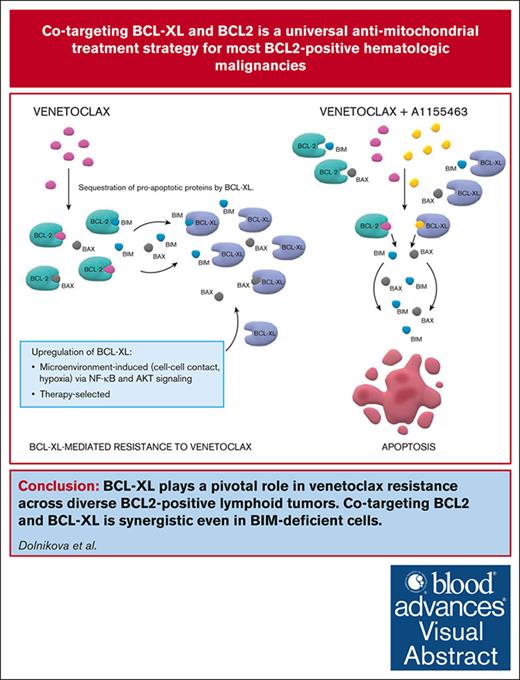
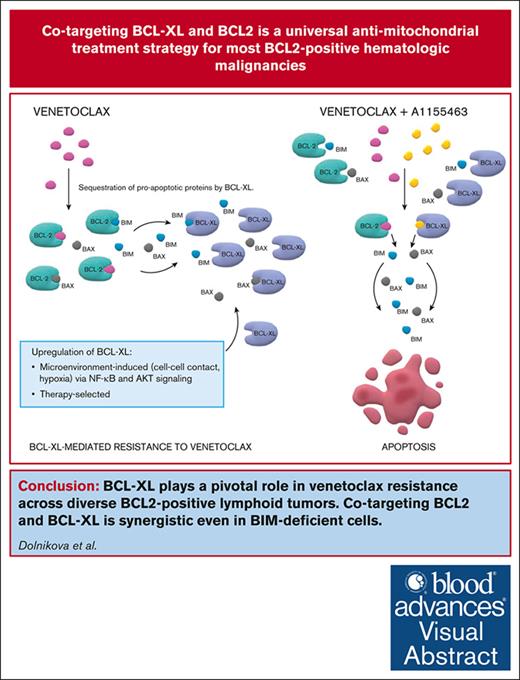
Key Points
BCL-XL belongs to key factors responsible for inherent and acquired resistance to VEN across diverse BCL2+ lymphoid neoplasms.
Interrupted targeting of BCL-XL with A1155463 induces synergy with VEN even in BIM-deficient cells and diminishes platelet toxicity.
Visual Abstract
Venetoclax (VEN), a B-cell lymphoma 2 (BCL2) inhibitor, has a promising single-agent activity in mantle cell lymphoma (MCL), acute lymphoblastic leukemia (ALL), and large BCLs, but remissions were generally short, which call for rational drug combinations. Using a panel of 21 lymphoma and leukemia cell lines and 28 primary samples, we demonstrated strong synergy between VEN and A1155463, a BCL-XL inhibitor. Immunoprecipitation experiments and studies on clones with knockout of expression or transgenic expression of BCL-XL confirmed its key role in mediating inherent and acquired VEN resistance. Of note, the VEN and A1155463 combination was synthetically lethal even in the cell lines with lack of expression of the proapoptotic BCL2L11/BIM and in the derived clones with genetic knockout of BCL2L11/BIM. This is clinically important because BCL2L11/BIM deletion, downregulation, or sequestration results in VEN resistance. Immunoprecipitation experiments further suggested that the proapoptotic effector BAX belongs to principal mediators of the VEN and A1155463 mode of action in the BIM-deficient cells. Lastly, the efficacy of the new proapoptotic combination was confirmed in vivo on a panel of 9 patient–derived lymphoma xenografts models including MCL (n = 3), B-ALL (n = 2), T-ALL (n = 1), and diffuse large BCL (n = 3). Because continuous inhibition of BCL-XL causes thrombocytopenia, we proposed and tested an interrupted 4 days on/3 days off treatment regimen, which retained the desired antitumor synergy with manageable platelet toxicity. The proposed VEN and A1155463 combination represents an innovative chemotherapy-free regimen with significant preclinical activity across diverse BCL2+ hematologic malignancies irrespective of the BCL2L11/BIM status.
Introduction
B-cell lymphoma 2 (BCL2) inhibitor venetoclax (VEN) revolutionized the therapy of chronic lymphocytic leukemia (CLL) and acute myeloid leukemias (AML).1-3 In contrast, the efficacy of single-agent VEN in aggressive lymphoid tumors, for example, mantle cell lymphoma (MCL), diffuse large BCL (DLBCL), and acute lymphoblastic leukemia (ALL) was rather disappointing.4-8 Despite promising overall response rates, remissions induced with single-agent VEN were generally short, underlining the need for rational drug combinations.9,10
Several cell-intrinsic and -extrinsic mechanisms of VEN resistance have been reported in the literature, including overexpression of antiapoptotic BCL2 proteins MCL1 and BCL-XL, deletion of proapoptotic BIM, mutations of BCL2, BAX, TP53, SMARCA4, and other genes, deregulated oxidative phosphorylation, as well as multiple microenvironmental factors.11-17 We and others have reported a strong synergy between VEN and diverse MCL1 inhibitors.18-20 Development of clinical-grade MCL1 inhibitors, however, has been hampered by adverse side effects, including cardiotoxicity and hepatotoxicity.21-25 Another pivotal antiapoptotic protein of the BCL2 family, BCL-XL, has been repeatedly associated with VEN resistance in several cancer types.26,27 A new generation of dual BCL2/BCL-XL inhibitors has demonstrated promising preclinical and clinical activity in hematologic and solid cancers.28-30
In this study, we bring conclusive evidence that concurrent blockage of BCL-XL and BCL2 with specific nanomolar inhibitors A1155463 and VEN, respectively, induces synthetic lethality across a wide range of BCL2+ aggressive lymphoproliferative neoplasms including MCL, DLBCL, and ALL.
Methods
Cell lines, primary lymphoma samples, and patient-derived xenograft models
UPF1H, UPF29TO, UPF24F, UPF25R, UPF26K, UPF27L, UPF31A, UPF34N, and UPF35S cell lines were derived in our laboratory from patients with treatment-refractory MCL (UPF1H), double-hit DLBCL (UPF29TO), B-ALL (UPF24F, UPF25R, UPF26K, UPF27L, UPF31A, and UPF35S), and T-ALL (UPF34N). These patient–derived lymphoma cell lines were cultured in Iscove's Modified Dulbecco's Medium supplemented with 20% human plasma (Macopharma, France), 1% penicillin/streptomycin, and 0.4% heparin. Commercially available cell lines were obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen or American Type Culture Collection cell banks and authenticated by Multiplexion. Patient-derived xenografts (PDXs) were established in our laboratory from patients with treatment-refractory MCL, DLBCL, or B-ALL/T-ALL as described previously.18,31,32
HS5 CD40L cell line was provided by M. Mraz (M.U.) and generated by transduction of HS5 cells (Deutsche Sammlung von Mikroorganismen und Zellkulturen) by CDS for human CD40L and blasticidin resistance cloned into the pEZ-Lv197 lentiviral vector (plasmid EX-G0117-Lv197, GeneCopoeia).
Establishment of lymphoma cell clones with K/O of expression and transgenic re-expression of selected BCL2 genes
Lymphoma cell clones with knockout (K/O) of BCL2L1 gene (coding for antiapoptotic BCL-XL) were generated using CRISPR/CRISPR-associated protein 12a (Cas12a) system. A synthetic oligonucleotide containing Cas12a direct repeats and CRISPR RNA-encoding DNA sequences targeting exon 2 and surrounding introns (GATGCCCGGGAGGTGATCCCCAT: exon 2, TACCCCCGTCTTCTCCGAAATGC: intron 1-2, and GCCTCTGGTCAGAGATCCCCAAC: intron 2-3) was cloned into Cas12a-expressing plasmid pX AsCpf1-Venus-NLS and electroporated into BCL cell lines. Twenty-four hours after transfection, the cells were single-cell sorted, and fluorescent Venus-expressing cells were cultivated further. Elimination of BCL-XL in the clonal cultures was assessed by western blotting using anti–BCL-XL antibody (catalog no. 2764; Cell Signaling Technology, MA). Inducible re-expression of BCL-XL in the derived clones with K/O of BCL-XL gene (designated BCL-XL K/O R clones) was achieved using the Sleeping Beauty transposon system.33BCL2L1 gene coding for BCL-XL was polymerase chain reaction (PCR)-amplified from pCDH-puro-BCL-XL plasmid (catalog no. 46972; Addgene, MA) using primers with SfiI overhangs (forward: TAGCGGCCTCTGAGGCCACCATGTCTCAGAGCAACCGGGAGC and reverse: ATGCGGCCTGACAGGCCTCATTTCCGACTGAAGAGTGAGCC) and cloned into pSBtet-Pur plasmid (catalog no. 60507; Addgene). Resulting pSBtet-Pur-BCL-XL plasmid was coelectroporated together with transposase-carrying plasmid pCMV(CAT)T7-SB100 (catalog no. 34879; Addgene) and pmax green fluorescent protein(GFP) (2 μg, 3 μg, and 1 μg, respectively) into 1.5 million cells per sample. After 96 hours, the cells were sorted using fluorescence-activated cell sorting (FACS) for GFP expression and selected under 2 μg/mL puromycin (Serva, Germany). The transduced cells were incubated with 0.01 to 0.2 μg/mL doxycycline (Duchefa Biochemie, The Netherlands), and BCL-XL induction was confirmed by western blotting.
Lymphoma cell clones with a K/O of BCL2L11 gene (coding for proapoptotic inducer BIM) and/or K/O of BAK1 gene (coding for proapoptotic effector BAK) were generated using CRISPR/Cas9 according to methodology described by Cong et al.34 Specific single guide RNA were cloned into a pX330 recombinant plasmid (catalog no. 42230; Addgene) carrying Cas9 nuclease. We targeted exon 4 of BCL2L11 gene with 2 guide sequences (GTTCTGATGCAGCTTCCATGAGG and TCCTTGCATAGTAAGCGTTAGGG) and exon 3 of BAK1 gene (CGTTTTTTACCGCCATCAGCAGG and GCAGGTGAGCTACAACCGCTGGG). Using Neon transfection system, 6 μg of each pX330 plasmid was electroporated into the cells, along with 5 μg of plasmid cloning DNA-EmGFP reporter plasmid. GFP+ cells were sorted using BD FACS Aria (NJ) and seeded into 96-well plate (1 cell per well). After establishing clonal cultures, genomic DNA and protein lysates were isolated to confirm the deletion by both PCR and western blotting.
Apoptosis measurement
VEN and A1155463 were purchased from MedChemExpress (NJ), dissolved in dimethyl sulfoxide (Carl Roth, Germany) at 10 mM concentration, and stored at –20°C. A percentage of apoptotic and necrotic cells was determined after 24-hour incubation with the indicated treatment by flow cytometry (BD FACS Canto II, NJ) using Annexin V fluorescein isothiocyanate (EXBIO, Czech Republic) and propidium iodide (Sigma-Aldrich, MO). Percentage was calculated using the following formula: (measured apoptosis − basal apoptosis)/(100 − basal apoptosis) ∗ 100 (%). Drug concentrations that induced apoptosis in 50% of cells after 24 hours were determined by nonlinear regression algorithms using GraphPad Prism software. CompuSyn version 1.0 software (ComboSyn) was used to assess drug synergism between VEN and A1155463. The combination index for 2-drug combination was calculated for different concentrations of drugs considering various cell sensitivity.
Western blotting
Cells were lysed in radio-immunoprecipitation assay lysis buffer (1% Triton X-100, 0.1% sodium dodecyl-sulfate (SDS), 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), and 50 mM Tris-HCl pH 7.4) supplemented with 1% protease (Protease Inhibitor Cocktail; Sigma-Aldrich) and 10% phosphatase inhibitors (PhosSTOP; Roche, Switzerland). Protein concentration in the collected supernatants was determined by the bicinchoninic acid assay (BCA) protein assay (Pierce BCA Protein Assay, Thermo Scientific, MA) according to the manufacturer’s protocol. Lysate samples (25 μg) were combined with SDS loading buffer containing 2-mercaptoethanol and boiled for 5 minutes. Samples were separated on 10% to 15% SDS-polyacrylamide gel electrophoresis (PAGE) minigels in Tris-glycine buffer (Bio-Rad, CA). Proteins were transferred onto polyvinylidene fluoride membranes (Bio-Rad). Membranes were washed in 1× phosphate-buffered saline buffer (VWR, PA) containing 0.1% Tween-20 and incubated in 10% nonfat dried milk (Carl Roth, Germany) for 30 minutes. β-Actin was used as the loading control (1:10 000; Abcam, United Kingdom). Primary antibodies were purchased from Cell Signaling Technology (anti-BAK, anti–BCL-XL, anti-BIM, and anti-MCL1) or BD Biosciences (anti-BCL2) and diluted 1:1000 or 1:2000, respectively (summarized in supplemental Table 1). After thorough washing in blocking buffer, a secondary horseradish peroxidase-conjugated anti-mouse and/or anti-rabbit antibody (both from Jackson ImmunoResearch Laboratories, Inc, PA) was added (concentration 1:10 000). The signal was detected using western blotting detection kit (WesternBright ECL; Advansta Inc, CA), and membranes were visualized by ChemiDoc Imaging System (Bio-Rad).
Protein coimmunoprecipitation
Cell pellets (107) were lysed at 4°C for 25 minutes in a nondenaturing lysis buffer (1% [w/v] Triton X-100, 50 mmol/L Tris-HCl [pH 7.4], 300 mmol/L NaCl, 5 mmol/L EDTA, and 0.02% [w/v] sodium azide supplemented with protease inhibitor) and centrifuged (16 000g, 4°C, 15 minutes). Protein concentrations of cell extracts were measured as outlined above, using the BCA protein assay (Thermo Scientific). First, protein samples were precleared with Protein protein A and protein G (A/G) Agarose bead slurry (Pierce Protein A/G Agarose; Thermo Scientific), which was incubated with anti-IgG antibody (Normal Rabbit IgG; EMD Millipore Corp, MA) for 30 minutes at 4°C, followed by centrifugation (16 000g, 4°C, 2 seconds). Preclearing was repeated 2 more times. The cell lysates were split and incubated with 10% bovine serum albumin (Sigma-Aldrich) and Protein A/G Agarose beads with either a specific antibody or a corresponding isotype control immunoglobulin bound to them. Incubation took 1 hour at 4°C. Immunocomplexes were then centrifuged (16 000g, 4°C, 2 seconds), washed 3 times in ice-cold wash buffer (0.1% Triton X-100, 50 mmol/L Tris-HCl [pH 7.4], 300 mmol/L NaCl, 5 mmol/L EDTA, and 0.02% sodium azide) and once more in ice-cold 1% phosphate-buffered saline. Subsequently, the samples were fractionated by 15% SDS-PAGE, followed by western blotting detection, in which proteins in the gel were transferred to polyvinylidene fluoride membrane (Bio-Rad). To detect BIM interaction with BCL2 or BCL-XL, anti-BIM (Cell Signaling Technology) antibody was used for the immunoprecipitation, and the precipitates were subjected to immunoblot analysis using anti-BCL2 (BD Biosciences) or anti-BCL-XL (Abcam) antibodies (summarized in supplemental Table 1).
Real-time PCR
Total RNA was isolated from cell pellets (5 × 106) using RNeasy Mini Kit (QIAGEN, Germany) by following the manufacturer's instructions. Reverse transcription and PCR were performed in a single well provided by 2-phase hot-start mechanism with the QuantiNova Probe RT-PCR Kit (QIAGEN, Germany) in combination with TaqMan Gene Expression Assays (BCL2L1: Hs00236329_m1 and B2M: Hs00187842_m1) on QuantStudio 7 Pro Real-Time PCR System (Applied Biosystems, MA). The following quantitative PCR conditions were used: for initiation of complementary DNA synthesis, 10 minutes at 45°C; activation of DNA polymerase, 5 minutes at 95°C; and amplification, 40 cycles of 5 seconds at 95°C and 35 seconds at 60°C. Quantification cycle values of BCL2L1 gene were normalized to the reference gene B2M.
Experimental therapy of lymphoma-bearing mice
The experimental design was approved by the Institutional Animal Care and Use Committee (MSMT-1712/2021-2). NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice (referred to as NSG mice) were purchased from The Jackson Laboratory (ME). Adult female NSG mice were used for all experiments. NSG mice were subcutaneously inoculated with ∼10 × 106 lymphoma or leukemia cells. Therapy was initiated when all mice developed palpable tumors (designated as day 1 [D1]). At D1, all mice were stratified so that all cohorts contained mice with comparable calculated tumor volumes. Each cohort of mice contained 6 animals. VEN (100 mg/kg, once daily by oral gavage) and A1155463 (10 mg/kg, once daily intraperitoneally) were administered on days 1 to 4 and 8 to 11. Tumor volumes were calculated using the following formula: π/6 × length × width × height. Experimental mice were euthanized when size of subcutaneous tumors exceeded 2 cm in the largest diameter. The data were analyzed using the GraphPad Prism software.
Evaluation of mouse platelet counting with an automated hematology
The common site for blood collection in mice was retro-orbital sinus, and blood was collected into EDTA capillary tubes (Vitrex, Denmark). Blood cell counts in mice on A1155463 therapy were analyzed using the BC-5300 Auto Hematology Analyzer (Mindray, China).
Statistical analyses
To assess the “practical significance” of treatment effectiveness, the charts with growth curves were plotted indicating group mean tumor volumes accompanied by expert opinion on the differences observed. For the purpose of attaching the label of “statistical significance” on treatment effectiveness, we made an assumption that the calculated differences (between mean tumor volumes in the compared groups) were generated by a process that includes a deterministic linear trend in the following form: , in which Yt denotes random variables in the data-generating stochastic process of the analyzed differences; t = 1, 2, …T is a time variable; T signifies the length of the experiment (not the same for all experiments) in days; and εt is the Gaussian independent and identically distributed white noise. For the daily time series of these differences, statistical hypothesis tests of linear trend slopes equality to 0 were carried out. The Bonferroni correction was used to smooth the significance level for multiple simultaneous statistical hypothesis tests.
Primary lymphoma and leukemia cells were obtained from 28 patients with MCL, DLBCL, CLL, or B-/T-ALL according to the Declaration of Helsinki. Informed written consent was obtained from each participant. The experimental design was approved by the ethics committee of the General University Hospital Prague under number 60/20.
Results
Cotargeting BCL-XL with A1155463 and BCL2 with VEN is synthetically lethal across BCL2+ lymphoproliferative malignancies
Several studies demonstrated a critical role of BCL-XL in mediating inherent and acquired resistance to VEN.15,27,35-37 Using a panel of 21 cell lines, including MCL (n = 7), DLBCL (n = 7), and ALL (n = 7), we demonstrated strong in vitro synergy between VEN and BCL-XL inhibitor A1155463 (Table 1). The synergy was also confirmed on ex vivo cultured primary lymphoma and leukemia cells obtained from patients not only with newly diagnosed or treatment-refractory MCL (n = 7), DLBCL (n = 4), and ALL (n = 2) but also other BCL2+ hematologic malignancies including CLL (n = 7), follicular lymphoma (FL; n = 3), AML (n = 2), and chronic myeloid leukemia (n = 3) (supplemental Table 4). In all the tested primary cell samples, the combination index between VEN and A1155463 was <1, indicating a synergistic effect (supplemental Tables 2 and 3).
BAX is a key apoptotic trigger in BIM-deficient lymphoma and leukemia cells exposed to the VEN and A1155463 combination
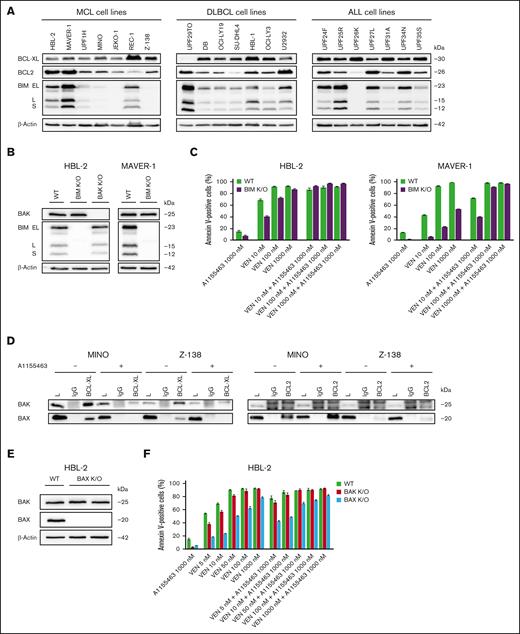
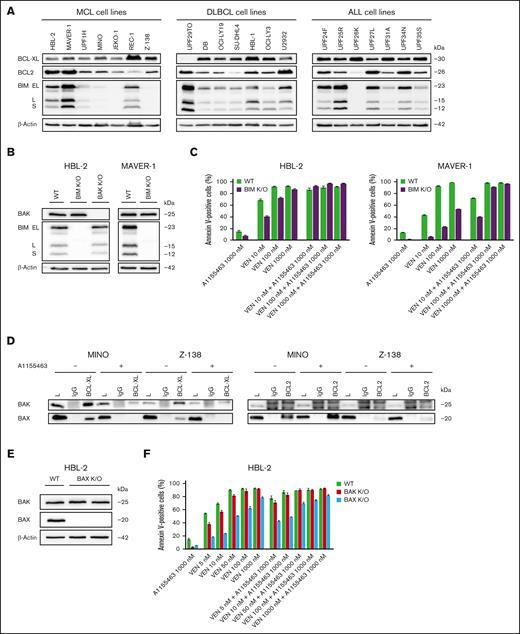
The proapoptotic inducer BCL2L11/BIM (referred to as BIM) has been repeatedly reported as the key mediator of VEN antitumor activity.38,39 In line with this, BIM deletion, sequestration, or loss of expression have been associated with VEN resistance.35,40,41 In this study, the cytotoxic synergy of the VEN and A1155463 combination was observed even in BIM-deficient lymphoma and leukemia cell lines, which are inherently resistant or less sensitive to VEN (JEKO-1, Z-138, MINO, and UPF26K; Figure 1A; Table 1). Similarly, lymphoma clones with K/O of BIM (BIM K/O clones) became VEN resistant (VEN-R; or significantly less sensitive) but retained sensitivity to the tested proapoptotic VEN and A1155463 combination (Figure 1B-C). The data clearly suggested that other proapoptotic BCL2 molecule(s) contribute(s) to the synthetic lethality between VEN and A1155463. Immunoprecipitation experiments in BIM-deficient Z-138 and MINO cells (characterized by strong synthetic lethality between VEN and A1155463) detected proapoptotic effectors BAX and BAK bound to BCL-XL. Both BAX and BAK were released from BCL-XL upon exposure to A1155463. However, only BAX was enriched on BCL2 upon exposure of the cells to A1155463, whereas BAK binding to BCL2 was not detected (Figure 1D). In addition, VEN-induced apoptosis was more suppressed in HBL-2 BAX than BAK K/O cells. Last but not least, BAX K/O, but not BAK K/O, partially block apoptosis triggered by the VEN and A1155463 combination (Figure 1E-F).
BAX belongs to key mediators of cytotoxicity triggered by the combination of VEN and A1155463 in BIM-deficient lymphoma and leukemia cells. (A) Western blot analysis of selected BCL2 family proteins in the tested lymphoma and leukemia cell lines. (B) Western blot confirmation of absence of BIM and/or BAK proteins in the HBL-2 and MAVER-1 lymphoma cell clones with K/O of BIM and BAK genes. (C) K/O of BIM in lymphoma cell lines (HBL-2 and MAVER-1 BIM K/O clones) resulted in increased VEN-R, whereas sensitivity to the combination was largely retained; sensitivity of wild-type (WT) cells is shown for comparison. (D) Immunoprecipitations (IPs) of 2 BIM-deficient cell lines (MINO and Z-138) exposed for 3 hours to A1155463 (1000 nM) or medium only with anti–BCL-XL and anti-BCL2 antibodies and subsequent western blot detection of BAX and BAK proteins bound on BCL-XL and BCL2. (E) Western blot confirmation of absence of BAX protein expression in HBL-2 cell clone with K/O of BAX gene. (F) Apoptosis essays after exposure to the defined VEN and A1155463 concentrations reveal that HBL-2 cells with BAX K/O are significantly more resistant to VEN and to the VEN + A1155463 combination than HBL-2 cells with BAK K/O.
BAX belongs to key mediators of cytotoxicity triggered by the combination of VEN and A1155463 in BIM-deficient lymphoma and leukemia cells. (A) Western blot analysis of selected BCL2 family proteins in the tested lymphoma and leukemia cell lines. (B) Western blot confirmation of absence of BIM and/or BAK proteins in the HBL-2 and MAVER-1 lymphoma cell clones with K/O of BIM and BAK genes. (C) K/O of BIM in lymphoma cell lines (HBL-2 and MAVER-1 BIM K/O clones) resulted in increased VEN-R, whereas sensitivity to the combination was largely retained; sensitivity of wild-type (WT) cells is shown for comparison. (D) Immunoprecipitations (IPs) of 2 BIM-deficient cell lines (MINO and Z-138) exposed for 3 hours to A1155463 (1000 nM) or medium only with anti–BCL-XL and anti-BCL2 antibodies and subsequent western blot detection of BAX and BAK proteins bound on BCL-XL and BCL2. (E) Western blot confirmation of absence of BAX protein expression in HBL-2 cell clone with K/O of BAX gene. (F) Apoptosis essays after exposure to the defined VEN and A1155463 concentrations reveal that HBL-2 cells with BAX K/O are significantly more resistant to VEN and to the VEN + A1155463 combination than HBL-2 cells with BAK K/O.
BCL-XL is a critical modulator of VEN sensitivity
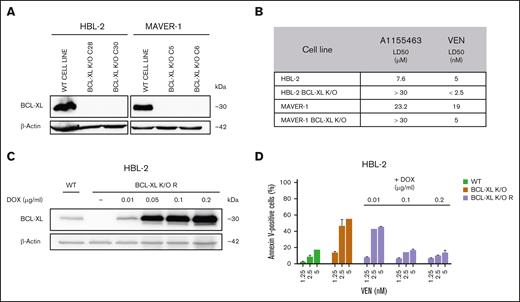
To better understand the mechanistic role of BCL-XL in mediating susceptibility to VEN, we generated clones with BCL-XL K/O (BCL-XL K/O) from 2 lymphoma cell lines (HBL-2 and MAVER-1) using the CRISPR/Cas9 technology. Both clones became significantly more sensitive to VEN than the parental cell lines (Figure 2A-B). In addition, transgenic re-expression of BCL-XL in the HBL-2 BCL-XL K/O cells decreased their sensitivity to VEN. The extent of doxycycline-induced transgenic re-expression of BCL-XL negatively correlated with the extent of sensitivity to VEN (Figure 2C-D). Although BCL-XL K/O did not influence the engraftment and growth of lymphoma cells in vivo, the therapy of the mice bearing HBL-2 BCL-XL K/O tumors with single-agent VEN was significantly more effective than the combinatorial therapy (ie, VEN and A1155463) of mice xenografted with the wild-type HBL-2 parental lymphoma cells. Of note, 3 of 6 mice remained without any signs of lymphoma 6 months after the initiation of therapy (supplemental Figure 1).
BCL-XL belongs to key mediators of sensitivity/resistance to VEN-induced apoptosis. (A) Representative western blot analysis confirms absence of BCL-XL protein expression in the HBL-2 and MAVER-1 lymphoma clones with K/O of BCL-XL gene. (B) Calculated lethal dose 50 (LD50) for A1155463 and VEN in the HBL-2 and MAVER-1 cell clones with BCL-XL K/O compared with the WT lymphoma cell lines. (C) Western blot analysis confirms doxycycline-induced dose-dependent re-expression of BCL-XL protein in HBL-2 BCL-XL K/O clone with transgenic re-expression of BCL-XL (designated BCL-XL K/O R); expression of BCL-XL in the WT HBL-2 cell line is shown for comparison. (D) Extent of the doxycycline-induced transgenic re-expression of BCL-XL in the HBL-2 BCL-XL K/O R clone under doxycycline promotor negatively correlates with sensitivity to VEN; sensitivity to HBL-2 WT cell line and HBL-2 BCL-XL K/O clone is shown for comparison. DOX, doxycycline.
BCL-XL belongs to key mediators of sensitivity/resistance to VEN-induced apoptosis. (A) Representative western blot analysis confirms absence of BCL-XL protein expression in the HBL-2 and MAVER-1 lymphoma clones with K/O of BCL-XL gene. (B) Calculated lethal dose 50 (LD50) for A1155463 and VEN in the HBL-2 and MAVER-1 cell clones with BCL-XL K/O compared with the WT lymphoma cell lines. (C) Western blot analysis confirms doxycycline-induced dose-dependent re-expression of BCL-XL protein in HBL-2 BCL-XL K/O clone with transgenic re-expression of BCL-XL (designated BCL-XL K/O R); expression of BCL-XL in the WT HBL-2 cell line is shown for comparison. (D) Extent of the doxycycline-induced transgenic re-expression of BCL-XL in the HBL-2 BCL-XL K/O R clone under doxycycline promotor negatively correlates with sensitivity to VEN; sensitivity to HBL-2 WT cell line and HBL-2 BCL-XL K/O clone is shown for comparison. DOX, doxycycline.
Microenvironmental factors are critical triggers of BCL-XL expression
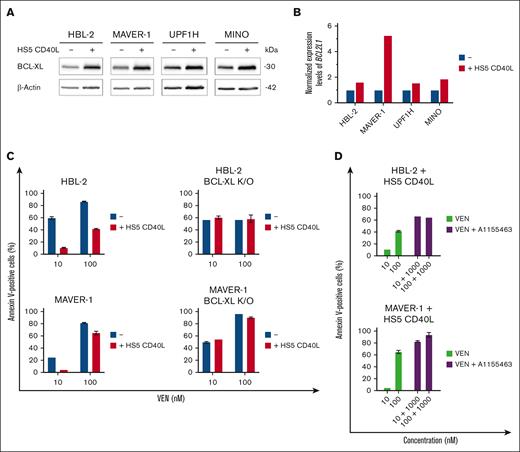
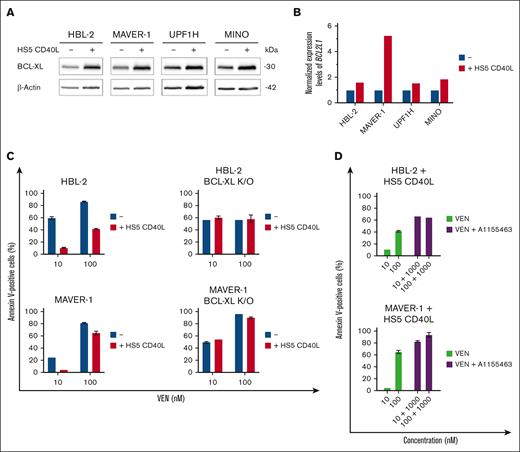
Multiple studies have shown that B- and T-cell interactions in the lymphoma/leukemia microenvironment support the survival of malignant B cells (Hoferkova E, Seda V, Kadakova S, et al, unpublished data, 2024), including CD40L-triggered NF-κB activity leading to overexpression of BCL-XL.36,37 Indeed, we confirmed that cocultures of VEN-sensitive lymphoma cells with CD40L-expressing stromal cells (HS5-CD40L) resulted in overexpression of BCL-XL messenger RNA and protein leading to VEN resistance (Figure 3A-C). In contrast, the coculture of BCL-XL K/O HBL-2 and MAVER-1 clones with HS5-CD40L did not change their sensitivity to VEN. Cotreatment of the unmanipulated (parental) lymphoma cell lines cocultured with HS5-CD40L with the combination of VEN and A1155463 overcame the observed microenvironment–mediated VEN resistance (Figure 3D).
Upregulation of BCL-XL induced by microenvironmental factors belongs to key mediators of VEN resistance in vitro. (A) Western blot analysis of BCL-XL protein after 24-hour coculture of the tested lymphoma cell lines (HBL-2, MAVER-1, UPF1H, and MINO) with HS5 CD40L feeder cells with stable transgenic expression of human CD40 ligand (HS5 CD40L). (B) Bar charts showing the differences of normalized expression levels of BCL2L1/BCL-XL in the tested WT lymphoma cell lines (blue bars) and after 24-hour coculture with HS5 CD40L feeder cells (red bars). (C) Coculture of HBL-2 and MAVER-1 WT lymphoma cell lines with HS5 CD40L feeder cells for 24 hours resulted in VEN resistance as measured by numbers of apoptotic cells after exposure to VEN (10 and 100 ng/mL); in contrast, coculture of HBL-2 and MAVER-1 BCL-XL K/O clones with HS5 CD40L did not change their sensitivity to VEN. (D) Combined treatment of the WT lymphoma cell lines cocultured for 24 hours on HS5 CD40L feeder cells with the combination of VEN (10 and 100 nM) and A1155463 (1000 nM) overcame the microenvironment-induced VEN resistance.
Upregulation of BCL-XL induced by microenvironmental factors belongs to key mediators of VEN resistance in vitro. (A) Western blot analysis of BCL-XL protein after 24-hour coculture of the tested lymphoma cell lines (HBL-2, MAVER-1, UPF1H, and MINO) with HS5 CD40L feeder cells with stable transgenic expression of human CD40 ligand (HS5 CD40L). (B) Bar charts showing the differences of normalized expression levels of BCL2L1/BCL-XL in the tested WT lymphoma cell lines (blue bars) and after 24-hour coculture with HS5 CD40L feeder cells (red bars). (C) Coculture of HBL-2 and MAVER-1 WT lymphoma cell lines with HS5 CD40L feeder cells for 24 hours resulted in VEN resistance as measured by numbers of apoptotic cells after exposure to VEN (10 and 100 ng/mL); in contrast, coculture of HBL-2 and MAVER-1 BCL-XL K/O clones with HS5 CD40L did not change their sensitivity to VEN. (D) Combined treatment of the WT lymphoma cell lines cocultured for 24 hours on HS5 CD40L feeder cells with the combination of VEN (10 and 100 nM) and A1155463 (1000 nM) overcame the microenvironment-induced VEN resistance.
In vivo acquired resistance to VEN is associated with upregulation of BCL-XL and increased binding of BIM to BCL-XL
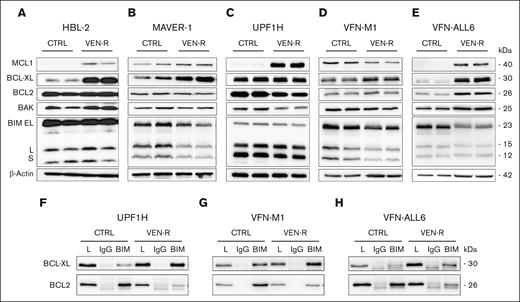
VEN-sensitive cell lines (HBL-2, MAVER-1, and UPF1H) and PDX cells (VFN-M1 and VFN-ALL6) were subcutaneously xenografted into immunodeficient NSG mice and treated with VEN (100 mg/kg per daily) until the development of VEN-R tumors. The most frequently observed changes in the expression of BCL2 family of proteins comprised upregulation of antiapoptotic proteins BCL-XL, MCL1, and BCL2 and downregulation of the proapoptotic protein BIM (Figure 4A-E). Real-time PCR analysis showed upregulation of BCL-XL messenger RNA in all tested VEN-R samples (supplemental Figure 2). Immunoprecipitation experiments confirmed that BCL-XL serves as a buffer for the BIM protein released from BCL2 after exposure of lymphoma cells to VEN (Figure 4F-H).
Sequestration of BIM to upregulated BCL-XL belongs to hallmarks of acquired VEN resistance. (A-E) Western blot analysis of selected BCL2 family proteins (MCL1, BCL-XL, BCL2, BAK, and BIM) in cell lysates isolated from the subcutaneous cell line–based xenograft (CDX) tumors (HBL-2, MAVER-1, and UPF1H), and subcutaneous patient-derived xenograft (PDX) tumors (VFN-M1 and VFN-ALL6) obtained from the untreated animals (CTRL) and from the animals with acquired VEN resistance. (F-H) IP of the selected CDX or PDX tumors with anti-BIM antibody and subsequent detection of BCL-XL and BCL2 bound on BIM; 1 CDX model (UPF1H) and 2 PDX models (VFN-M1 and VFN-ALL6) were analyzed; the mice were euthanized and tumors analyzed 24 hours after the last dose of VEN.
Sequestration of BIM to upregulated BCL-XL belongs to hallmarks of acquired VEN resistance. (A-E) Western blot analysis of selected BCL2 family proteins (MCL1, BCL-XL, BCL2, BAK, and BIM) in cell lysates isolated from the subcutaneous cell line–based xenograft (CDX) tumors (HBL-2, MAVER-1, and UPF1H), and subcutaneous patient-derived xenograft (PDX) tumors (VFN-M1 and VFN-ALL6) obtained from the untreated animals (CTRL) and from the animals with acquired VEN resistance. (F-H) IP of the selected CDX or PDX tumors with anti-BIM antibody and subsequent detection of BCL-XL and BCL2 bound on BIM; 1 CDX model (UPF1H) and 2 PDX models (VFN-M1 and VFN-ALL6) were analyzed; the mice were euthanized and tumors analyzed 24 hours after the last dose of VEN.
Combined inhibition of BCL2 and BCL-XL is strongly synergistic in vivo on a panel of 4 cell line–based xenograft and 9 PDX models of BCL2+ aggressive lymphoid neoplasms
Clinically, a blockage of BCL-XL with navitoclax, a dual BCL2/BCL-XL inhibitor, led to thrombocytopenia.1 Similarly, A1155463, a specific BCL-XL inhibitor, induced thrombocytopenia in mice after 4 consecutive days of therapy with A1155463 (Table 2). Importantly, after 3 days of therapy cessation, the levels of platelets rebounded to normal range. This led us to use an interrupted “4 days on/3 days off” dosing schedule in all in vivo experiments implemented in this study.
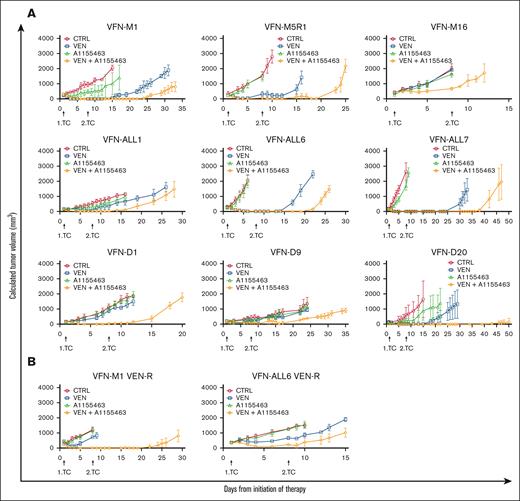
In vivo, the combination of VEN and A1155463 more effectively inhibited tumor growth than respective monotherapies, as demonstrated on a panel of 4 cell line–based xenografts (HBL-2, MAVER-1, UPF1H, and MINO) and 9 PDX models obtained from patients with MCL (VFN-M1, M5R1, and M16), BCL2+ DLBCL (VFN-D1, D9, and D20), B-ALL (VFN-ALL1, VFN-ALL6), and T-ALL (VFN-ALL7). Of note, the combination was effective even in the tumors inherently resistant to both VEN and A1155463 monotherapies (VFN-M16, VFN-D1, and VFN-D9), and in the tumors with acquired VEN-R, including HBL-2, MAVER-1, UPF1H, VFN-M1, and VFN-ALL6 (Figure 5; supplemental Figure 3).
Cotargeting of BCL2 and BCL-XL with VEN and A1155463 is synthetically lethal on a panel of PDX models derived from patients with MCL, ALL, and DLBCL. (A) Cotargeting of BCL2 and BCL-XL with VEN and A1155463 was tested on a panel of 9 PDX models including MCL (VFN-M1, VFN-M5R1, and VFN-M16), ALL (VFN-ALL1, VFN-ALL6, and VFN-ALL7), and DLBCL (VFN-D1, VFN-D9, and VFN-D20); x-axis shows days from initiation of therapy; y-axis shows calculated tumor volumes ± standard deviations of PDX tumors; therapy was administered on days 1 to 4 and 8 to 12 (for details, see “Methods”). (B) Cotargeting of BCL2 and BCL-XL with VEN and A1155463 was effective even in the PDX models with acquired VEN resistance (VFN-M1 VEN-R and VFN-ALL6 VEN-R); VEN-R PDX tumors were derived from animals, in which originally VEN-sensitive PDX tumors grew on continued VEN therapy; such VEN-R PDX tumors were re-engrafted into secondary recipients and subjected to retreatment; each cohort of mice comprised 6 animals.
Cotargeting of BCL2 and BCL-XL with VEN and A1155463 is synthetically lethal on a panel of PDX models derived from patients with MCL, ALL, and DLBCL. (A) Cotargeting of BCL2 and BCL-XL with VEN and A1155463 was tested on a panel of 9 PDX models including MCL (VFN-M1, VFN-M5R1, and VFN-M16), ALL (VFN-ALL1, VFN-ALL6, and VFN-ALL7), and DLBCL (VFN-D1, VFN-D9, and VFN-D20); x-axis shows days from initiation of therapy; y-axis shows calculated tumor volumes ± standard deviations of PDX tumors; therapy was administered on days 1 to 4 and 8 to 12 (for details, see “Methods”). (B) Cotargeting of BCL2 and BCL-XL with VEN and A1155463 was effective even in the PDX models with acquired VEN resistance (VFN-M1 VEN-R and VFN-ALL6 VEN-R); VEN-R PDX tumors were derived from animals, in which originally VEN-sensitive PDX tumors grew on continued VEN therapy; such VEN-R PDX tumors were re-engrafted into secondary recipients and subjected to retreatment; each cohort of mice comprised 6 animals.
Discussion
In recent years, a large body of evidence has suggested that BCL-XL belongs to the pivotal factors of both inherent and acquired VENresistance.15,27,35-37 Microenvironmental factors, such as the CD40L-induced NF-κB signaling, and hypoxia-induced HIF1-alpha have been reported to induce BCL-XL expression.37 Although the precise mechanisms of BCL-XL upregulation during the establishment and regrowth of VEN-R tumors upon or after VEN therapy remain largely elusive, we suppose that survival, overgrowth and expansion of BCL-XL (over)expressing cells (VEN-R) is a result of selective pressure of VEN in a Darwinian way.
The tested combination of VEN and A1155463 might appear as a return to the old agent navitoclax, a dual, BCL2 and BCL-XL inhibitor, which had failed to demonstrate better antitumor activity than the BCL2-specific VEN.42 However, navitoclax was a weak inhibitor of BCL2, and a strong inhibitor of BCL-XL, thereby inducing deep thrombocytopenia while eliciting only moderate antileukemia effects. In contrast, the combined strong inhibition of both BCL2 and BCL-XL leads to significant antitumor synergy, which was confirmed in our study on a large panel of diverse PDX models derived from various BCL2+ hematologic malignancies. Curiously, the combination of VEN and low-dose navitoclax had promising antitumor activity in patients with ALL/lymphoblastic lymphoma.43 A new generation of dual BCL2/BCL-XL inhibitors have also demonstrated preclinical and clinical activity.28-30 In contrast to the dual inhibitors, a combination of 2 separate highly specific nanomolar inhibitors would enable a continued BCL2 blockage with an interrupted blockage of BCL-XL to mitigate thrombocytopenia, as well as separate management of potential adverse side effects of either agent. The interrupted dosing strategy proposed here was inspired by previous studies with navitoclax, in which not only a ramp-up dosing of BCL-XL but also an interrupted dosing schedule blunted the depth of thrombocytopenia.42 Moreover, sequential administration of the 2 inhibitors (eg, first single-agent VEN, followed by the VEN + A1155463 combination) might substantially decrease the risk of tumor lysis syndrome, a known life-threatening side effect of VEN.
It was reported that the affinity of BIM follows the order BCL2 > BCL-XL > MCL1.15,27 This observation underlined the importance of BCL-XL in mediating VEN resistance compared with MCL1. Mechanistically, our data confirmed that BCL-XL indeed serves as a buffer for BIM released from the BCL2 protein upon exposure to VEN. K/O of BCL-XL significantly increased sensitivity to VEN, whereas transgenic re-expression of BCL-XL rendered lymphoma cells VEN-R. This is in line with evidence provided by previous studies.37,44 Furthermore, acquired resistance to VEN in PDX models caused by prolonged in vivo therapy with single-agent VEN resulted in overexpression of BCL-XL and/or increased binding of BIM to BCL-XL, which rendered VEN-R leukemia and lymphoma cells primed for the BCL-XL–targeting BH3-mimetic A1155463. Consequently, the VEN-R tumors could be effectively re-treated with the combination of A1155463 and VEN, even though the combo was less effective compared with the original (VEN-sensitive) tumors. Of note, the therapy of mice xenografted with the HBL-2 BCL-XL K/O clone with single-agent VEN was significantly more effective than the combinatorial therapy (ie, VEN + A1155463) of mice xenografted with the parental HBL-2 cells. Three of 6 mice remained without any signs of lymphoma 6 months after the initiation of therapy. This not only underscores key importance of BCL-XL in mediating susceptibility to VEN but also suggests that next-generation, more-specific BCL-XL–targeting agents might be associated with even better synthetic lethality.
BIM was repeatedly reported as the principal mediator of VEN mode of action.38 BIM deletion, its sequestration, or decreased expression were all associated with VEN resistance.41,45-48 Indeed, our lymphoma clones with BIM K/O became significantly less sensitive to VEN. Importantly, the tested VEN and A1155463 combination was effective even in the BIM-deficient cell lines and the derived clones with BIM K/O, which were all resistant to single-agent VEN. This observation suggested that other proapoptotic BCL2 protein(s) (than BIM) can drive the proapoptotic activity of the VEN and A1155463 combination. Our immunoprecipitation data demonstrated that both BAX and BAK effectors are bound on BCL-XL in the BIM-deficient cell lines, but only BAX was detected enriched on BCL2 upon exposure to A1155463. The data from the BAX and BAK K/O clones further supported key roles of BAX and, to a lesser extent, BAK as important mediators/effectors of VEN proapoptotic activity. In BIM-proficient cells, BIM-activated BAX/BAK pore formation can be initiated after VEN-mediated release of these proteins from BCL2 only. In BIM-deficient cells, however, BAX and BAK release from both BCL2 and BCL-XL is requisite for triggering the mitochondrial apoptosis.
Besides the upregulation of BCL-XL, other mechanisms might contribute to the acquired VEN resistance. Although this was not the focus of this study, some of these mechanisms, in particular the overexpression of MCL1, might cause resistance even to the combination of VEN and A1155463. Indeed, the combination of VEN and A1155463, despite proving more effective than the respective monotherapies was not capable of long-term eradication of the engrafted PDX tumors. We can only speculate that prolonged treatment schedules, continued exposure to VEN, or increased dosing of 1 or both agents might be more effective than the tested “4 days on/3 days off” regimen. It is, however, plausible that the observed molecular changes would eventually lead to drug resistance to the combination of both BH-3 mimetics. There exist several potential 3-drug combinations to avoid the expected development of drug resistance mediated by MCL1 overexpression. Agents that block cell cycle progression are known to downregulate MCL1 protein, including cyclin-dependent kinase inhibitors (palbociclib) or antibody-drug conjugates (polatuzumab vedotin and inotuzumab ozogamicin) with small molecule mitotic poisons attached to the antibody carriers as toxic payloads. In addition to MCL1-downregulating agents, drugs with known synergy with VEN (eg, azacytidine) or agents with nonoverlapping modes of action and nonoverlapping adverse side effects including therapeutical monoclonal antibodies (eg, anti-CD20 rituximab or obinutuzumab), bispecifics (eg, glofitamab or epcoritamab), or targeted agents (eg, Bruton tyrosine kinase inhibitors) might be effective components of such experimental multiagent chemotherapy-free regimen.
In summary, cotargeting BCL-XL and BCL2 with A1155463 and VEN appears as a universal antimitochondrial treatment strategy not only for MCL, ALL, and BCL2+ DLBCL but potentially for most BCL2+ hematologic malignancies (including CLL, FL, AML, and chronic myeloid leukemia). Our data provide a sound preclinical rationale for early clinical trials in patients.
Acknowledgments
The authors thank all patients who donated samples for this study.
The study was supported by the Ministry of Health of the Czech Republic (grant AZV NU21-03-00386 and NU23-08-00448), all rights reserved, Grant Agency of the Czech Republic (GA23-05474S), and the project National Institute for Cancer Research (Programme EXCELES, ID project number LX22NPO5102), funded by the European Union, NextGenerationEU. This study was also supported by a grant from the Leukemia & Lymphoma Society (MCL 7005-24), a Cooperation Program, research area “Hemato-oncology,” and Charles University graduate students research program (acronym SVV; number 260634/2023).
Authorship
Contribution: A.D. did most of the experiments and participated in manuscript preparation; D.K. took part in derivation of clones; M.K. contributed in manuscript preparation; E.P. helped with in vivo experiments; D.S. and C.D.K. took part in derivation of the clones; L.T. contributed to protein analysis; E.H. and M.M. prepared the CD40L expressing fibroblasts; K.H. did statistical analyses; N.C. and K.M.P. provided primary cell samples; L.A. and M.T. contributed to data analysis and preparation of the manuscript; and P.K. designed the experiment and supervised manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pavel Klener, Charles University, First Faculty of Medicine, Institute of Pathological Physiology, and University General Hospital Prague and First Faculty of Medicine, First Department of Medicine-Hematology, Charles University, U Nemocnice 5, Prague 2, N/A 12853, Czech Republic; email: pavel.klener2@lf1.cuni.cz.
References
Author notes
Data are available on request from the corresponding author, Pavel Klener (pavel.klener2@lf1.cuni.cz).
The full-text version of this article contains a data supplement.