Visual Abstract
Comprehensive cancer treatment approach incorporating Social Determinants of Health. Created with BioRender.com.
Comprehensive cancer treatment approach incorporating Social Determinants of Health. Created with BioRender.com.
Abstract
Social determinants of health (SDHs) have been reported as relevant factors responsible for health inequity. We sought to assess clinical data from observational studies conducted in the United States evaluating the impact of SDHs on the outcomes of patients with hematologic malignancies. Thus, we performed a systematic review in 6 databases on 1 September 2021, in which paired reviewers independently screened studies and included data from 41 studies. We assessed the risk of bias using the Joanna Briggs Institute appraisal tools and analyzed the data using a descriptive synthesis. The most common SDH domains explored were health care access and quality (54.3%) and economic stability (25.6%); others investigated were education (19%) and social and community context (7.8%). We identified strong evidence of 5 variables significantly affecting survival: lack of health insurance coverage or having Medicare or Medicaid insurance, receiving cancer treatment at a nonacademic facility, low household income, low education level, and being unmarried. In contrast, the reports on the effect of distance traveled to the treatment center are contradictory. Other SDHs examined were facility volume, provider expertise, poverty, and employment rates. We identified a lack of data in the literature in terms of transportation, debt, higher education, diet, social integration, environmental factors, or stress. Our results underscore the complex nature of social, financial, and health care barriers as intercorrelated variables. Therefore, the management of hematologic malignancies needs concerted efforts to incorporate SDHs into clinical care, research, and public health policies, identifying and addressing the barriers at a patient-based level to enhance outcome equity (PROSPERO CRD42022346854)
Introduction
Over the last decades, there have been significant improvements in the survival expectation of patients with hematologic malignancies.1 Such progress in outcomes has been possible through a combination of advances in cancer biology research, the implementation of more accurate risk and prognostic scoring systems, and the breakthrough of novel drugs and subsequent lines of therapies.2,3 Nevertheless, this enhancement has not been experienced equally across all populations, and certain groups experience disproportionately higher mortality rates.4
The prevalence of cancer outcome inequity can be attributed to the complex interaction among multiple aspects, including genetic factors, health behaviors, and social determinants of health (SDHs).5 SDHs are defined as the set of nonbiological factors and systems that shape the environment of daily life, such as the conditions where people are born, grow, work, live, and age, and affect health outcomes. SDHs are categorized into 5 key domains: health care access and quality, education access and quality, social and community context, economic stability, and neighborhood and built environment.6
Economic instability, a lower education level, decreased access to health care, residential segregation, discrimination, and a lack of social support systems have been linked to lower cancer screening rates, diagnosis at advanced stages, and worse cancer survival.7-10 Although that evidence has enormously contributed to our understanding of the factors that underlie the inequity, the studies reporting on this topic have varied on the specific SDH analyzed, the scope and size of the population studied, and the reported influence of SDHs.
To better assess the impact of SDHs on cancer-treatment outcomes, we performed a systematic review to understand the degree to which multiple factors may contribute to cancer outcome disparities. Identifying these influences is an important step in building strategies and interventions to address the SDHs and accelerate progress toward health equity in cancer. Our broad review included all cancer types, and this article focused on reporting the findings for hematologic malignancies affecting adult patients.
Methods
We conducted and reported this systematic review in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines (supplemental Tables 1 and 2).11 We registered our protocol at the Prospective Register of Systematic Reviews, which can be accessed through the registration number CRD42022346854.
Eligibility criteria
We based our inclusion criteria on the PECOS approach (population, exposure, comparison, outcome), consisting of observational studies investigating any SDH's impact on cancer-treatment survival. Because of the cross-national differences in social behaviors, economic conditions, and education and health systems, we limited our review to those studies, including only data from the United States from 2002 onward. We focused our review on recognizing actionable SDHs that clinicians could identify and address in routine clinical practice. Thus, we excluded studies for the following reasons: (1) studies that evaluated sex, age, race/ethnicity, geography, and rurality as SDH; (2) studies that did not assess the relationship between SDHs and cancer survival; (3) studies in which the results according to cancer type cannot be individualized; (4) studies written in languages other than English; and (5) reviews, letters, conference articles, personal opinions, and book chapters.
Information sources and search strategy
We performed a customized literature search using PubMed, Cochrane, EMBASE, Scopus, and the Web of Science databases. We conducted an additional search in the gray literature using Google Scholar and examined the reference lists of included studies (supplemental Table 3). Our searches included articles published on or after 1 January 2002 until 1 September 2022. We used the reference manager software EndNote 20.2.1 (Clarivate Analytics)12 to export references and remove duplicate articles.
Studies selection process
We identified relevant studies in 2 phases. In phase 1, we imported references into Rayyan Systems Inc software,13 where 2 authors (M.M.G. and K.C.T.) independently screened the titles and abstracts for eligibility. We excluded those articles that did not meet our inclusion criteria. In phase 2, we accessed the full text of the selected studies independently by the same 2 reviewers. We resolved disagreements in any phase by discussion among reviewers and, when necessary, arbitration by a third author (J.E.C.) (supplemental Table 4).
Data collection process and data items
One author (M.M.G.) extracted the data from the studies that fulfilled the inclusion criteria through the NVivo software. The second author (K.C.T.) cross-checked the data and verified their accuracy, and other authors (J.E.C., E.A. B., and G. A.) were involved when necessary, according to their area of expertise.
Risk of bias by individual studies
We independently assessed the risk of bias for the included studies by 2 reviewers (M.M.G. and K.C.T.) using the Joanna Briggs Institute checklist for analytical cross-sectional studies.14 We categorized the studies according to the scores of items “yes” as high risk (<49%), moderate risk (50%-69%), and low risk (>70%).
Effect measures
Our outcomes were any cancer-treatment survival measures, such as early mortality, disease-free survival, cancer-specific survival, and overall survival (OS).
Synthesis of results
We conducted a qualitative synthesis of the data by categorizing studies across the 5 domains of SDH.6 We next grouped the results into the following categories: (1) significant association between SDH and any cancer-treatment survival in multivariable-adjusted analysis, (2) significant association in multivariable-adjusted analysis only in a subgroup of the study population (such as according to age or cancer subtype), (3) significant association only in the unadjusted analysis, and (4) not significant association. We considered significant any association that was found to be statistically significant, between SDHs and survival by the authors of each manuscript. In case the statistical significance criterion was not described in the manuscript, we set the statistical significance as confidence intervals of hazard ratio not including 1. Because of the variability of methods, we did not attempt a uniform definition. For cases in which multiple SDHs, types of cancer, databases, and/or outcomes were studied, we extracted results for each variable separately and reported them individually. We presented the outcomes as hazard ratio with stated confidence interval as measures of significance.
Results
Study selection
We identified 38 654 records from the electronic databases we searched. These were reduced to 15 319 after removing duplicate studies. After screening of the title and abstract, 1477 records meet our inclusion criteria. We excluded 719 reports that presented data from countries other than the United States. We then selected the 28 studies that assessed hematologic malignancies in adult patients. In addition, we retrieved 13 additional studies from the reference list. After reading the full text, we retained 41 studies for data extraction (supplemental Figure 1).
Characteristics of included studies
Twenty-five of the 41 studies we included in our analysis evaluated national cohorts (61%), whereas 8 comprised state data studies (19.5%) and 6 single-center cohorts (14.6%). Two studies (4.9%) analyzed 2 different cohorts, national and institutional data. Studies used multiple data sources, including 17 studies evaluating the nationwide US databases, such as the National Cancer Database (NCDB; 41.5%), 7 used the Surveillance, Epidemiology, and End Results (SEER; 17.1%), and 1 used the Center for International Blood and Marrow Transplant Research (CIBMTR; 2.4%). State studies assessed cancer registries: 4 studies from California (11.1%), 1 from Florida (2.2%), 1 from New York (2.2%), 1 from North Carolina (2.2%), and 1 from Virginia (2.2%). Institutional studies retrieved data from the patients’ medical records.
Owing to the diversity of study settings, the sample size ranges widely, from 95 to 132 402 patients (mean, 24 353; SD, 32 330), with a median value of 7073 (IQR, 3461-42 718). Overall, studies addressed the impact of SDHs on cancer outcomes for a variety of hematologic malignancies as follows: lymphoma in 16 studies (34.1%), myeloma in 11 (31.7%), and leukemia in 14 (34.2%). Five studies analyzed a mixed group of patients, in which 4 studies evaluated 2 cancer types and 1 evaluated 4 types of hematologic malignancies.
Most of the studies in our analysis (n = 30, 73.2%) examined more than 1 SDH, ranging from 1 to 8. Thus, this systematic review covered the analysis from 132 variables (median, 2; IQR, 2-4) of SDHs assessed in the 41 included manuscripts. The most common SDH domains explored were health care access and quality (n = 70, 53%) and economic stability (n = 33, 25%); others investigated were education (n = 19, 14.4%) and social and community context (n = 10, 7.6%). Although most of the studies evaluated a unique outcome (n = 28, 68.3%), 12 studies explored 2 outcomes (29.3%), and 1 assessed 3 outcomes (2.4%). The primary outcome was OS in 41 studies (73.2%). Other outcomes measured less frequently were early mortality in 6 studies (10.7%), cancer-specific survival in 5 (8.9%), progression-free survival in 2 (3.6%), disease-free survival in 1 (1.8%), and transplant-related mortality in 1 (1.8%) (supplemental Tables 5 and 6).
Risk of bias
The risk of bias from individual studies had low risk except for 1 study that was considered a moderate risk (supplemental Figure 2).
Synthesis of results
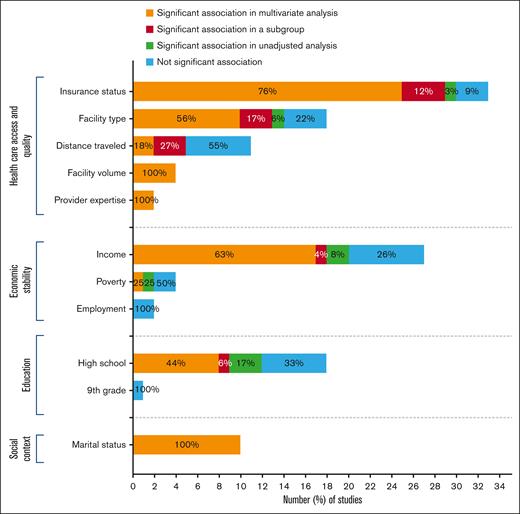
We presented the synthesis of our results in Tables 1-4 and Figure 1.
Distribution of the studies according to the impact of SDH within the major domains on cancer-treatment outcomes.
Distribution of the studies according to the impact of SDH within the major domains on cancer-treatment outcomes.
Health care access and quality
Included studies encompassed a variety of variables to evaluate health care access and quality, including health insurance coverage (n = 33, 47.1%), treatment facility type (n = 20, 28.5%), distance traveled from residence to treatment facility (n = 11, 15.7%), provider case volume (n = 4, 5.7%), and provider expertise (n = 2, 2.8%) (supplemental Table 6).
Health insurance coverage
Health insurance coverage at diagnosis was the most studied SDH across all included manuscripts. There was variability in the grouping of insurance status across studies. However, broadly, the cohorts were compared in terms of private, or managed care vs uninsured, Medicare or Medicaid. Most studies (n = 30, 90.9%) found a significant association between health insurance coverage and survival in multivariable, univariate, and/or subgroup analysis. Overall, patients with Medicaid, Medicare, or other government insurance or who are uninsured have inferior survival rates compared with those with private or military insurance coverage. Of note, one of these studies reported that the difference was significant only in univariate analysis. Data source from that study was single-center data with a small sample size (652 patients) compared with other studies included.15 In 3 out of the 30 studies, insurance coverage influenced survival only in patients younger than 65 years old.16-18 In contrast, 3 studies (9.1%) reported no association between insurance status and cancer outcomes, including 2 studies of acute myeloid leukemia (AML)19,20 and 1 of non-Hodgkin lymphoma (NHL)21 (Table 1).
Facility type
Twenty studies examined the impact of the type of facility on cancer outcome, including treatment facility type (n = 18, 64.3%), being diagnosed at National Cancer Institute (NCI)–designated cancer centers (n = 1, 3.6%), and access to NCI- and National Comprehensive Cancer Network (NCCN)–designated cancer centers (n = 1, 3.6%). Fourteen of 18 studies (77.8%) demonstrated a significant improvement in early and OS for patients treated at academic/research cancer centers compared with those receiving treatment at community cancer centers, comprehensive community cancer centers, or integrated network cancer programs. Of those, 1 study for NHL22 found a statistically significant association in unadjusted analysis for patients treated at academic cancer centers (vs nonacademic centers) that was not confirmed in multivariate analysis. Three studies described significant association in multivariable-adjusted analysis only in subgroup analysis. Two of them17,18 observed that treatment facility type influenced survival in patients older than 65, but this difference did not translate to patients younger. Another study23 classified plasmacytomas as originating in bone (P-bone) or extramedullary tissue (P-EM) and noted a significant association between facility type and mortality only in the P-bone cohort.
Two studies24,25 evaluated and reported better outcomes among patients treated at NCI–designated cancer centers vs non-NCI–designated cancer centers. However, one other study26 did not observe differences in survival rates based on whether patients were diagnosed at an NCI–designated cancer center or not. In 1 study exploring the impact of NCI/NCCN CC access on outcomes of patients with multiple myeloma (MM),27 access to 1 NCCN CC or 2 or more NCI CCs correlated with OS improvement in a multivariable-adjusted analysis. Finally, 1 study28 established that patients diagnosed and treated at the same facility had an increased risk of death than those diagnosed at one facility and treated at another (Table 2).
Distance traveled to the treatment center
The relationship between distance traveled to the treatment center and cancer outcomes was investigated in 11 studies with inconsistent results. None of the studies specified how distance was measured (eg, straight line vs car driving distance). Unexpectedly, 5 studies (45.5%) identified that 1-month and overall mortality were higher for those patients who traveled shorter distances (range, 6-40 miles), whereas 6 studies (54.5%) did not find a significant association. Three of the 5 studies identified significant differences in multivariable-adjusted analysis in subgroup analyses. Two studies categorized patients according to age (<65 vs ≥65 years old) and found contradictory results. Although in the cohort of Dhakal et al,17 distance traveled affected 1-month mortality only in patients younger than 65 years old, Chohan et al18 reported that this SDH was significantly associated with lower OS in the unadjusted analysis exclusively in subjects older than 65. Similar to the previous results in the insurance status section, Ghiassi-Nejad et al23 described that distance traveled influenced mortality in the unadjusted analysis in patients with P-bone but not in the P-EM cohort (Table 2).
Treatment facility volume
Four studies evaluated facility volume as determined by the mean annual volume of newly diagnosed patients with the disease of interest treated at each treatment facility. All 4 studies supported a volume-outcome relationship: patients treated at lower-volume facilities had a higher mortality risk (Table 2).
Provider expertise
Two studies examining whether provider expertise influences cancer-treatment outcomes reported a significant relationship in multivariable-adjusted analysis. Shanafelt29 showed that patients treated by hematologists/oncologists who specifically focused on their specific malignancy had superior OS than those treated by general hematologists/oncologists. Loberiza et al30 observed a higher risk of death among rural patients treated by community-based providers vs rural patients treated by university-based providers (Table 2).
Economic stability
Economic stability was investigated in 33 studies assessed by median household income (n = 27, 81.8%), poverty (n = 4, 12.1%), and unemployment rate (n = 2, 6.1%) (supplemental Table 6).
Median household income
Household income was typically categorized into quartiles based on the patient’s zip code. Low-income and high-income quantiles varied among studies from 30 000 to 63 332 and 30 000 to 68 500, respectively. Twenty of the 27 studies (74.1%) assessing this SDH demonstrated an influence of income on survival. Collectively, the results show an income gradient where lower income was associated with the shortest survival probability. Two of the 20 studies18,31 (7.4%) only found a difference in the unadjusted model. Of those 7 studies (25.9%) that found no significant correlation between income and outcomes, 5 used state databases with a mean sample size of 849.4 patients (range, 213-2330).26,30,32-34 The remaining 2 studies were population-based and reported no relationship between earnings16,35 (Table 3).
Poverty rate
Four studies (12.1%) evaluated poverty rate–related differences in cancer survival and showed significant heterogeneity in the results. A state-level study36 and a population-based study31 concluded that patients living in areas with the highest poverty levels (≤10.1%) had worse OS than those residing in low-poverty neighborhoods. In contrast, Freeman et al32 and Perry et al16 reported no significant difference in mortality among poverty levels (Table 3).
Unemployed rate
Social context
Patients' social context was measured directly and exclusively through marital status in 10 studies (supplemental Table 6). Results showed that marital status consistently and significantly influences OS throughout all studies evaluated in multivariable-adjusted analysis. Overall, unmarried patients, including single, divorced, widowed, and/or separated, had a significantly higher probability of dying compared with married patients. In addition, 2 studies evaluating patients with MM24,37 and 1 with AML22 also attempted to understand whether marital status played a role in early mortality. Both MM studies found higher early mortality among unmarried vs married patients. In contrast, the 1 AML analysis38 found no significant effect of marital status on early mortality (Table 4).
Education
Nineteen studies analyzed whether education level played a role in cancer survival. Most of the studies (n = 18, 94.7%) evaluated education level as determined by the percentage of adults without a high school diploma for the patient's geographical area, whereas 1 study16 (5.3%) assessed education through the proportion of the population without ninth grade education (supplemental Table 6). Overall, 12 studies (66.7%) found that residing in low-education areas correlates with higher mortality. Three of those 12 studies22,31,39 reported statistical significance only in the unadjusted mortality analysis. Six other studies (5.6%)16,17,21,32,35,40 reported no significant relationship between the level of education and patients' outcomes. Finally, Perry16 performed a comparative survival analysis between patients residing in areas above and below the median without ninth grade education and found no statistically significant difference between the cohorts (Table 4).
Discussion
Health care disparities affect patients with cancer in general and hematologic malignancies in particular. The role of SDHs in the outcome of patients with hematologic malignancies has been scarcely studied. In our review of 41 studies assessing patients with hematologic malignancies, we identified strong evidence of 5 variables of SDHs affecting survival: lack of health insurance or having Medicare or Medicaid insurance, receiving cancer treatment at a nonacademic facility, low household income, low education level, and being unmarried. Our findings have meaningful clinical applications, prompting physicians to identify patients who have been economically and socially marginalized and implement focused interventions.
Cumulative evidence assessing insurance status indicates that uninsured patients and those with Medicaid or Medicare insurance had a higher mortality risk than their private or military insurance counterparts. This might be explained by the fact that uninsured patients are less likely to receive preventive care, have more advanced disease at diagnosis, have more comorbid conditions, and have lower odds of receiving guideline-based treatment, likely driven by the cost of care and costly medications.8,41,42 Given the protective financial role that insurance coverage exerts, the disparities found between patients insured by Medicare and Medicaid and those with private insurance are perhaps surprising and may be related to specific line items covered by various insurance plans. It is also possible that other factors (including other SDH) associated with being covered by Medicaid and Medicare are at least in part responsible for these differences.
The analysis of the type of facility where patients receive treatment and provider case volume revealed a similar impact on cancer survival. Overall, patients receiving treatment at academic/research cancer centers and high-volume facilities achieve better outcomes. This imbalance could be related to academic centers and high-volume facilities having multidisciplinary care teams, disease-specific expertise of physicians, adherence to guidelines, and access to new technologies and therapies, including clinical trials.43,44 Consistent with this notion, evidence for our review revealed that provider expertise, access to NCI- and NCCN–designated cancer centers, and treatment at NCI–designated cancer centers provide a survival advantage. All things considered, our findings suggest that addressing health care access barriers through increasing access to preventive health care, reducing delayed cancer diagnosis and treatments, and expanding access to novel evidence–based therapies are focused actions on eliminating cancer-treatment disparities. Shared-care models where there is access to specialized centers for the care areas that most need it and care with local oncology clinics in close communication with the academic institutions would be optimal. The feasibility of such shared-care models for patients with hematologic malignancies has been shown to improve access to curative therapies, such as stem cell transplant (SCT), and the quality of life of patients, without compromising outcomes.45,46 This requires, among other things, increased communication, true integration of electronic health records, more access to telehealth, and decreasing barriers to practice across state lines.
Another important finding in our review is that unmarried patients present a higher mortality risk than married ones. Although the cause of this effect is multifactorial, the literature suggests the effect comes mainly through emotional and social support.47 Married patients seek prompt medical care encouraged by spouses, display higher treatment adherence, and present fewer mental health conditions such as depression, anxiety, and emotional distress.48-50 Furthermore, the caregiver role that spouses provide is an undervalued but critical component of the health care system. Spouses may support patients in different ways, including by assisting with personal care, making household chores, managing finances and legal matters, making medical appointments, assisting with mobility, and administering medications.
On economic stability and education, our results suggest a protective effect of higher median household income and education levels on survival. Importantly, education access and quality were measured as the percentage of adults who did not graduate from high school. We did not identify any study that evaluated the role of postsecondary degrees, such as undergraduate, graduate, and postgraduate. In addition, this has been assessed at the community level rather than at the individual level. There is a close correlation between the level of education and economic status.51,52 Higher educational levels are linked to more stable employment, higher salaries, and access to work with additional benefits,53 including insurance with better coverage.54
The reports on the effect of poverty level on patients' outcomes are, in contrast, contradictory. It is difficult to draw any conclusion because of the limited number of studies that evaluated this variable. Altogether, the mechanisms involved in the influence of education and economic stability on cancer survival are multifactorial, complex, and strongly related to other domains of SDHs.
One of the most striking results from our analysis is the role of distance traveled to treatment facilities. Nearly half of the evaluated studies reported higher mortality rates for patients who traveled shorter distances. Although those results need to be interpreted carefully, a possible explanation might be that some patients prefer to be treated at high-quality cancer centers instead of local centers, even if that means traveling long distances and incurring additional economic expenses. This assumption is intercorrelated with other SDHs, including having a more outstanding financial position and job flexibility that allows for such travel.
Our results highlight the need to identify patients who have been economically and socially marginalized and need psychosocial support and to invest in socially targeted interventions as part of a comprehensive cancer-treatment approach. Some progress has been made in designing screening tools to identify patients’ social and economic needs.55 These should be routinely assessed in clinical practice and recorded in patients’ charts. Required actions must focus on multidimensional strategies to provide patients with a social network that assists, encourages, and offers emotional and informative support that positively affects cancer outcomes.56,57
Our analysis is subject to some limitations that need to be acknowledged. First, there is a lack of individual patient data on most SDHs. Most studies used data routinely collected for other purposes and implemented a census-based approach. Second, there is a risk of data duplication considering those studies that analyzed national or state databases. Third, we identified a lack of data in the literature in terms of transportation, debt, higher education, diet, social integration, environmental factors, or stress. Our search strategy may not identify studies that do not identify variables as SDHs. In addition, how SDHs are measured and reported and what SDHs are reported vary across studies, making assessment of the interconnectivity of various factors challenging. However, the strengths of our review compensate for these limitations. To the best of our knowledge, this is the first systematic review assessing the role of SDHs on the outcomes strictly of patients affected by hematologic malignancies. We also restricted our analysis to only studies conducted in the United States because of the particular context of each country regarding health systems, education, the economy, and social behaviors.
In conclusion, our review highlights the extension of disparities among different populations in health care access and quality, financial burden, and social support. Furthermore, these results indicate the major effect of SDHs on survival outcomes in patients with hematologic malignancies. SDHs are a complex matrix of social, financial, and cultural elements as multiple intercorrelated variables. They represent a potential area of intervention independent of tumor biology or therapeutic efficacy. Strategies to incorporate SDHs into clinical care, research, and public health policies are needed, identifying and addressing social barriers at a patient-based level to enhance cancer equity.
Authorship
Contributions: J.E.C., M.M.G., and K.C.T. conceptualized the study; J.E.C., E.A.B., and G.A., were responsible for methodology; M.M.G., K.C.T., E.A.B., and G.A. curated the data; M.M.G. and J.E.C. wrote the original draft; J.E.C., E.A.B., and G.A. supervised the study; and M.M.G., K.T., E.A.B., G.A., and J.E.C. wrote and reviewed the manuscript.
Conflicts of interest disclosures: The authors declare no competing financial interests.
Correspondence: Jorge E. Cortes, Georgia Cancer Center, Augusta University, 1410 Laney Walker Blvd, CN2222 Augusta, GA 30912; e-mail: jorge.cortes@augusta.edu.
References
Author notes
Data that support the findings of this study are available on request from the corresponding author, Jorge E. Cortes (jorge.cortes@augusta.edu).
The full-text version of this article contains a data supplement.