Key Points
Treatment with pembrolizumab after alloHCT is feasible but can be associated with severe immune-related adverse events.
Treatment efficacy was observed in the post-alloHCT setting in lymphomas harboring PD-L1 gene alterations; however, no responses were observed in myeloid malignancies.
Abstract
A failed graft-versus-tumor (GVT) effect is a common mechanism of relapse after allogeneic hematopoietic cell transplantation (alloHCT). Although targeting the PD-1/PD-L1 axis may restore GVT effects, PD-1 blockade exacerbates graft-versus-host disease (GVHD) in murine models, and severe GVHD can occur in patients treated with anti-PD-1 therapy after alloHCT. Therefore, we developed a prospective study to assess the safety and efficacy of pembrolizumab in patients relapsing after alloHCT. Eligible patients received pembrolizumab (200 mg every 3 weeks) for up to 2 years. Twelve patients were enrolled (8 patients with acute myeloid leukemia, 1 patient with myelodysplastic syndrome, 1 patient with classical Hodgkin lymphoma, and 2 patients with diffuse large B-cell lymphoma [DLBCL]). All participants received reduced-intensity preparative regimens with in vivo T-cell depletion. The median time from alloHCT to enrollment was 587 days (range, 101-4211). Three participants (25%) experienced grade 3 to 4 immune-related adverse events (irAE) (pneumonitis, 2 patients; hyperthyroidism, 1 patient), all occurring after 1 to 2 cycles, and resolving after pembrolizumab discontinuation and corticosteroid treatment. irAEs of any grade occurred in 5 patients (42%). No treatment-emergent GVHD was observed. Overall and complete response (CR) rates were 22% (2/9). Both patients achieving CRs had PD-L1 gene–amplified lymphomas and diffuse PD-L1 expression on pretreatment biopsies. An acquired EZH2 mutation was identified at relapse in a patient with DLBCL who achieved an initial CR to pembrolizumab, which was associated with downregulated HLA expression on malignant B cells, implicating EZH2 mutations as a potential immune escape mechanism after PD-1–blockade therapy. In conclusion, after alloHCT, treatment with pembrolizumab is feasible and associated with objective responses in relapsed lymphoid malignancies but can induce severe irAEs, requiring vigilant monitoring. This trial was registered at www.clinicaltrials.gov as #NCT02981914.
Introduction
Allogeneic hematopoietic cell transplantation (alloHCT) represents a potentially curative treatment option for a variety of hematologic malignancies.1-4 Unfortunately, more than one-third of patients will experience disease relapse after alloHCT, and treatment options are very limited in this setting. In fact, most patients who relapse after alloHCT will die within a year, and disease relapse represents the most common cause of death among alloHCT recipients.5 The development of effective therapies for hematologic malignancies relapsing after alloHCT is therefore an area of significant clinical unmet need.
A major mechanism contributing to disease relapse after alloHCT is a failed graft-versus-tumor (GVT) effect.6 Interestingly, preclinical studies have demonstrated that therapeutic blockade of immune checkpoints, such as PD-1, can potently augment GVT responses, and retrospective analyses have reported that anti-PD-1 therapies induce high clinical response rates in patients with classical Hodgkin lymphoma (cHL) relapsing after alloHCT.7-11 Despite this, post-alloHCT PD-1 blockade therapy has also been associated with severe graft-versus-host disease (GVHD) in murine models,12 and serious and sometimes fatal GVHD has been observed in patients treated with anti-PD-1 therapy after alloHCT.13-15 Indeed, GVHD was observed in 30% to 55% of patients with lymphoma, treated with PD-1 blockade after alloHCT in 2 separate retrospective analyses and was fatal in 33% to 47% of the cases.7,9 These observations have led to a Food and Drug Administration package insert warning regarding the administration of anti-PD-1 antibodies after alloHCT and have catalyzed the development of prospective studies aimed at more accurately assessing the safety profile of PD-1 blockade in this setting. To date, one such phase 1 clinical trial of PD-1 blockade in the post-alloHCT relapse setting has been published. In that study, the safety and efficacy of a reduced dose of nivolumab was evaluated, demonstrating an overall response rate (ORR) of 32%, including durable responses in some patients with lymphoid malignancies, but a relatively high incidence of new or worsening GVHD was seen, including 2 fatal cases of acute GVHD of the liver and gut.14
Based on these findings, it is clear that significant immune-related toxicities may occur when PD-1 blockade is administered after alloHCT. Reliable predictive biomarkers of response and toxicity are needed to mitigate risk and identify patients most likely to benefit from checkpoint blockade therapies in this context. Moreover, whether standard doses of anti-PD-1 antibodies will yield enhanced clinical activity (or increased toxicity) as was observed with post-alloHCT CTLA-4 blockade therapy,16,17 is not known. The latter will be particularly important to guide off-label dosing recommendations for physicians considering the use of anti-PD-1 antibodies for relapse in patients after alloHCT. Here, we report the results of a pilot study evaluating the safety and preliminary efficacy of the standard dose of pembrolizumab for the treatment of hematologic malignancies relapsing after alloHCT.
Methods
Patient eligibility
Adults with mature B-cell lymphoma, acute myeloid leukemia (AML), or myelodysplastic syndrome (MDS) with histologically confirmed disease relapse after alloHCT were eligible. Participants were required to be removed for >90 days from alloHCT at the time of enrollment and had to be off all immunosuppressive medications for a minimum of 2 weeks. Patients could not have a history of grade 3 or 4 acute GVHD or any stage of chronic GVHD. All patients needed to have adequate organ function as outlined in the supplemental Appendix protocol and an Eastern Cooperative Oncology Group performance status of 0 or 1. Participants with malignant central nervous system involvement, HIV infection, noninfectious pneumonitis or colitis, autoimmune disorders requiring systemic treatment in the past 2 years, or donor lymphocyte infusion within 8 weeks were also excluded. Although not required for study enrollment, all patients who were treated in this study received in vivo T-cell depletion as part of GVHD prophylaxis in accordance with the standard institutional guidelines at the University of Chicago. A full list of inclusion and exclusion criteria can be found in the accompanying protocol.
Study objectives
The primary objective of the study was to determine the tolerability of pembrolizumab in patients with relapsed hematologic malignancies after alloHCT. Secondary objectives included assessment of response rate, duration of response, and overall survival after receiving treatment with pembrolizumab.
Study design and treatment
All eligible patients enrolled in the study were treated with a fixed dose of pembrolizumab (200 mg IV every 3 weeks for up to 2 years), provided that neither disease progression nor the development of a dose-limiting toxicity (DLT) occurred. DLT was defined as the development of grade 3 or 4 acute GVHD (defined by the Gluckenberg scale18), graft rejection, the development of any unexpected grade ≥3 toxicity felt to be related to pembrolizumab, or the development of >grade 2 dysfunction of a vital organ felt to be secondary to an immune-related adverse event (irAE) within 90 days of the initiation of pembrolizumab. For irAEs that did not meet the criteria for a DLT but did meet the criteria for treatment-limiting toxicity, for example, grade 2 acute GVHD, administration of pembrolizumab was held until the toxicity decreased to grade ≤1, and the participants were on a dosage of ≤10 mg of prednisone equivalent per day. Pembrolizumab was permanently discontinued if delayed by >12 weeks for all non-DLTs. For patients who developed GVHD or an irAE while on pembrolizumab, corticosteroid therapy and potentially other immunomodulatory agents were initiated per guidance from the clinical protocol as deemed necessary by the treating physician.
Adverse events were monitored every 3 weeks throughout the trial and graded in severity according to the guidelines outlined in the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Disease response assessment was performed in accordance with protocol-driven, disease-specific guidelines. It was conducted every 3 months and at any time relapse was suspected. All patients who received at least 1 dose of pembrolizumab were evaluable for toxicity assessment. All safety and efficacy analyses were performed using descriptive statistics.
The study was approved by the University of Chicago Institutional Review Board and conducted in accordance with the Good Clinical Practices guidelines. All participants provided written informed consent. All the authors had access to the primary clinical trial data, and data analysis was performed by J.G. and T.K. This trial was registered at www.clinicaltrials.gov as #NCT02981914.
Correlative analyses
PD-L1 fluorescent in situ hybridization (FISH) was performed as previously described.19 FISH probes targeted PD-L1 (CD274, Empire Genomics), centromere 9 (CEP9, Abbott), and a region centromeric to PD-L2 (RP11-610G2, Empire Genomics) to assess PD-L1 translocations.
HLA-I (EMR8-5, Abcam), HLA-II (EPR11226, Abcam), and Pax5 (1H9, BD Biosciences) protein expression was assessed on archived formalin-fixed paraffin-embedded (FFPE) tissue samples using multiparameter immunofluorescence imaging. The extent of CD4 (SP35, Sigma-Aldrich) and CD8 (SP16, Sigma-Aldrich) T-cell infiltration of lymphoma specimens was determined using immunohistochemistry (IHC).
Whole exome sequencing was performed as previously discussed.19 Briefly, DNA was extracted from FFPE biopsy specimens using the AllPrep DNA/RNA FFPE kit (Qiagen). Paired germline DNA was obtained from peripheral blood using the DNeasy Blood/Tissue kit (Qiagen). Whole exome and untranslated regions were captured using the Agilent SureSelect All Exon V6 plus UTR kit, and 101 bp paired-end reads were generated using Illumina HiSeq2500 at Theragen Etex Bio Institute. FASTQ files were aligned to GRCh38 using Burrows-Wheeler Aligner–MEM (version 0.7.17) and quality assessed using FastQC (version 0.11.5). Duplicates were marked and subsequent Base Quality Score Recalibration was performed with Genome Analysis Toolkit 4.1.3.0. Mutect2 through Genome Analysis Toolkit was then used to detect somatic variants, using a panel of normals generated from the 1000 Genomes Project. Variants that passed Mutect2 filters were annotated using Variant Effect Predictor (version 102) and the resulting vCard files were converted to mutation annotation format files using vcf2maf. Only the variants predicted to be deleterious and published in a previous list of diffuse large B-cell lymphoma (DLBCL) driver genes20 were considered for downstream analysis.
Results
Patient characteristics
Twelve participants were enrolled. Patient characteristics are provided in Table 1. Eight participants had AML, 2 had DLBCL, and 1 each had MDS and cHL. The median age was 54 years (range, 27-62 years), and the median number of preceding treatments excluding alloHCT was 4 (range, 1-9). Seven patients (58%) received alloHCT from matched related donors, and 5 (42%) received transplants from haploidentical-umbilical cord blood donors. All participants received reduced-intensity chemotherapy preparative regimens comprising fludarabine and melphalan. GVHD prophylaxis comprised alemtuzumab and tacrolimus for matched related donor transplants, and antithymocyte globulin, tacrolimus, and mycophenolate mofetil for haploidentical-umbilical cord blood transplants. Five participants (42%) had a history of grade 1 to 2 acute GVHD, and by design, none were receiving active immune suppression for GVHD at the time of study enrollment. The median time-off treatment for GVHD was 275 days (range, 28-788 days) in the patients with a prior history of GVHD. The median time from alloHCT to enrollment was 587 days (range, 101-4211 days).
Safety
All 12 patients received at least 1 cycle of treatment with pembrolizumab and were assessable for toxicity. A median of 3.5 cycles of pembrolizumab (range, 1-17 cycles) were administered, and at the time of data cutoff, all participants were off treatment. Adverse events observed among study participants are listed in Table 2. Overall, 3 participants (25%) experienced a DLT. These included 2 participants with grade 3 to 4 pneumonitis and 1 participant with grade 3 hyperthyroidism. All DLTs occurred after 1 to 2 cycles of pembrolizumab and resolved after the discontinuation of pembrolizumab and with treatment with corticosteroids. The 2 cases of pneumonitis started 7 and 15 days after receiving pembrolizumab. Both participants were hospitalized for hypoxia (low-flow oxygen in a participant and high-flow oxygen in the other) and had computed tomography (CT) scans with patchy infiltrates concerning for drug-induced pneumonitis. A CT-guided lung biopsy confirmed the diagnosis in 1 of the cases. Bronchoscopy with bronchoalveolar lavage was negative for infectious studies including pneumocystis for both patients. Each participant was started on broad-spectrum antibiotics and empiric steroids at a dose of approximately 1 mg/kg prednisone equivalent. Hypoxia resolved after 2 weeks of treatment in both participants, and steroids were successfully weaned off over 6 to 12 weeks.
Other grade 3 to 4 adverse events included fatigue (8%), fever (17%), hemolytic anemia (8%), and idiopathic thrombocytopenic purpura (8%). There was also a case of therapy-related MDS (t-MDS), not considered related to study treatment. No treatment-emergent GVHD was observed, but 5 participants developed irAEs of any grade. Reasons for the discontinuation of pembrolizumab included DLT in 3 participants, non-DLT toxicity (nausea and vomiting) in 1 participant, t-MDS in 1 participant, and disease progression in 7 participants.
Efficacy and immune correlatives
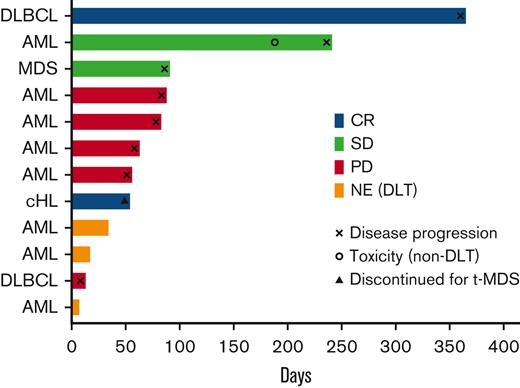
Nine participants were assessable for efficacy end points. The ORR was 22% (2/9). The best overall responses included complete response (CR) in 2 participants (DLBCL and cHL), stable disease in 2 participants, and progressive disease in 5 participants. No objective responses were observed in participants with myeloid malignancies, with 2 participants achieving stable disease (1 AML and 1 MDS). Three participants were not evaluable for response due to the development of early DLT. After a median follow-up of 4.4 years, a total of 9 participants died. The median duration of response, progression-free survival, and overall survival was not estimable, 2.9 months, and 23.3 months, respectively. The most common cause of death was progressive disease (6 participants). One participant died from infection (unrelated to pembrolizumab), 1 from GVHD after a second alloHCT, and 1 from t-MDS (cHL patient with an ongoing CR of the lymphoma at the time of death). No participant remained on treatment. Response characteristics are shown in Figure 1. Interestingly, both participants achieving CR to pembrolizumab had PD-L1 gene–amplified lymphomas and strong PD-L1 protein expression on lymphoma cells in pretreatment biopsies (Figure 2). Peripheral blood CD3+ donor chimerism before treatment and at the time of response for the 2 patients achieving CR were 100% at both time points for the participant with DLBCL and 37% and 17% for the participant with cHL.
Response information. Swimmer plot demonstrating response information for patients in the study. CR, complete response; NE, not evaluable; PD, progressive disease; SD, stable disease.
Response information. Swimmer plot demonstrating response information for patients in the study. CR, complete response; NE, not evaluable; PD, progressive disease; SD, stable disease.
PD-L1 FISH and PD-L1 IHC in a patient with response to pembrolizumab. (A) Representative PD-L1 FISH demonstrating a PD-L1 gene amplification in a patient with DLBCL. PD-L1 and centromere 9 FISH probes are shown in orange and blue, respectively. (B) Representative PD-L1 IHC demonstrating intense PD-L1 protein expression on tumor cells from the corresponding case in panel A.
PD-L1 FISH and PD-L1 IHC in a patient with response to pembrolizumab. (A) Representative PD-L1 FISH demonstrating a PD-L1 gene amplification in a patient with DLBCL. PD-L1 and centromere 9 FISH probes are shown in orange and blue, respectively. (B) Representative PD-L1 IHC demonstrating intense PD-L1 protein expression on tumor cells from the corresponding case in panel A.
The participant with DLBCL, who achieved CR, relapsed 1 year after pembrolizumab therapy was initiated. We performed whole exome sequencing on this participant’s pre-pembrolizumab and post-pembrolizumab biopsies to identify a potential genetic basis for acquired pembrolizumab resistance. Canonical lymphoma driver mutations that were present before pembrolizumab treatment included TET2, GNAS, and XPO1 among others (Figure 3A). The mutational landscape of the lymphoma shifted at the time of progression of pembrolizumab treatment, with the acquisition of several new mutations involving SETD1B, TP53, and EZH2 (Figure 3A-B). Of these, we were particularly interested in the missense EZH2E317K mutation as a potential mediator of pembrolizumab resistance. EZH2 is a proto-oncogene and master regulator of the germinal center response, and activating EZH2 mutations promote malignant B-cell transformation through aberrant histone methyltransferase activity and repression of normal B-cell differentiation.21 Activating EZH2 missense mutations can also result in epigenetic downregulation of major histocompatibility complex (MHC) expression, leading to a cold or noninflamed DLBCL microenvironment.22 We, therefore, speculated that the acquired EZH2 mutation may have resulted in pembrolizumab resistance through downregulation of MHC expression and, thus, diminished presentation of lymphoma antigens. Multiparameter immunofluorescence analysis was, therefore, performed on pre-pembrolizumab and post-pembrolizumab treatment biopsies from this participant to measure HLA class I and II expression (Figure 3C). Consistent with prior studies,22 we identified significant downregulation of HLA-I on Pax5+ malignant B cells at the time of relapse. HLA-II expression was also decreased on tumor cells, but this was apparent at both time points. No other alterations in canonical genes involved in HLA expression were identified at the time of progression (B2M, CIITA, CREBBP, NLRC5, HLA, etc) (Figure 3A). The biopsy taken at the point of failure of pembrolizumab treatment demonstrated marked CD8+ T-cell infiltration with a sparse CD4+ T-cell infiltration (Figure 3D). Thus, based on these data, we speculate that despite robust tumor infiltration by CD8+ T cells, the lymphoma ultimately progressed on pembrolizumab therapy as a consequence of EZH2-mediated epigenetic downregulation of HLA-I expression and decreased recognition of lymphoma antigens by CD8+ T cells.
Mutational profile and HLA tumor cell expression before pembrolizumab treatment and at disease relapse following an initial CR in a participant with DLBCL. (A) Comparison of the tumor mutational profile of a DLBCL tumor before pembrolizumab and at the time of relapse after a 1-year CR. (B) Line plot demonstrating changes in variant allele frequency of lymphoma driver mutations before pembrolizumab treatment and at the time of acquired resistance to pembrolizumab. (C) Multiparameter immunofluorescence staining revealing decreased HLA-I expression on Pax5+ tumor cells at the time of pembrolizumab relapse compared with baseline in the participant with DLBCL, with an acquired EZH2 mutation at disease progression. (D) Degree of tumor infiltration by CD4+ and CD8+ T cells at the time of pembrolizumab progression as assessed by IHC.
Mutational profile and HLA tumor cell expression before pembrolizumab treatment and at disease relapse following an initial CR in a participant with DLBCL. (A) Comparison of the tumor mutational profile of a DLBCL tumor before pembrolizumab and at the time of relapse after a 1-year CR. (B) Line plot demonstrating changes in variant allele frequency of lymphoma driver mutations before pembrolizumab treatment and at the time of acquired resistance to pembrolizumab. (C) Multiparameter immunofluorescence staining revealing decreased HLA-I expression on Pax5+ tumor cells at the time of pembrolizumab relapse compared with baseline in the participant with DLBCL, with an acquired EZH2 mutation at disease progression. (D) Degree of tumor infiltration by CD4+ and CD8+ T cells at the time of pembrolizumab progression as assessed by IHC.
Discussion
Treatment of relapsed hematologic malignancies after alloHCT is associated with extremely poor outcomes and remains an area of unmet clinical need.5 Here, we demonstrated that PD-1 blockade with pembrolizumab is feasible and can elicit objective responses in participants with hematologic malignancies relapsing after alloHCT. However, IrAEs, including severe toxicities, were observed in 42% of participants treated with pembrolizumab. These findings are consistent with a recent phase 1 study that evaluated low-dose nivolumab in a similar context.14 In that study, the ORR was 32%, but irAEs were seen in 35% of the participants, including 2 grade 5 toxicities. Similarly, a phase 1 trial investigating the anti–CTLA-4 antibody, ipilimumab, reported a 32% ORR, but irAEs (including severe toxicities) were seen in 21% of the participants when ipilimumab was administered after alloHCT.16
Although irAEs occurred commonly in each of these 3 prospective studies evaluating checkpoint blockade therapy in the post-alloHCT setting, there has been variability in the reported incidence of new or worsening GVHD. For example, we observed no new GVHD cases and no exacerbations of GVHD after the administration of pembrolizumab. Moreover, ipilimumab was associated with only a 14% incidence of GVHD in the post-alloHCT setting.16 In contrast, low-dose nivolumab given in the post-alloHCT setting was associated with new or worsening GVHD in 39% of the participants, including 2 fatal cases of acute GVHD of the liver and gut.14 Potential explanations for these discrepancies could relate to differences in study inclusion criteria, as participants with a history of chronic GVHD were excluded from our study but were eligible for the other 2. In addition, all patients enrolled in our study had in vivo T-cell depletion as part of GVHD prophylaxis, which may have mitigated the risk of developing classical GVHD after PD-1 blockade, as has been suggested in retrospective analyses.7,9,23 However, all 3 studies required participants to be at least 3 to 6 months removed from alloHCT, off immune suppression for >2 weeks with no active GVHD, and to have no history of grade 3 to 4 acute GVHD before enrollment. Thus, physicians should proceed with extreme caution when considering off-label use of PD-1 blockade therapy for patients not meeting these criteria, particularly as a history of acute GVHD and a shorter time from alloHCT to administration of checkpoint blockade therapy were previously identified as potential risk factors for developing immune-related toxicities from PD-1 blockade in retrospective series.7,9,23
A consistent finding across prospective and retrospective series evaluating PD-1 blockade therapy in the post-alloHCT setting is that when immune-related toxicities occur, they almost invariably do so within the first 1 to 2 cycles of treatment. Therefore, early vigilant monitoring is warranted in patients receiving off-label treatment. We would also recommend considering immediate cessation of anti-PD-1 therapy and early initiation of corticosteroids (and potentially other immunosuppressant medications) for any patient developing irAEs given the severe and refractory toxicities that have been reported.7,9,13,14 Indeed, in our clinical trial, all participants had resolution of grade 3 to 4 irAEs with prompt discontinuation of pembrolizumab and treatment with a corticosteroid.
Overall, the results from this and other studies suggest that PD-1 blockade therapy is associated with modest clinical activity but severe and potentially life-threatening toxicities when used after alloHCT. Thus, future efforts should be focused on identifying biomarkers and other strategies that favorably modulate the risk-benefit ratio of treatment. One potential strategy to mitigate risk is to administer lower doses of treatment. To this effect, a recent phase 1 study of nivolumab identified a reduced dose of 0.5 mg/kg every 2 weeks as the maximum-tolerated dose in the post-alloHCT setting (the standard dose is 3 mg/kg every 2 weeks or a flat dose of 240 mg or 480 mg every 2 or 4 weeks, respectively).14 However, a reduced dose of nivolumab was still associated with a high rate of irAEs (35%) and new or worsening GVHD (39%). Moreover, the administration of a reduced dose of treatment raises the theoretical concern of decreased efficacy as was observed with post-alloHCT CTLA-4 blockade.16,17 Moving forward, it might be most appropriate to risk stratify patients by assessing a combination of clinical factors. In support of this notion, various clinical parameters have been implicated with a risk of post-alloHCT anti-PD-1 therapy toxicity, including early administration of treatment after alloHCT, a history of GVHD, and use of GVHD prophylaxis regimens that lack in vivo T-cell depletion or post-transplant cyclophosphamide.23 Regarding biomarkers of response, previous retrospective and prospective studies of anti-PD-1 therapy after alloHCT have demonstrated high response rates in hematologic malignancies known to be responsive outside of the alloHCT setting (namely cHL and primary mediastinal lymphomas), with evidently lower activity in myeloid malignancies.7-9,14 We similarly observed that objective responses were limited to participants with lymphoma, and in particular, those with high PD-L1 protein expression and PD-L1 gene alterations. This suggests that PD-L1 protein expression and/or PD-L1 gene alterations could also be incorporated into clinical decision making when weighing the risks and benefits of PD-1 blockade treatment after alloHCT. Moreover, the low response rates to treatment with pembrolizumab after alloHCT in myeloid malignancies suggest that further investigation of PD-1 blockade in this setting is unwarranted given the potentially severe toxicity.
Finally, an interesting observation from our study was the acquisition of a putative activating EZH2 mutation at the time of relapse in a participant with DLBCL achieving an initial CR to pembrolizumab. EZH2 is the functional enzymatic component of the polycomb repressive complex 2 and an important epigenetic regulator of oncogenic pathways in DLBCL and other lymphomas.24 Activating EZH2 mutations have been associated with reduced MHC expression on tumor cells from primary DLBCL samples and decreased T-cell tumor infiltration in preclinical DLBCL models.22 We similarly observed diminished HLA expression in a participant with DLBCL at the time of pembrolizumab relapse and concomitant acquisition of an EZH2 mutation. These data therefore implicate EZH2 mutations as a putative mechanism of acquired resistance to the administration of pembrolizumab after alloHCT, potentially resulting from epigenetic downregulation of tumor-antigen presentation. This would be consistent with a previous AML study that similarly observed epigenetic downregulation of MHC expression on leukemia cells at the time of relapse after alloHCT.25 It is interesting to speculate that augmented GVT effects invoked by pembrolizumab in this participant with DLBCL induced selective pressure favoring the acquisition and outgrowth of EZH2-mutant lymphoma cells more equipped to withstand GVT targeting due to reduced HLA expression. Moving forward, combining pembrolizumab with pharmacologic EZH2 inhibitors may therefore represent a rational approach to improve the efficacy of pembrolizumab because EZH2 inhibitors can enhance MHC expression in DLBCL cell lines and may prevent relapse resulting from epigenetic MHC downregulation as was suspected in our patient.22,26 However, it is important to recognize that these findings are correlative and that definitive experiments are needed to demonstrate a potential causal relationship between this participant’s EZH2 mutation, decreased HLA expression, and acquired resistance to pembrolizumab.
We also acknowledge that there are limitations to this study. For example, the study comprised a small cohort of participants with a variety of hematologic malignancies, which may limit the broad generalization of our findings. We would for instance caution the extrapolation of our results to patients treated with T-cell-replete alloHCT because all patients in this study received in vivo T-cell depletion as part of GVHD prophylaxis.
In conclusion, treatment with pembrolizumab is feasible and associated with objective responses in lymphoid malignancies relapsing after alloHCT but is associated with a high incidence of irAEs in this setting. Future efforts should be focused on identifying strategies that limit the risk of developing irAEs and developing biomarkers that tailor treatment to the patients most likely to derive clinical benefit.
Acknowledgments
The authors thank the patients and their families who participated in the clinical trial.
This study was supported in part by a research grant from Investigator-Initiated Studies Program of Merck Sharp & Dohme LLC. The opinions expressed in this article are those of the authors and do not necessarily represent those of Merck Sharp & Dohme LLC.
Authorship
Contribution: J.G. and J.K. designed the study, wrote the manuscript, and enrolled patients onto the study; M.T. contributed to the design of the study and reviewed the manuscript; T.K. performed data analysis and reviewed the manuscript; H.L., E.C., A.A., P.A.R., W.S., S.M.S., and M.R.B. enrolled patients onto the study and edited the manuscript; J.Y. and A.C. performed the genomic analyses for the study; G.V. completed correlative IHC analyses; and C.F. performed and analyzed PD-L1FISH testing.
Conflict-of-interest disclosure: S.M.S. has served as a consultant for MorphoSys/Incyte, Janssen, Bristol Myers Squibb (BMS), Karyopharm, TGTX, and Celgene and has received research funding from FortySeven, TG Therapeutics, Pharmacyclics, Acerta, Karyopharm, Portola, Celgene, Novartis, Genentech/Roche, and Epizyme. M.R.B. receives research support from Kite/Gilead, Novartis, Arcellx, and CRISPR Therapeutics; has served on advisory boards for Kite/Gilead, Novartis, Arcellx, CRISPR Therapeutics, Autolus, Juno, and Celgene; and serves on speaker bureaus for BMS, Incyte, Sanofi, and Kite/Gilead. P.A.R. receives research support from Kite/Gilead, Novartis, Celgene/BMS, MorphoSys, and Calibr; has served on advisory boards or provided consulting for Bayer, Novartis, Kite/Gilead, Karyopharm, Verastem, and Celgene/BMS; and serves on speaker bureaus for Bayer and Kite/Gilead. H.L. has served on the advisory board of Agios, Pfizer, CTI Biopharm, and Nkarta; provided consulting services to BeiGene and NGM Biopharma; and received research support from Karyopharm and BMS. J.K. receives research support from Merck, Verastem, and iTeos; has served on a speakers bureau for Kite/Gilead; and has served on advisory boards for Verastem, Seattle Genetics, MorphoSys, and Karyopharm. The remaining authors declare no competing financial interests.
Correspondence: Justin Kline, University of Chicago, 900 East 57th St, Chicago, IL 60637; e-mail: jkline@medicine.bsd.uchicago.edu.
References
Author notes
The protocol will be attached as a supplementary document. All other data can be requested by contacting the corresponding author, Justin Kline (jkline@medicine.bsd.uchicago.edu).
The full-text version of this article contains a data supplement.