for efficacy. At a median follow-up of 44 months, the median progression-free survival (PFS) was 13 months and overall survival (OS) 44 months. Twenty-eight patients received Dara-KPd. The rate of nCR or better after 4 cycles was 11/28 (39%; 95% CI, 22%-59%), meeting the statistical threshold for efficacy. As the best response to Dara-KPd, the ORR was 25/28 (89%) and the rate of measurable residual disease negativity by flow cytometry (10−5) was 17/26 (65%). At a median follow-up of 26 months, the median PFS and OS for Dara-KPd were not reached. Dara-KPd induced deeper and more durable responses than KPd without compromising safety in a predominantly high-risk, lenalidomide-refractory population, warranting further evaluation of this quadruplet. This trial is registered at www.clinicaltrials.gov as #NCT01665794.
Introduction
Survival in patients with multiple myeloma (MM) has increased significantly since the introduction of proteasome inhibitors (PIs) and immunomodulatory imides (IMiDs), along with improvements in supportive care for autologous stem cell transplantation (ASCT).1,2 The incorporation of all 3 of these therapies in the frontline setting has led to substantially increased durations of response, with as many as 35% of patients still alive and without progression 8 years after diagnosis.3 Although the first line of therapy in MM typically leads to a patient’s longest duration of response, most patients will eventually experience disease progression, and there are significant attrition rates with each progression.4 For these reasons, there is a need to optimize earlier lines of therapy, including options in early relapse in MM.
Given that most patients in the United States have been treated upfront with a PI, IMiD, and dexamethasone, potentially followed by ASCT with high-dose melphalan and then lenalidomide maintenance, the great majority of patients who experience disease progression will be lenalidomide-refractory and bortezomib-exposed. Carfilzomib,5 a second-generation PI, and pomalidomide,6 a third-generation IMiD, have both been shown to be effective in lenalidomide-resistant disease. Daratumumab (Dara), a CD38-targeting humanized monoclonal antibody, was also shown to have single-agent activity in MM, even in heavily pretreated patients.7 Dara was subsequently studied in earlier lines of therapy as separate combinations with bortezomib,8 carfilzomib,9,10 and pomalidomide,11,12 each of which showed improvements in progression-free survival (PFS) compared with in that without dara. Several other monoclonal antibody–containing quadruplet combinations have shown promising efficacy and potential superiority over their triplet counterparts.13-15
Considering the efficacy of carfilzomib and pomalidomide in patients with lenalidomide-refractory disease, we initiated a nonrandomized phase 1/2 study of carfilzomib, pomalidomide, and dexamethasone (KPd) in relapsed/refractory MM with prior lenalidomide. With the emergence of the role of quadruplets in the treatment of MM,13-15 we subsequently opened a second cohort combining KPd with Dara (Dara-KPd) to evaluate a novel quadruplet in relapsed/refractory MM. We present the findings of both cohorts here.
Methods
Study design and participants
This was a multicenter, open-label, phase 1b/2 study in patients with relapsed/refractory MM, conducted within the Multiple Myeloma Research Consortium. Enrollment criteria were the same for all cohorts. Patients aged 18 years or older who had received one or more prior lines of therapy were eligible. Patients with only 1 prior line of therapy were required to have lenalidomide-refractory disease, defined as progression within 60 days of the completion of at least 2 cycles of lenalidomide. Patients with ≥2 prior lines of therapy were required to be lenalidomide-exposed regardless of refractoriness. Receipt of prior carfilzomib was permitted, but prior daratumumab was not. Other key eligibility criteria included an absolute neutrophil count ≥1 × 109/L, hemoglobin ≥8 g/dL, platelet count ≥75 × 109/L, and a creatinine clearance >30 mL/min. Complete eligibility criteria can be found in the supplement. The University of Chicago institutional review board approval was obtained. All patients provided written informed consent. This trial was registered at www.clinicaltrials.gov as #NCT01665794.
Treatment
The study included 2 sequential cohorts: the first cohort received KPd, and the second received Dara-KPd. During the phase 1b portion (KPd cohort only), KPd was administered at 3 dose levels in 28-day cycles using the time-to-event continual reassessment method (CRM).16 Dose levels and dose-limiting toxicities (DLTs) are defined in Table 1. Carfilzomib was administered IV on days 1, 2, 8, 9, 15, and 16 for the first 8 cycles, then on days 1, 2, 15, and 16 for subsequent cycles. Oral pomalidomide was taken daily on days 1 to 21. For cycles 1 to 4, oral dexamethasone 40 mg was administered on days 1, 8, 15, and 22, and 20 mg for cycles 5 and thereafter. During the phase 2 portion, the KPd cohort was treated at the maximum tolerated dose (MTD; dose level 3). Patients in the Dara-KPd cohort were treated at the MTD (dose level 3) of the KPd cohort and received split-dose dexamethasone (20 mg on days 1, 2, 8, 9, 15, 16, 22, and 23) along with daratumumab intravenously on days 1, 8, 15, and 22 for cycles 1 to 2, on days 1 and 15 for cycles 3 to 6, and then on day 1 for each cycle thereafter. Treatment was administered until disease progression or unacceptable toxicity. Thromboprophylaxis was mandatory and included full-dose aspirin, prophylactic dose low-molecular weight heparin, or prophylactic dose direct oral anticoagulant as indicated.
Statistical analysis
The initial KPd cohort followed a phase 1/2 model. The primary objective for the phase 1 portion was the determination of the MTD using a single-parameter logistic model using the CRM (time-to-event CRM).17 The MTD was defined as the dose at which the probability estimate of a DLT was closest to but not exceeding 25%. The primary end point for the KPd phase 2 cohort was the rate of partial response (PR) or better (also known as the overall response rate [ORR]) after 4 cycles, with 50% to be considered promising and 25% unworthy of further study. In a minimax 2-stage design, 15 patients were to be accrued to the first stage, with at least 5 patients reaching more than PR necessary to accrue to the second stage. The second stage would enroll an additional 15 patients. At the conclusion, if at least 13 of the 30 patients enrolled reached more than PR, the null hypothesis was to be rejected in favor of the alternative, that the KPd regimen has a response rate of at least 50% in this patient population with 80% power and 5% type I error.
The Dara-KPd cohort was added as an amendment and opened after the completion of enrolment into the phase 2 KPd cohort. Patients were treated as part of the phase 2 portion of the study and followed a minimax 2-stage design. The primary end point was the rate of near complete response (nCR) or better after 4 cycles (CR independent of paraprotein on immunofixation),18 with a rate of at least 35% to be considered promising and 15% unworthy of further study. These rates were chosen based upon the rates of response in the previously accrued KPd cohort. Enrollment of 21 patients was required in the first stage, with at least 4 needing to reach more than or equal to nCR after 4 cycles to allow expansion into the second phase for a total target enrolment of 34 patients. If at least 10 of 34 patients enrolled reached more than nCR, the null hypothesis would be rejected in favor of the alternative, that the Dara-KPd regimen has a more than nCR after 4 cycles of at least 35% in this patient population with 80% power and 5% type I error. Twenty-eight patients were enrolled to this cohort; at that point, study enrolment was closed given that the primary end point had been met.
Secondary end points for both cohorts included the best ORR, depth of response, PFS, and overall survival (OS). As an exploratory end point for the Dara-KPd cohort, minimal residual disease (MRD) negativity as the best response and sustained MRD negativity (2 consecutive MRD-negative assessments at least 1 year apart) were assessed by 10-color flow cytometry, with a limit of detection <10−5.
Results
Patient characteristics
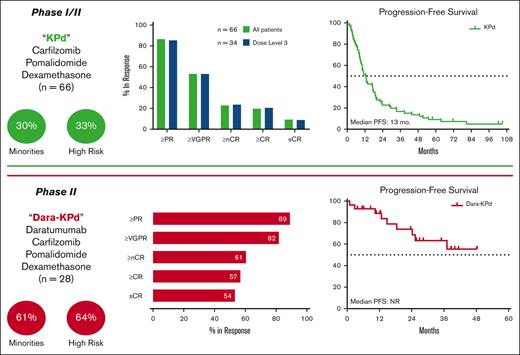
A total of 66 patients enrolled in the KPd cohort from November 2012 to April 2017, and 28 patients enrolled in the Dara-KPd cohort from August 2017 to October 2021 (Figure 1). The data cutoff was 1 March 2022. Baseline characteristics can be found in Table 2. The median age at the start of therapy was 63 years (range, 44-79; 44% >65 years old) for KPd and 62 years (range, 37-74; 39% >65 years old) for Dara-KPd. A total of 28 of 94 patients (30%) identified themselves as Black, including 17 of 28 (61%) within the Dara-KPd cohort.
The median time from diagnosis was 5 and 6 years for the KPd and Dara-KPd cohorts, respectively. The median prior lines of therapy was 2 for KPd (range, 1-7; 24% with 1 prior line, 38% with 2 prior lines, and 38% with ≥3 prior lines of therapy) and 1 for Dara-KPd (range, 1-3; 75% with 1 prior line and 25% with ≥2 prior lines). High-risk cytogeneticsm as defined by the International Myeloma Working Group,19 were present in 22 (33%) of the KPd and 18 (64%) of the Dara-KPd cohorts. The majority of patients (85% KPd and 86% Dara-KPd) had a prior ASCT, were refractory to lenalidomide (91% KPd and 82% Dara-KPd), and exposed to a PI but not refractory (72% KPd and 64% Dara-KPd) (Table 2).
DLTs
For the KPd cohort, a total of 46 patients were enrolled in the phase 1 portion and 20 in the phase 2 portion. There were 3 patients treated at dose level 1, 29 patients treated at dose level 2 with 26 evaluable for DLT, and 34 patients treated at dose level 3 (13 evaluable for DLT in phase 1, 1 nonevaluable for DLT in phase 1, and 20 in phase 2 portion). A DLT occurred in 1 of 3 patients (33%) at dose level 1, with a probability estimate of a DLT of 12.9%, in 4 of 26 patients (14%) at dose level 2 (probability estimate of 19%), and in 4 of 13 patients (31%) at dose level 3 (probability estimate of 24.8%). All DLTs were asymptomatic cytopenias (8 were grade 3 neutropenia and 1 was grade 4 thrombocytopenia). There were no treatment-related deaths. No patient was enrolled in dose level 4 because the maximum probability of a DLT was met with dose level 3 (24.8%). Therefore, dose level 3 (carfilzomib 20/27 mg/m2 and pomalidomide 4 mg at the schedule above) was identified as the MTD for the phase 2 portion for both KPd and Dara-KPd.
Overall safety
Hematologic adverse events (AEs) were common in both cohorts (Table 3). For KPd (n = 66), 17 (26%) experienced neutropenia (17% grade 3+), 21 (32%) thrombocytopenia (9% grade 3+), and 26 (39%) anemia (12% grade 3+). For Dara-KPd (n = 28), 19 (68%) had neutropenia (64% grade 3+), 8 (29%) had thrombocytopenia (7% grade 3+), and 13 (46%) had anemia (7% grade 3+).
The most common nonhematologic AEs were fatigue (59% KPd and 71% Dara-KPd), upper respiratory tract infections (58% KPd and 50% Dara-KPd), dyspnea (50% KPd and 29% Dara-KPd), diarrhea (44% KPd and 64% Dara-KPd), hypertension (30% KPd and 39% Dara-KPd), and hyperglycemia (27% KPd and 61% Dara-KPd). Cardiovascular toxicities were typically of low grades; in particular, reduced ejection fraction was seen in 5 (8%) of KPd and 5 (18%) of Dara-KPd, with only 1 grade 3 event in each cohort and none leading to treatment discontinuation, suggesting the asymptomatic nature and/or reversibility of this toxicity. Most dyspnea cases were grade 1 to 2 (Table 3). Thromboembolic events occurred in 2 (3%) of the KPd cohort and in 1 (4%) of the Dara-KPd cohort. There was 1 episode of grade 3 hemolytic uremic syndrome in the Dara-KPd cohort.
Dose reductions occurred in 30 of 66 (45%) of the KPd and 17 of 28 (61%) of the Dara-KPd cohort.
Reasons for discontinuing therapy in the KPd cohort were progressive disease (55/66, 83%), AE (5/66, 8%), elective withdrawal (4/66, 6%), nonadherence (1/66, 1.5%), and death (1/66, 1.5%), which was not treatment-related. In the Dara-KPd cohort, 11 of 28 patients (39%) had discontinued therapy: 8 (29%) because of progression, 2 (7%) because of elective withdrawal, and 1 (4%) because of death, which was not treatment-related.
Efficacy
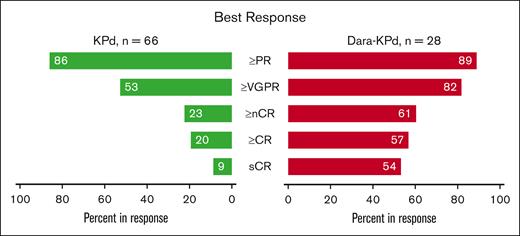
For the 34 patients treated at the MTD in the KPd cohort, the ORR after 4 cycles was 27 of 34 patients (79%; 95% confidence interval [CI], 62%-91%), meeting the predefined statistical threshold for efficacy. The ORR after 4 cycles for the entire KPd cohort was 50 of 66 patients (76%; 95% CI, 64%-85%) and as best response 57 of 66 patients (86%). The rate of CR or better was 13 of 66 patients (20%) (Figure 2). The median times to PR, very good PR (VGPR), and CR were 0.9, 3.7, and 7.3 months, respectively. At a median follow-up of 44 months, the median PFS and OS were 13 and 44 months, respectively (Figure 3). There were no differences in best response or PFS by dose level (supplemental Figure 1). No patients in the KPd cohort were being treated on study at the data cutoff.
Response rates. Best response in the KPd and Dara-KPd cohorts. sCR, stringent complete response.
Response rates. Best response in the KPd and Dara-KPd cohorts. sCR, stringent complete response.
Progression free survival and overall survival. PFS (A) and OS (B) for the KPd cohort. PFS (C) and OS (D) for the Dara-KPd cohort. NR, not reached.
Progression free survival and overall survival. PFS (A) and OS (B) for the KPd cohort. PFS (C) and OS (D) for the Dara-KPd cohort. NR, not reached.
For the Dara-KPd cohort, the rate of more than nCR after 4 cycles was 1 of 28 patients (39%; 95% CI, 22%-59%), also meeting the predefined statistical threshold for efficacy. As best response, the ORR was 25 of 28 patients (89%) and the rate of more than CR was 16 of 28 patients (57%) (Figure 2). The median times to PR, VGPR, CR, and stringent CR were 1, 3.9, 5.5, and 7.8 months, respectively. A total of 26 patients were assessed for MRD by 10-color multiparametric flow cytometry; 17 of 26 patients (65%) achieved MRD negativity at a sensitivity threshold of 10−5, and 14 of 26 (53%) were in CR plus MRD negativity as best response. The median time to MRD negativity was 5.5 months. Sustained MRD negativity, defined as consecutive MRD-negative results at least 1 year apart, was identified in 11 of 26 patients (42%); of these 11 patients, only 1 patient had progressed at the data cutoff. At a median follow-up of 26 months, the median PFS and OS were not reached, with 1- and 2-year PFS rates of 89% and 73%, and 1- and 2-year OS rates of 92% and 83%, respectively (Figure 3). A total of 17 of 28 patients (61%) remained on study at the data cutoff.
When stratified by cytogenetic risk, there were no differences in PFS between those with standard-risk and high-risk disease in the Dara-KPd. Though there was a nominal difference in median PFS for the KPd cohort at 17 vs 10 months for standard-risk vs high-risk disease, respectively, it did not reach statistical significance (hazard ratio 0.62; 95% CI, 0.35-1.09; P = .09) (supplemental Figure 2).
Discussion
This phase 1/2 study assessed the safety and efficacy of both KPd and Dara-KPd in the treatment of patients with relapsed/refractory MM, most of whom were lenalidomide-refractory. Four cycles of KPd led to a high ORR at the MTD (79%), thereby providing the primary end point of efficacy for the KPd cohort. Dara-KPd led to deeper responses and also achieved the primary end point, with a rate of 39% more than nCR after 4 cycles, a more than CR rate of 57% as best response, and an MRD negativity (10−5) rate of 65%.
The response rates and outcomes from the KPd cohort compare favorably with the results of another KPd study, which reported an ORR of 59% with a median PFS of 7.2 months in more heavily pretreated patients (6 prior lines of therapy).20 A once weekly KPd regimen was studied in a phase 1/2 fashion among patients who were lenalidomide-refractory; at the MTD for carfilzomib of 27 mg/m2 weekly, the ORR was 62%, and the median PFS 10.3 months.21 Sonneveld et al investigated KPd with ASCT in patients with bortezomib and lenalidomide refractoriness, which yielded a 92% ORR and a median PFS of 26 months.22 Here, we showed a similarly high ORR of 86% but a median PFS of 13 months, which is only modestly improved compared with the other studies of KPd without ASCT. The ongoing SELECT study (#NCT04191616) is studying weekly KPd in early relapsed lenalidomide-refractory disease, which will provide additional evidence on this regimen.
As this study was enrolling, the data on daratumumab in the relapsed/refractory setting was rapidly evolving, leading to its incorporation as a separate cohort. This study was not designed to directly compare the KPd and Dara-KPd cohorts but included a predefined efficacy threshold that would be considered statistically promising for the effect of an addition of daratumumab to KPd. Even with a higher proportion of patients with high-risk cytogenetics (64% vs 33%), Dara-KPd led to high rates of deep response, exceeding the predefined statistical threshold for promising efficacy. This resulted in a median PFS and OS not reached at 26 months of follow-up, with an estimated 2-year PFS for Dara-KPd of 73%; as reference, the 2-year PFS for KPd was 22%. Not only did Dara-KPd lead to a high rate of MRD negativity, but this was sustained for most patients; only 1 patient had disease progression among those with sustained MRD negativity. Although the rates and severity of neutropenia were significantly higher with Dara-KPd than with KPd, encouragingly the rates of infection were similar between the 2 cohorts. Similarly, although cardiotoxicity was observed and appeared slightly higher in Dara-KPd cohort, the rates of G3 cardiotoxicity were similarly low in both cohorts and the absolute numbers were small which limits comparison between the 2 cohorts.
Most newly diagnosed patients with MM in the United States receive a PI, an IMiD, and a corticosteroid as frontline therapy, meaning that at relapse, their disease will likely be resistant to lenalidomide and possibly bortezomib as well. Effective second-line therapy has evolved to include triplet therapy, but the optimal regimen in this setting is not established. The phase 3 CASTOR trial comparing Dara-Vd with Vd (bortezomib and dexamethasone) showed that in those receiving Dara-Vd, the ORR was 83% with 59% achieving more than VGPR, 19% achieving more than CR, and a median PFS of 16.7 months.8,23 However, the median PFS was much lower for those who had lenalidomide-refractory disease (7.8 months). The phase 3 OPTIMISMM trial that compared VPd (bortezomib, pomalidomide, and dexamethasone) with Vd among lenalidomide-exposed patients found a median PFS of 11.2 months for those receiving VPd.24 Our nonrandomized results with KPd suggest a similar, if not superior, level of PFS benefit.
The use of an anti-CD38 monoclonal antibody was further investigated in the lenalidomide-refractory population specifically; the phase 3 APOLLO and ICARIA trials identified a median PFS of 12.4 months for Dara-Pd12 and 11.5 months for isatuximab (Isa)–Pd.25 KPd appears on par with these triplet combinations in the lenalidomide-refractory setting with similar prior lines of therapy but falls short of the combinations of anti-CD38 monoclonal antibodies with carfilzomib, which had less pretreated patients with lower rates of lenalidomide refractoriness. The phase 3 CANDOR trial resulted in a median PFS of 28.6 months for Dara-Kd, including a median PFS of 25 months in patients who were lenalidomide-refractory with 1 prior line of therapy.10 The phase 3 IKEMA trial found a median PFS of 35.7 months for Isa-Kd (41.7 months with censoring of PFS events occurring >8 weeks after last assessment),26-28 including a median PFS of 38 months in patients with 1 prior line of therapy; there is no data specifically on patients with lenalidomide-refractory disease.
With a median follow-up of 26.3 months in a patient population with 64% high-risk disease, the median PFS with Dara-KPd had not been reached, suggesting that the addition of pomalidomide with half the dose of carfilzomib (27 mg/m2) can induce and maintain remarkably deep and durable responses; a randomized comparison of Dara-KPd vs Dara/Isa-Kd would help to confirm this finding. The lower dose of carfilzomib in our study design may help to reduce the number of treatment discontinuations, as 7% of patients electively discontinued therapy with Dara-KPd (14% with KPd) compared with 28% with Dara-Kd in CANDOR, which was almost entirely attributed to toxicity from carfilzomib.
There are important differences between patients in the Dara-KPd cohort and those receiving Dara-Kd and Isa-Kd in the trials above. Both CANDOR and IKEMA used carfilzomib 56 mg/m2 twice weekly, compared with 27 mg/m2 twice weekly in our study. In the Dara-KPd cohort, 75% had 1 prior line of therapy, which is higher than that seen in CANDOR (46%) and IKEMA (44%). However, it is notable that 100% of patients receiving Dara-KPd were lenalidomide-exposed (vs 39% and 40% in CANDOR and IKEMA) and 82% were lenalidomide-refractory (vs 32% in both CANDOR and IKEMA), which suggests that our study did not preferentially enroll patients with more indolent disease compared with other studies.
Although the debate over frontline regimens in MM will continue to focus on triplets vs quadruplets, there are key issues at relapse for patients treated with either combination. For those treated without an anti-CD38 monoclonal antibody upfront, is KPd an effective regimen in the second line, and should quadruplet therapy with an anti-CD38 monoclonal antibody be pursued? This study helps to support the use of both KPd and Dara-KPd in earlier lines of therapy for relapsed/refractory MM. Which one leads to superior efficacy will require randomized prospective evaluation; our results suggest that Dara-KPd may be the preferred option, especially after standard induction with VRd with or without ASCT and lenalidomide maintenance. Although the study was not designed nor powered to evaluate the efficacy of KPd or Dara-KPd in high-risk disease, the results with the Dara-KPd quadruplet in those with high-risk disease seem to indicate that this is a highly effective regimen for high-risk disease at relapse.
Limitations of this study include its nonrandomized design and the use of nCR as a primary end point. Although this avoided issues with having to discern immunoglobulin Gκ paraprotein from daratumumab when a paraprotein was detected by immunofixation alone, this may be considered outdated as nCR is typically regarded as a VGPR according to the International Myeloma Working Group response criteria. With that said, the rate of VGPR or better with Dara-KPd was 82%, which compares favorably with the rates of VGPR or better for Isa-Pd (38%), Dara-Pd (51%), Dara-Kd (69%), and Isa-Kd (73%).10,12,25,26 The dose of carfilzomib 27 mg/m2 twice weekly was chosen in this study because the maximum probability of a DLT allowed (25%) was met with this dose, and it is congruent with the Food and Drug Administration package insert dosing recommendation. The published ARROW study showing superiority of carfilzomib 70 mg/m2 weekly to 27 mg/m2 twice weekly was not published until June 2018 (after both cohorts opened), and this was in combination with dexamethasone only.29 The MANHATTAN and MASTER trials published in 2021 used carfilzomib 56 mg/m2 weekly dosing as part of quadruplet therapy, which appears to have been effective but did not have direct supporting clinical data before that.30,31 A study of Dara-KPd with weekly carfilzomib at 56 mg/m2 is ongoing (#NCT04176718), whose interim analysis showed a 95% ORR and 12-month PFS of 86%.32 Strengths include the high proportion of patients with high-risk cytogenetic abnormalities and prior PI/IMiD exposure, along with a diverse patient population that includes one of the highest reported rates of minority enrollment in MM (30% overall, 61% for Dara-KPd).
Given the trend toward the use of anti-CD38 monoclonal antibodies upfront, a major unanswered question is, “what is the best option at first relapse?” Both KPd and Dara-KPd may be logical choices given the patterns of refractoriness that may develop. Additional studies to compare Dara-KPd to an investigator’s choice of triplet therapy in the second line in MM would be well served, especially for those with high-risk disease. Even with the increasing use of daratumumab in frontline therapy, its benefit in maintenance therapy remains inconclusive. It is possible that, like what is observed for bortezomib-based inductions, an anti-CD38 monoclonal antibody could be used during induction/consolidation and then revisited in the first relapse after a hiatus.
The MM treatment landscape is quickly evolving with an increasing proportion of patients becoming refractory to multiple PIs, IMiDs, and an anti-CD38 monoclonal antibody after just 2 lines of therapy. Yet, highly effective immunotherapies, such as chimeric antigen receptor T-cell therapy, are currently approved in the United States for relapsed and refractory myeloma after 4 prior lines of therapy, and other novel immunotherapies are coming to earlier lines as well. Notwithstanding anticipated progress and further changes in the treatment landscape, our results suggest that KPd is an attractive option for triple-class exposed MM and that Dara-KPd may be among the most preferred options for treatment of relapsed/refractory MM, whereas further studies of immunotherapies in earlier lines in MM are in progress.
Acknowledgments
The authors thank the patients and their families for participating in this study. The authors thank Luis Alcantar, Evangelia Andreatos, Christine Gleason, Martha Gorski, Bernadette Libao, Sarah Major, Allison Marthaler, Amanda McIver, Megan Whelan, and Brittany Wolfe at the University of Chicago for their assistance in conducting this study.
This study was supported, in part, by Amgen, Bristol Myers Squibb, and the Multiple Myeloma Research Consortium. The funders were involved in the design of the study. The funders had no role in the collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; nor decision to submit the manuscript for publication.
Authorship
Contribution: A.J.J. conceived and designed the trial; B.A.D., A.T.S., and A.J.J. analyzed and interpreted the data and drafted the original manuscript; and all authors collected and assembled the data and critically reviewed and approved the final manuscript.
Conflict-of-interest disclosure: B.A.D. provided consultancy for Janssen, Sanofi, and COTA Healthcare, and received honoraria from Plexus Communications and MJH Life Sciences. J.Z. provided consultancy for Intellia, Amgen, Takeda, Janssen, Regeneron, Alnylam, Caelum, and Oncopeptides, and received research funding from Bristol Myers Squibb (BMS), and Celgene. D.R. provided consultancy for Janssen, BMS, Takeda, and Karyopharm; received honoraria from Janssen, BMS, Sanofi, Amgen, GlaxoSmithKline (GSK), and Takeda; and received research funding from BMS, Janssen, and Sanofi. C.C. provided consultancy for AstraZeneca, PRIME Education, Sanofi, and Oncopeptides, and received research funding from GSK. J.B. provided consultancy for AbbVie, Amgen, BioClinica, bluebird bio, BMS, Celgene, Celularity, Constellation, CRISPR Therapeutics, CURIS, Eli Lilly, EMD Sorono, Genentech, Glenmark, Ichnos, Janssen, Kesios, Legend, SecuraBio, and Servier, and received resarch funding from 2seventy bio, AbbVie, Acetylon, Amgen, bluebird bio, BMS, Celgene, Celularity, Constellation, CRISPR Therapeutics, Janssen, Karyopharm, Kite Pharma, Novartis, Poseida, Prothena, Sanofi, Takeda, and Teva. A.T.S. reports employment at SimBioSys. J.J. reports employment at Janssen. A.J.J. reports consultancy and advisory board membership for AbbVie, Adaptive, Amgen, BMS/Celgene, GSK, Janssen, Juno, and Karyopharm. The remaining authors declare no competing financial interests.
Correspondence: Andrzej J. Jakubowiak, Multiple Myeloma Program, University of Chicago, 900 E 57th St, Chicago, IL 60637; e-mail: ajakubowiak@medicine.bsd.uchicago.edu.
References
Author notes
Data are available on request from the corresponding author, Benjamin A. Derman (bderman@medicine.bsd.uchicago.edu).
The full-text version of this article contains a data supplement.