Key Points
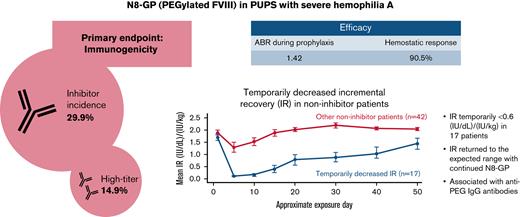
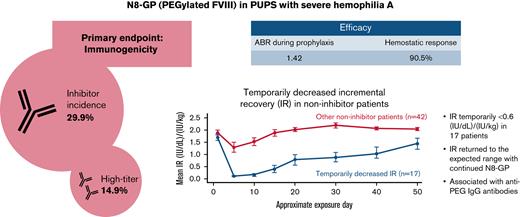
The inhibitor incidence of N8-GP in previously untreated patients was 29.9% (14.9% high-titer).
In 17 patients, temporarily decreased incremental recovery without inhibitors was observed within the first 5 exposure days to N8-GP.
Abstract
N8-GP (turoctocog alfa pegol) is a recombinant, glycoPEGylated, extended half-life, factor VIII replacement product. Here, we examined the immunogenicity, safety, and efficacy of N8-GP in previously untreated patients (PUPs). pathfinder6 is an ongoing, open-label, phase 3 trial that enrolled PUPs with severe hemophilia A and were aged <6 years. The primary end point was the incidence of factor VIII inhibitors (≥0.6 Bethesda units [BU]). Eighty patients received ≥1 N8-GP dose and were included in this analysis; ≥50 patients had ≥50 exposure days to N8-GP. The inhibitor incidence was 29.9% (14.9% high-titer [>5 BU]). Sixty-five patients received N8-GP prophylaxis for an average of 2.17 years with a median annualized bleeding rate (interquartile range) of 1.42 (0.76; 3.13) and a 90.5% hemostatic success rate. Temporarily decreased incremental recovery (IR), defined as ≥2 consecutive measurements of IR <0.6 (IU/dL)/(IU/kg) but no inhibitors, was observed in 17 patients within 5 exposure days to N8-GP and had a strong temporal correlation with anti–polyethylene glycol immunoglobulin G antibody titers. IR returned within the expected range with continued N8-GP dosing. During the period of decreased IR, hemostatic response was similar to that of the overall trial population, and no hypersensitivity related to N8-GP or unexpected new adverse events were reported. N8-GP prophylaxis was efficacious for the prevention and treatment of bleeding episodes in PUPs with severe hemophilia A. The inhibitor incidence was 29.9%. All patients with temporarily decreased IR continuing on N8-GP dosing returned within the expected range and had no evident lack of efficacy. This trial was registered at www.clinicaltrials.gov as #NCT02137850.
Introduction
N8-GP (turoctocog alfa pegol; Esperoct [Novo Nordisk A/S, Bagsvaerd, Denmark]) is a recombinant extended half-life factor VIII (FVIII) replacement product designed for the treatment of hemophilia A. The half-life of N8-GP is extended ∼1.6-fold in adults and 1.9-fold in children through the 40-kDa polyethylene glycol (PEG) molecule attached to the B domain of turoctocog alfa.1,2 During activation by thrombin, the truncated B domain of N8-GP with the PEG molecule is cleaved, leaving the native structure of activated FVIII intact.
Pivotal phase 3 clinical trials in previously treated children, adolescents, and adults, in which patients were treated for up to 6.6 years, showed that N8-GP was efficacious for the treatment and prevention of bleeding episodes.3,4 The median annualized bleeding rates (ABRs) for previously treated adults and adolescents was 0.84 for every-fourth-day prophylaxis,4 and previously treated children had a median ABR of 0.81 on a twice-weekly regimen.3 Based on these data, N8-GP has been approved by the European Medicines Agency for prophylaxis and the treatment of bleeding episodes of adults and adolescents with hemophilia A and additionally for children of all ages by the US Food and Drug Administration.
Inhibitory FVIII antibodies (inhibitors) bind FVIII, impeding activity and decreasing or abolishing the effectiveness of FVIII replacement therapy, depending on inhibitor titer.5 Overall, in the N8-GP pathfinder clinical program, >270 previously treated patients (PTPs) have been exposed to N8-GP for >1000 patient-years. A single PTP developed an inhibitor to N8-GP.6 Furthermore, in clinical trials, the presence or development of anti-PEG antibodies had no effect on FVIII activity in adult patients or PTPs.3,4
Previously untreated patients (PUPs) with severe hemophilia A are at the highest risk for inhibitor development during the first 50 to 75 exposure days (EDs) to FVIII replacement products. The cumulative risk of inhibitor development is ∼30%.7,8 Approximately 16% to 24% of PUPs with severe hemophilia A develop high-titer inhibitors (>5 Bethesda units [BU]).7 In the current study, we examine the immunogenicity, safety, and efficacy of N8-GP in PUPs with severe hemophilia A.
Materials and methods
Patients and trial design
pathfinder6 (#NCT02137850) is an ongoing, multinational, single-arm, open-label, phase 3 trial investigating the immunogenicity, safety, and efficacy of N8-GP in pediatric PUPs with hemophilia A that includes centers in 14 countries. pathfinder6 was initiated on 26 June 2014; recruitment is complete and expected trial completion is in November 2021. The cutoff date for this interim analysis was 31 August 2020, when ≥50 patients had ≥50 EDs, corresponding to the requirement for PUP trials until recently required by the European Medicines Agency for authorization of a new FVIII product in PUPs.9
Before initiation, this trial was approved by local authorities and all relevant independent review boards. This trial was conducted in accordance with the principles of the Declaration of Helsinki and the International Council for Harmonisation Good Clinical Practice, including the archiving of essential documents. Informed consent was obtained from the patients’ legally acceptable representatives before initiation of any trial-related activities.
Male patients, <6 years old, diagnosed with severe hemophilia A (FVIII activity <1%) with no prior use of purified FVIII-containing products, and ≤5 prior EDs to blood components were included in this trial. Key exclusion criteria included: history of FVIII inhibitors; known or suspected hypersensitivity to the trial product; previous participation in this trial; receipt of any investigational medical product within 30 days of screening; any chronic disorder or severe disease that, in the opinion of the investigator, might jeopardize the patient’s safety or compliance with the protocol; or other coagulation disorders. This trial comprised a main phase and an extension phase (supplemental Figure 1). The main phase comprised the first 50 EDs (defined as any day on which a patient was dosed with N8-GP, including prophylaxis, treatment of bleeding episodes, surgery, immune tolerance induction [ITI], or pharmacokinetic assessment) and included 9 study visits. After the main phase, patients could proceed to the extension phase. After a patient achieved 100 total EDs, they could continue N8-GP prophylaxis until the end of trial. Information on approximate timing of each study visit is presented in supplemental Figure 1.
Surgery details are provided in the supplemental Materials and methods.
End points and assessments
The primary end point was the incidence of FVIII inhibitors (≥0.6 BU). Samples for inhibitor testing were taken before dosing approximately every 5 EDs until 20 EDs and every 10 EDs until the patient reached 100 EDs. Further samples for inhibitor testing could be taken at any time, at the investigator’s discretion. Inhibitors were assessed by using a heat-modified Nijmegen FVIII Bethesda assay validated according to international recognized guidelines.10 A second, confirmatory sample (≥0.6 BU), preferably drawn <2 weeks apart, was required to categorize a patient as positive. Inhibitors ≥0.6 to 5 BU were considered low-titer and >5 BU were considered high-titer. Secondary safety end points included the frequency of adverse events (AEs) and serious adverse events (SAEs). Secondary efficacy end points included ABRs, the hemostatic effect of N8-GP in the treatment of bleeding episodes as measured on a 4-point scale (excellent, good, moderate, and none), the consumption of N8-GP for prophylaxis or the treatment of bleeding episodes, total N8-GP consumption, and ITI outcome (see also “Treatment”).
At each visit (except visit 2), FVIII activity predose and 30 minutes postdose (for calculation of incremental recovery [IR]) was determined by chromogenic assay using a product-specific standard as a calibrator, and, when plasma volume allowed, anti-PEG immunoglobulin G (IgG) and IgM were analyzed by using a direct enzyme-linked immunosorbent assay according to Yang et al.11 All patient samples were tested for anti–N8-GP-binding antibodies at all trial visits using a radioimmunoassay. During confirmatory inhibitor testing, sampling for assessments of FVIII activity (trough and recovery), anti–N8-GP-binding antibodies, lupus anticoagulant, and PEG antibodies were also performed. Details for the in vitro FVIII chromogenic assay to assess the neutralizing effect of anti-PEG IgG are presented in the supplemental Materials and methods. F8 genotype was documented at visit 0. In the case that F8 genotype was not available, genotyping was offered at the investigator’s discretion and according to local law.
Treatment
The standard prophylaxis regimen was ∼60 IU/kg IV twice weekly, or every 3 or every 7 days. Further information about increasing or decreasing dosing frequency based on a patient’s bleeding pattern is included in the supplemental Materials and methods. For patients <24 months old, pre-prophylaxis (defined as low dose or less frequent administration as a single bolus IV dose with >1 week between doses and/or on-demand treatment of bleeding episodes with N8-GP) was allowed at the investigator’s discretion. Patients could be started on regular prophylaxis at any time but had to be switched to prophylaxis by 24 months of age or after 20 EDs to N8-GP (whichever came first). Bleeding episodes during both pre-prophylaxis and prophylaxis were to be treated with 20 to 75 IU/kg IV N8-GP.
For patients with confirmed FVIII inhibitors, ITI treatment with N8-GP was permitted in this trial. The ITI regimen was at the investigator’s discretion. Further details about ITI regimen and success criteria are provided in the supplemental Materials and methods.
Statistical analyses
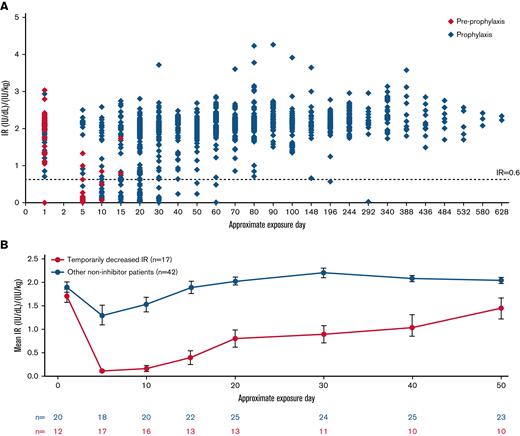
The incidence of inhibitory antibody development was calculated by dividing the number of patients with inhibitors by the number of all patients with ≥10 EDs to N8-GP plus all patients who had developed an inhibitor. Inhibitor incidence is reported with a one-sided 97.5% upper confidence limit based on an exact calculation for binomial distribution. For post hoc analysis of IR observations, temporarily decreased IR was defined as patients with at least 2 consecutive measurements of IR <0.6 (IU/dL)/(IU/kg) in the absence of an inhibitor. This definition was based on the lowest IR value observed in PTPs of the same age. Further statistical methods are presented in the supplemental Materials and methods.
Results
Patients and exposure
Overall, 80 patients received ≥1 dose of N8-GP and are included in this interim analysis. Of those, 54 patients started the trial with a pre-prophylaxis regimen. As of this analysis, 65 patients have started on or have been switched to N8-GP prophylaxis. Patients on pre-prophylaxis had a mean ± standard deviation age of 5.4 ± 5.2 months, and patients on prophylaxis were aged 11.7 ± 11.6 months at baseline. Further baseline and demographic characteristics are displayed in Table 1. Patients had a median (range) of 112.0 (7; 669) EDs to N8-GP during a median 1.8 (0.1-6.0) years of treatment. During the trial, 27 minor surgeries and 2 major surgeries were performed in 25 patients. Patient flow is presented in supplemental Figure 2. The mean trough FVIII activity of 287 samples from patients on a twice-weekly or every third day regimen was 0.015 IU/mL (95% confidence interval, 0.011; 0.020). A total of 104 measurements were below the lower limit of quantification (0.0045 IU/mL).
Development of inhibitory antibodies
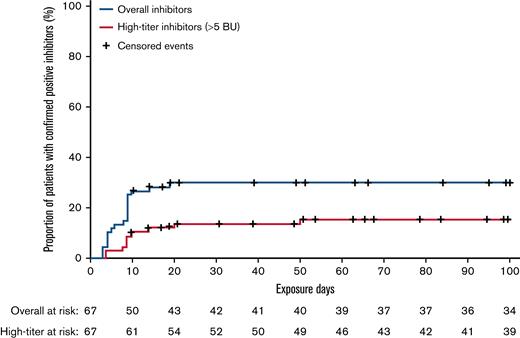
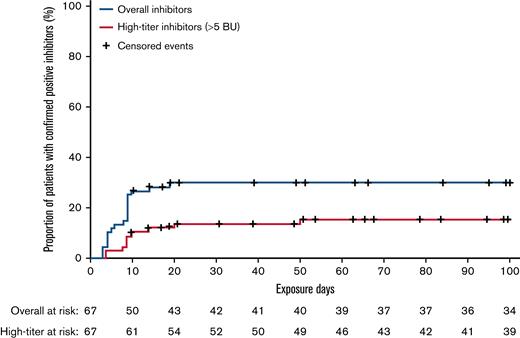
Of the 67 patients eligible for inhibitor analysis (patients with ≥10 EDs to N8-GP and all patients who developed inhibitors), 20 developed confirmed inhibitors, resulting in an overall incidence of 29.9% with a one-sided 97.5% upper confidence limit of 42.3. One case of inhibitors transitioned from low-titer to high-titer inhibitors during the study. Including this case, 10 of 20 inhibitor cases were high-titer (>5 BU), resulting in an incidence of 14.9% with a one-sided 97.5% upper confidence limit of 25.7. All instances of inhibitors occurred within the first 20 EDs to N8-GP (Figure 1). One-half of the inhibitor occurred during pre-prophylaxis and the other half during prophylaxis. Post hoc analysis showed that of the 12 inhibitor patients who had F8 genotyping, 9 (75%) had a high-risk mutation (supplemental Table 1). Furthermore, 4 of 9 patients with a family history of inhibitors developed an inhibitor.
Kaplan-Meier analysis of time to confirmed inhibitors and high-titer inhibitors in pathfinder6.
Kaplan-Meier analysis of time to confirmed inhibitors and high-titer inhibitors in pathfinder6.
Of the 20 patients who developed inhibitors, 7 initiated ITI therapy (4 patients had high-titer). Thus far, ITI was successful in 5 patients (median time to success, 1.15 years), ITI is ongoing in 1 patient, and 1 patient has been withdrawn from the trial. Further information on ITI regimens are presented in the supplemental Results. Of the 13 inhibitor patients who did not start ITI, 5 patients remained on regular N8-GP prophylaxis (in 4 patients the inhibitor resolved; 1 patient developed a high-titer inhibitor), and 8 patients were withdrawn from the trial.
Efficacy of N8-GP for prophylaxis and treatment of bleeding episodes
Sixty-five patients received N8-GP prophylaxis for an average of 2.17 years. During that period, 54 prophylaxis patients (83.1%) experienced 266 bleeding episodes. Most bleeds (80.8%) in patients on prophylaxis were traumatic, with nearly one-half (44.7%) affecting the skin. Overall, 97.0% of bleeding episodes were mild or moderate, with only 8 bleeds (3.0%) considered severe (supplemental Table 2). Across all treatments, 3 central nervous system bleeds were reported. During prophylaxis, patients had a median ABR (interquartile range [IQR]) of 1.42 (0.76; 3.13) compared with a median ABR of 6.63 (2.61; 13.04) during pre-prophylaxis (Table 2).
During N8-GP prophylaxis, patients had a 90.5% success rate for the treatment of bleeding episodes (82.7% on pre-prophylaxis). Furthermore, while on prophylaxis, 91.2% of bleeds were treated with ≤2 N8-GP injections (87.2% on pre-prophylaxis).
The hemostatic success in minor surgeries was 81.5%. The hemostatic responses for both major surgeries were recorded as “excellent” and “good,” resulting in a 100% hemostatic success rate.
Adverse events
A total of 689 AEs occurred in 75 (93.8%) of 80 patients exposed to N8-GP over a total of 166.3 patient-years in the trial (average of 4.1 AEs per patient-year). Seventy SAEs occurred in 45 patients (average of 0.4 SAEs per patient-year). The most frequent SAE was occurrence of an FVIII inhibitor (21 events in 21 patients). Two of these patients had only one positive measurement, with no subsequent confirmatory measurement and thus did not achieve the protocol definition of a confirmed inhibitor patient. Both patients also had an observation of temporarily decreased IR (discussed in the following text) and withdrew from the trial after 17 and 19 EDs, respectively. One event of a confirmed inhibitor was not reported as an SAE. Other SAEs that occurred in >1 patient were cerebral hemorrhage (2 events in 2 patients; in-depth information is provided in the supplemental Results), pyrexia (2 events in 2 patients), head injury (2 events in 2 patients), and positive anti-FVIII antibody (2 events in 2 patients [one with temporarily decreased IR and one with inhibitors]). No anaphylactic reactions or serious hypersensitivity events occurred. Only 3 SAEs occurred during ITI (all in 1 patient), and all were related to the port-a-cath (bacterial infection in relation to port, or port malfunction). No patients died, and there were no thromboembolic events (other than occlusion of venous access device).
Post hoc analysis of temporarily decreased IR
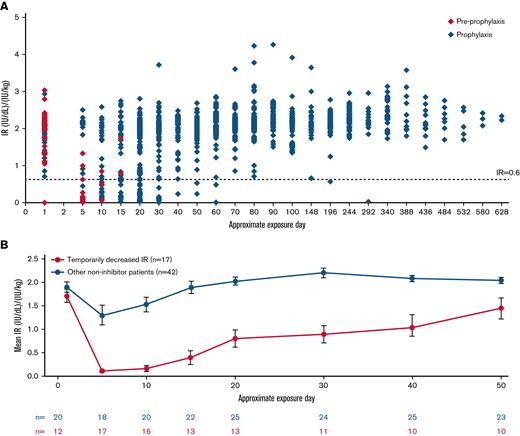
Temporarily decreased IR was observed in 17 patients (Figure 2). In all these patients, temporarily decreased IR occurred within the first 5 EDs to N8-GP. Four patients with temporarily decreased IR withdrew from the trial. In the remaining 13 patients, IR returned to the expected range with continued N8-GP dosing over time, in 9 of these patients by ED 30 from the start of N8-GP. Nine (75%) of 12 patients with an observation of temporarily decreased IR had an F8 gene mutation at high risk for inhibitor development (5 patients had missing data for F8 mutation) (supplemental Table 1).
IR at 30 minutes post–N8-GP dose in pathfinder6. (A) IR of all patients in pathfinder6. (B) Mean IRs of patients with temporarily decreased IR and all other non-inhibitor patients. IR at 30 minutes postdose was determined by chromogenic assay using a product-specific standard as calibrator. The EDs are approximated based on visit windowing and standard prophylaxis regimen. Temporarily decreased IR was defined as patients with an observation of 2 consecutive measurements of IR <0.6 (IU/dL)/(IU/kg) without inhibitor EDs.
IR at 30 minutes post–N8-GP dose in pathfinder6. (A) IR of all patients in pathfinder6. (B) Mean IRs of patients with temporarily decreased IR and all other non-inhibitor patients. IR at 30 minutes postdose was determined by chromogenic assay using a product-specific standard as calibrator. The EDs are approximated based on visit windowing and standard prophylaxis regimen. Temporarily decreased IR was defined as patients with an observation of 2 consecutive measurements of IR <0.6 (IU/dL)/(IU/kg) without inhibitor EDs.
All patients were on prophylaxis at some period during temporarily decreased IR (mean duration of 0.48 years). Two patients changed from pre-prophylaxis to prophylaxis during the time of temporarily decreased IR, two patients changed from an every 7 days regimen to a bi-weekly regimen, and one patient changed from a bi-weekly regimen to an every 3 days regimen. During time on prophylaxis, 14 (82.4%) of 17 patients experienced bleeding episodes, resulting in a median ABR (IQR) of 4.42 (1.17; 7.03) (Table 3). Bleeding episodes were mostly considered traumatic (71.1%), with 97.4% of bleeds occurring in the skin, joint, muscle, or mouth/nose/gums. Most bleeding episodes (86.8%) were of mild/moderate severity (supplemental Table 2). The estimated success rate for hemostatic response during the period of temporarily decreased IR was 83.2% (95% confidence interval, 71.5; 90.7). Furthermore, 7 minor surgeries were performed during the period of temporarily decreased IR, 5 of which were rated as successful (71.4%).
During the full analysis period, the 17 patients on N8-GP prophylaxis with temporarily decreased IR had a total mean observation time of 2.17 years and a median ABR (IQR) of 2.34 (1.23; 5.45). The 42 non-inhibitor patients with regular IR had a median ABR of 1.52 (0.80; 4.54) during the trial (Table 3).
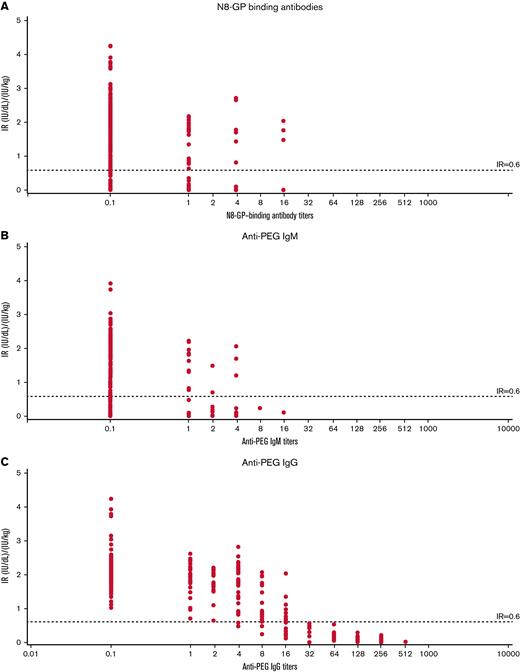
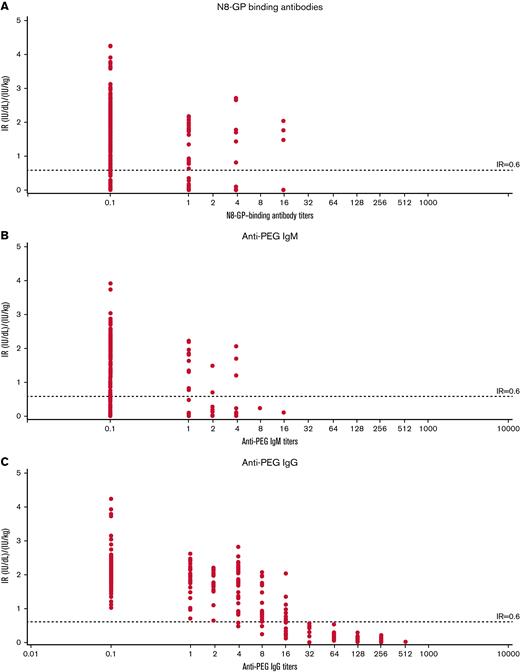
Transient anti–N8-GP, anti-PEG IgM, and anti-PEG IgG antibodies were observed during pathfinder6 (Figure 3). Compared with patients with regular IR, patients with temporarily decreased IR had markedly high titers of anti-PEG IgG with a strong temporal correlation with decreased IR. Analyses of the 17 patients with temporarily decreased IR showed that anti–N8-GP-binding, anti-PEG IgM, and anti-PEG IgG antibodies together explained 62.3% of the within-patient variance in IR. However, 58.4% of the within-patient variance was explained by anti-PEG IgG antibodies alone, whereas anti-PEG IgM and anti–N8-GP-binding antibodies explained only 10.3% and 14.2% of within-patient IR variance, respectively (Table 4).
IR of patients with temporarily decreased IR at 30 minutes vs antibody titer in pathfinder6. IR vs N8-GP binding (A), IR vs anti-PEG IgM (B), and IR vs anti-PEG IgG (C) antibody titers. IR at 30 minutes was determined by chromogenic assay using a product-specific standard as calibrator. Temporarily decreased IR was defined as patients with an observation of 2 consecutive measurements of IR <0.6 (IU/dL)/(IU/kg) without inhibitors.
IR of patients with temporarily decreased IR at 30 minutes vs antibody titer in pathfinder6. IR vs N8-GP binding (A), IR vs anti-PEG IgM (B), and IR vs anti-PEG IgG (C) antibody titers. IR at 30 minutes was determined by chromogenic assay using a product-specific standard as calibrator. Temporarily decreased IR was defined as patients with an observation of 2 consecutive measurements of IR <0.6 (IU/dL)/(IU/kg) without inhibitors.
Patient plasma containing various levels of anti-PEG IgG without inhibitors were tested for neutralizing capacity by spiking with FVIII or N8-GP and analyzed in a chromogenic FVIII activity assay. No inhibition of either N8-GP or FVIII was observed (supplemental Figure 3). In addition, N8-GP protein correlated with FVIII activity (data not shown), supporting a clearance mechanism in vivo rather than neutralization.
Overall, AEs during the period in which temporarily decreased IR was observed were generally mild and consistent with AEs experienced by the overall population. There were no reported events of serious hypersensitivity or hypersensitivity that were considered related to N8-GP. A single patient with temporarily decreased IR had an event of a generalized rash, classified as unlikely related to N8-GP.
Discussion
Although PUP studies are no longer required by authorities for approval of a new FVIII product, any replacement product used for the treatment of PUPs with hemophilia A should be examined for immunogenicity, safety, and efficacy in this population. This is the first report of a glycoPEGylated FVIII replacement product used for the treatment of PUPs with severe hemophilia A. Moreover, we are reporting a previously undescribed observation of temporarily decreased IR (associated with transient anti-PEG IgG) in a subset of patients shortly after initiating N8-GP.
In pathfinder6, 29.9% of PUPs exposed to N8-GP developed inhibitors and 14.9% developed high-titer inhibitors. Generally, 96% of inhibitor cases occur within the first 50 EDs of treatment12 and of the 67 patients in the inhibitor analysis, only 7 patients had <50 EDs. The pathfinder6 inhibitor incidence is in line with that of the only other extended half-life FVIII product to have published results in PUPs (recombinant FVIII Fc fusion protein; 31.1%).13 Furthermore, the inhibitor incidence was within the range of other recombinant FVIII products.(7,14-16) The incidence of high-titer inhibitor development (14.9%) in PUPs exposed to N8-GP was at the lower end of the expected range for recombinant products, similar to observations for plasma-derived products in the SIPPET (Survey of Inhibitors in Plasma-Product Exposed Toddlers) study.7,8,17 However, comparisons with other studies should be made with caution, as study designs vary (eg, real-world, retrospective, clinical trial), and some studies included minimally treated patients, patients with mild/moderate hemophilia A, or small populations.15,18 Of the 7 patients who received ITI during this trial, 5 completed ITI with N8-GP successfully, with ITI ongoing in 1 patient.
The median ABR for patients treated with prophylaxis in this trial (1.42) was similar to the median ABR seen in the main phase of the trial assessing N8-GP treatment in pediatric PTPs (1.95)2 and comparable to pediatric PTP studies on other extended half-life FVIII products.19,20 Furthermore, the hemostatic success rate in this trial for PUPs receiving prophylaxis was similar to the long-term hemostatic success rate in PTPs aged 0 to 5 years treated with N8-GP21 and in PUPs treated with other extended half-life FVIII molecules.13
During this trial, there were no reports of anaphylactic reactions or serious hypersensitivity events. The 2 events of intracranial bleeding were traumatic in nature. SAEs, other than FVIII inhibitors, were generally consistent with previously reported events after treatment of PTPs with N8-GP.3,4
There was a previously undescribed observation of temporarily decreased IR in the absence of inhibitors in 17 PUPs during this trial, occurring shortly after the first N8-GP exposure. These are the first observations of this kind during treatment of patients with N8-GP. Pediatric and adult PTPs largely maintained IR in the expected range from the first dose onward (unpublished data; pathfinder5 Clinical Trial Report S.D., #NCT01731600; pathfinder2, Clinical Trial Report S.D., #NCT01480180). Preexisting or anti-PEG antibodies developed during clinical trials in previously treated adults, adolescents, and children treated with N8-GP had no effect on FVIII activity and were not associated with AEs.2,4,22 This could be because PTPs may be tolerized to FVIII through several exposures. Furthermore, PEG is not presented by major histocompatibility complex II and is therefore unlikely to stimulate the T-cell response required to initiate a strong immune reaction toward either FVIII or PEG. PUPs, conversely, have not previously been exposed to FVIII and are therefore at risk of inducing a FVIII T-cell signal that can facilitate anti-FVIII or anti-PEG antibodies depending on the specificity of the available B cells.
In PUPs treated with N8-GP, temporarily decreased IR in the absence of FVIII inhibitors was chronologically associated with transient anti-PEG IgG antibodies. Temporarily decreased IR returned to the expected range with continued N8-GP dosing (by ED 15-30 from the start of N8-GP in 9 of 13 cases).
The association with anti-PEG IgG titers explained most within-patient variation in IR accounted for by combined anti–N8-GP-binding, anti-PEG IgM, and anti-PEG IgG. Because the first postdose visit was at 5 EDs, an initial anti-PEG IgM response may have occurred and faded by this time point. This contrasts with results seen in pediatric clinical trials of BAY94-9027, in which 12 PTPs discontinued due to a loss of efficacy within the first 4 EDs of exposure associated with hypersensitivity events and anti-PEG IgM.23
Research into PUPs and IR is limited, with few other single-product PUP studies measuring or reporting regular IR data, and none to the extent investigated in this study (ie, IR assessed at every study visit).14-16,18,24,25 Hence, knowledge on IR during the early stages of exposure of PUPs to FVIII products is scarce, and further research is needed to determine whether changes in IR occur in PUPs treated with other FVIII replacement products.
Antibodies, such as inhibitors, neutralize the activity of FVIII upon binding, impeding its interaction with the coagulation system. FVIII inhibitors also exert their inhibitory activity against FVIII in normal plasma, which is the basis for in vitro tests. However, other antibodies can cause increased clearance, an observation reported with other PEGylated therapeutic products.26 In this case, antibody binding causes decreased bioavailability of the antigen. Data from in vitro experiments indicate a reduction in IR potentially caused by accelerated blood clearance and removal of anti-PEG IgG/N8-GP complexes. In addition, FVIII activity was not decreased in patient samples spiked with N8-GP in our in vitro experiments, supporting a clearance mechanism rather than neutralization. Furthermore, patients who withdrew from pathfinder6 due to an observation of temporarily decreased IR, immediately had IRs within the expected range and had good clinical response when treated with another non-PEGylated FVIII replacement product (personal communication, C.M., August 2019). Given this evidence and the close correlation of anti-PEG IgG titers with decreased IR, we postulate that anti-PEG antibodies binding to N8-GP possibly caused rapid clearance from plasma.
During the time when patients had decreased IR, AEs were consistent with the overall trial safety results, and there were no anaphylactic reactions or serious hypersensitivity events. Furthermore, the proportion of bleeding episodes considered traumatic/spontaneous neared that of the overall population, and bleeding locations were similar. Patients also experienced a hemostatic success rate comparable with that observed in all patients during the trial. This hemostatic success rate is consistent with that reported in PTPs 0 to 5 years old treated with N8-GP prophylaxis in the main phase of the PTP pediatric trial.2 However, at 4.42, the median ABR during the time of temporarily decreased IR was higher than the median ABR for all patients during the trial (2.14). The median ABR during the period of temporarily decreased IR remained lower than the ABR of patients treated with pre-prophylaxis (6.63) over a similar mean treatment period (0.48 vs 0.47 year, respectively). Moreover, 71.4% of minor surgeries were rated as successful during the period of temporarily decreased IR. The 2 patients who did not experience success were later withdrawn from the trial because of suspicion of inhibitor development (one developed inhibitor after withdrawal, the other had a single episode of positive inhibitor measurement). The relatively short duration and the variability of bleeding frequency in small children does not allow for detection of meaningful differences in bleeding tendency during the period of temporarily decreased IR.
Assessed over the entire treatment duration, patients who had temporarily decreased IR had a somewhat higher ABR compared with the 42 non-inhibitor patients without an observation of temporarily decreased IR but within the range seen with prophylaxis regimens in pediatric PTP trials using other extended half-life FVIII products.19,20 Of the 4 patients with decreased IR who withdrew from the trial, 2 returned to normal IR on alternative FVIII replacement products, 1 later developed an inhibitor, and 1 is unknown (personal communications, C.M. and G.Y., August 2019). Of the 16 known patients with temporarily decreased IR, only 1 later developed an inhibitor (6.3%). Thus, temporarily decreased IR did not seem to be associated with inhibitor risk or any lasting clinical impact for the patients. In this study, all observations of temporarily decreased IR occurred early during the course of treatment (within 5 EDs to N8-GP). Thus, monitoring the IR of PUPs treated with N8-GP is recommended during this time to ensure optimal patient care.
There were several limitations to the current study. The pathfinder6 trial is ongoing. The inhibitor incidence is not expected to change significantly. However, caution should be taken when interpreting the exact rate of inhibitor development. Furthermore, 2 patients withdrew from this study without confirmatory inhibitor test results. Moreover, all analyses involving the group of patients with temporarily decreased IR were performed post hoc and in a relatively small subgroup. Thus, caution should be taken when comparing the efficacy of N8-GP in these patients vs those of other subgroups.
In conclusion, the incidence of inhibitors in PUPs treated with N8-GP was within the expected range, and the incidence of high-titer inhibitors was within the lower expected range. The use of N8-GP was efficacious in PUPs for both the prevention and treatment of bleeding episodes. Close monitoring of IR in patients led to the previously undescribed observation of temporarily decreased IR in a subset of PUPs without inhibitors shortly after treatment initiation that was associated with anti-PEG IgG antibody titers. There were no other unexpected AEs during this trial. During the period of temporarily decreased IR, no related hypersensitivity events occurred. IR returned to the expected range in all patients who continued N8-GP treatment, having little to no clinical impact and no lasting clinical effects.
Acknowledgments
The authors thank all patients and the families of patients who participated in pathfinder6.
This trial was sponsored by Novo Nordisk A/S. Medical writing for this manuscript was provided by Ashfield MedComms GmbH, funded by Novo Nordisk A/S.
Authorship
Contribution: All authors analyzed and interpreted the data in pathfinder6. All authors participated in the writing of this manuscript and approved the final version.
Conflict-of-interest disclosure: C.M. reports consultancy or speaker for Bayer, Biotest, CSL Behring, Grifols, Novo Nordisk, Roche, and Takeda; travel support from Bayer, Biotest, CSL Behring, and Novo Nordisk; and institutional research support from Bayer, Baxalta/Shire/Takeda, Biotest, CSL Behring, Novo Nordisk, and SOBI. G.K. reports grant and research support from Alnylam, Bayer, BPL, OPKO Biologics, Pfizer, Roche, and Takeda; and advisory boards and honoraria for consultancy/lectures from Alnylam, Bayer, BioMarin, CSL, Novo Nordisk, OPKO Biologics, Pfizer, Takeda, Roche, Sanofi, and uniQure. C.K. reports speaker or advisory boards for Bayer, Biotest, CSL Behring, Novo Nordisk, Roche/Chugai, Sanofi/Sobi, and Takeda; and institutional research support from Bayer, Biotest, CSL Behring, Intersero, Novo Nordisk, Pfizer, and Sanofi/Sobi. T.M. reports advisory boards for Baxalta/Shire/Takeda, Bayer, Novo Nordisk, Chugai, and Pfizer; received educational and investigational support from Chugai and Novo Nordisk; and received honoraria from Shire/Takeda, Bayer, Bioverativ/Sanofi, Chugai, CSL, JB: Japan Blood Products Organization, KM Biologics Co., Kirin, Nichiyaku, Novo Nordisk, Octapharma, and Sysmex. G.Y. has received consulting fees from ApcinteX, BioMarin, Genentech/Roche, Grifols, Novo Nordisk, Pfizer, Rani, Sanofi Genzyme, Spark, Takeda, and UniQure; and funds for research support from Genentech/Roche, Grifols, and Takeda. S.D., A.H.M., and M.Z. are employees of Novo Nordisk.
Correspondence: Christoph Male, Department of Paediatrics, Medical University of Vienna Waehringer Guertel 18-20 A-1090 Vienna, Austria; e-mail: christoph.male@meduniwien.ac.at.
References
Author notes
Subject-level analysis data sets are available from the corresponding author on reasonable request (e-mail: christoph.male@meduniwien.ac.at).
The full-text version of this article contains a data supplement.