Key Points
PTCy is associated with a lower risk of chronic GVHD, reduced relapse, and improved OS, PFS, and GRFS vs CNI-based prophylaxis.
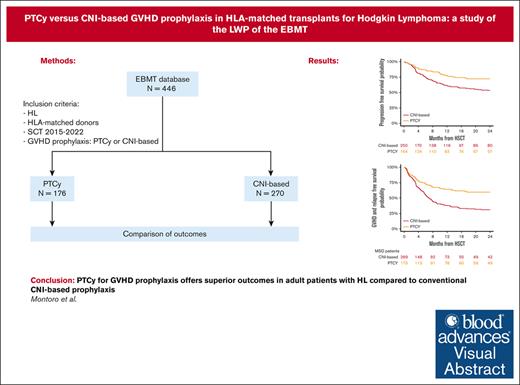
PTCy yields superior outcomes compared with CNI-based prophylaxis in adult patients with HL undergoing HSCT from HLA-matched donors.
Visual Abstract
Studies comparing the efficacy of posttransplant cyclophosphamide (PTCy) to conventional calcineurin inhibitor (CNI)–based graft-versus-host disease (GVHD) prophylaxis regimens in patients with Hodgkin lymphoma (HL) are scarce. This study aimed to compare the outcomes of patients with HL undergoing hematopoietic stem cell transplantation (HSCT) from HLA-matched donors who received GVHD prophylaxis with either PTCy- or conventional CNI-based regimens, using data reported in the European Society for Blood and Marrow Transplantation database between January 2015 and December 2022. Among the cohort, 270 recipients received conventional CNI-based prophylaxis and 176 received PTCy prophylaxis. Notably, PTCy prophylaxis was associated with delayed hematopoietic recovery but also with a lower risk of chronic (25% vs 43%; P < .001) and extensive chronic GVHD (13% vs 28%; P = .003) compared with the CNI-based cohort. The 2-year cumulative incidence of nonrelapse mortality and relapse was 11% vs 17% (P = .12) and 17% vs 30% (P = .007) for PTCy- and CNI-based, respectively. Moreover, the 2-year overall survival (OS), progression-free survival (PFS), and GVHD-free, relapse-free survival (GRFS) were all significantly better in the PTCy group compared with the CNI-based group: 85% vs 72% (P = .005), 72% vs 53% (P < .001), and 59% vs 31% (P < .001), respectively. In multivariable analysis, PTCy was associated with a lower risk of chronic and extensive chronic GVHD, reduced relapse, and better OS, PFS, and GRFS than the CNI-based platform. Our findings suggest that PTCy as GVHD prophylaxis offers more favorable outcomes than conventional CNI-based prophylaxis in adult patients with HL undergoing HSCT from HLA-matched donors.
Introduction
Posttransplant cyclophosphamide (PTCy) has emerged as a highly effective strategy for graft-versus-host disease (GVHD) prophylaxis, demonstrating success not only in the context of haploidentical (Haplo) hematopoietic stem cell transplantation (HSCT)1-3 but also extending its positive impact on HLA-matched donor transplants.4-9 As a result, PTCy is currently challenging the conventional calcineurin inhibitor (CNI)–based approach to GVHD prophylaxis.
Several studies have demonstrated the effectiveness and safety of PTCy compared with CNI-based GVHD prophylaxis in patients undergoing HLA-matched HSCT for various hematological malignancies.5,10-13 In a recent study conducted by the Lymphoma Working Party (LWP) of the of the European Society for Blood and Marrow Transplantation (EBMT), PTCy in patients with Hodgkin lymphoma (HL) undergoing HLA-matched HSCT showed low rates of GVHD and nonrelapse mortality (NRM), resulting in a significant improvement of overall survival (OS).14 Despite these compelling findings, a critical gap in the current literature exists, as no studies have explored the comparative efficacy of PTCy against standard GVHD prophylaxis regimens, specifically within the context of HLA-matched donors in patients with HL.
The aim of this study was to compare the outcomes of patients with HL undergoing HSCT from HLA-matched donors, which included matched sibling donors (MSDs) and matched unrelated donors (MUDs), using PTCy or conventional CNI-based GVHD prophylaxis.
Methods
Study design and data source
This is a retrospective registry-based analysis on behalf of the LWP of the EBMT. The EBMT is a voluntary working group of >600 transplantation centers that are required to report all consecutive HSCT and follow-up once a year. Audits are routinely performed to determine the accuracy of the data. All transplantation centers are required to obtain written informed consent before data registration with the EBMT, in accordance with the 1975 Declaration of Helsinki.
Patient eligibility
The study included all adult (≥18 years) patients diagnosed with HL who underwent their first allogeneic HSCT from full MSD or MUD between January 2015 and December 2022, and contemporarily received either PTCy or conventional CNI-based GVHD prophylaxis registered at the EBMT. HLA typing was determined at high-level resolution for 10 loci, including HLA-A, -B, -C, -DRB1, and -DQB1. The study excluded patients who received cord blood, ATG, alemtuzumab, or ex vivo graft manipulation.
Statistical analysis
The study end points were OS, progression-free survival (PFS), relapse incidence, NRM, engraftment, acute GVHD, chronic GVHD (cGVHD), and GVHD-free, relapse-free survival (GRFS). All end points were assessed at the time of transplantation. Engraftment was defined as achieving an absolute neutrophil count ≥0.5 × 109/L for 3 consecutive days. OS was defined as the time to death from any cause. PFS was defined as survival with no evidence of relapse or progression. NRM was defined as death from any cause without previous relapse or progression. GRFS events were defined as the first event among grades 3 to 4 acute GVHD, extensive cGVHD, relapse, or death from any other cause.15
Patient, disease, and transplant-related characteristics were compared between the 2 groups (PTCy and conventional CNI-based GVHD regimens) using the Mann-Whitney U test for numerical variables and the χ2 or Fisher exact test for categorical variables. The characteristics were described using the median and interquartile range for quantitative variables and frequency and percentage for categorical variables. The probabilities of OS, PFS, and GRFS were calculated using Kaplan-Meier estimates. Relapse incidence and NRM were calculated using cumulative incidence curves in a competing risk setting; death in remission being treated as a competing event for relapse. Early death was considered as a competing event for engraftment. To estimate the cumulative incidence of acute or cGVHD, relapse and death were considered as competing events. Univariate analyses were performed using the log-rank test for PFS and OS, whereas Gray test was used for the cumulative incidence.
Multivariate analyses were performed using the Cox proportional hazards regression model for survival outcomes and Fine and Gray test for cumulative incidence. Potential risk factors included in the model were transplant type, age, patients’ sex, disease status at HSCT, performance status at HSCT, and myeloablative conditioning. The results were expressed as the hazard ratio (HR) with a 95% confidence interval (95% CI). All P values were 2-sided, with a type 1 error rate fixed at .05. Statistical analyses were performed using the R statistical software version 4.0.2.
Results
Patient and transplantation characteristics
Table 1 summarizes the characteristics of the 270 and 176 patients from the conventional CNI-based and PTCy platforms, respectively. Patient and disease characteristics were comparable between the 2 groups, with the exception of a higher proportion of patients with a history of previous autologous HSCT in the conventional CNI-based platform (77% vs 67%; P = .01). Regarding transplant characteristics, in the conventional CNI-based cohort, 74% of patients underwent transplantation from MSD and 26% from MUD, whereas in the PTCy cohort, the distribution was 50% and 50%, respectively (P < .001).
In the PTCy cohort, most patients (72%) received GVHD prophylaxis with PTCy along with 2 additional immunosuppressive drugs (IS). Various combinations of IS drugs were used alongside PTCy, including cyclosporine A (CSA) plus mycophenolate mofetil (MMF) (n = 67), tacrolimus plus MMF (n = 34), sirolimus plus MMF (n = 20), and others (n = 5). A minority of the patients received a single IS drug, including CSA (n = 19), everolimus (n = 6), tacrolimus (n = 7), MMF (n = 4), methotrexate (n = 3), and sirolimus (n = 2). Regarding the conventional CNI-based platform, CsA plus Methotrexate (MTX) (n = 137, 51%), and CsA plus MMF (n = 74, 27%) were the most commonly used regimens . Unfortunately, data regarding patients who received treatment with PD-1 inhibitors before transplantation were available for only 20% of patients (n = 92). Among these, 36 (13%) and 56 (31%) patients had previously received PD-1 in the CNI-based and PTCy cohorts, respectively.
Engraftment
The cumulative incidence of neutrophil recovery at 30 days was 99% (95% CI, 96-100) in the conventional CNI-based group and 95% (95% CI, 90-98) in the PTCy cohort. The median time to neutrophil recovery was 13 days (95% CI, 12-14) and 20 days (95% CI, 19-20), respectively (P < .001; Table 2).
The 60-day cumulative incidence of platelet recovery was 97% (95% CI, 93-98) in the conventional CNI-based cohort and 92% (95% CI, 86-95) in the PTCy cohort. The median time to platelet engraftment was 15 days (95% CI, 14-16) and 21 days (95% CI, 18-23), respectively (P < .001; Table 2).
GVHD
The cumulative incidence of acute GVHD grade 2 to 4 at 100 days in the conventional CNI-based and PTCy cohorts was 31% (95% CI, 25-37) and 26% (95% CI, 19-33), respectively (P = .31), whereas for grade 3 to 4, it was 15% (95% CI, 11-20) and 10% (95% CI, 5-15), (P = .17; Table 2). In the multivariable analysis (Table 3), no statistically significant variables were identified as predictors of GVHD grades 2 to 4 or 3 to 4 GVHD.
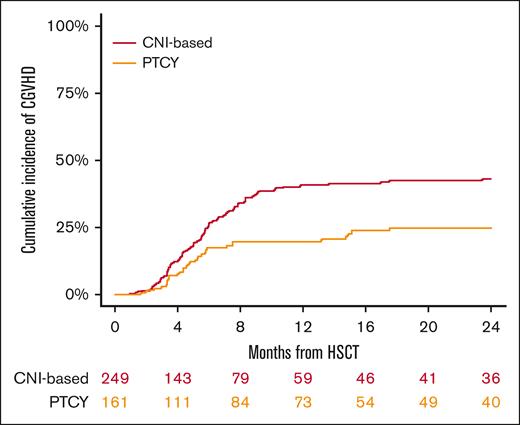
The 2-year cumulative incidence of cGVHD in the conventional CNI-based and PTCy cohorts was 43% (95% CI, 36-50) and 25% (95% CI, 18-33), respectively (P < .001; Figure 1), whereas for extensive cGVHD, it was 28% (95% CI, 22-34) and 13% (95% CI, 8-19) (P = .003), respectively (Table 2). In multivariable analysis (Table 3), PTCy prophylaxis demonstrated an independent association with a decreased risk of chronic (HR, 0.46; 95% CI, 0.3-0.7; P < .001) and extensive cGVHD (HR, 0.42; 95% CI, 0.2-0.8; P = .004) compared with the conventional CNI-based platform. Additionally, the use of myeloablative conditioning regimens (HR, 0.51; 95% CI, 0.2-0.9; P = .04) was associated with a reduced risk of extensive cGVHD.
NRM and relapse
Forty-nine and 17 patients died without experiencing disease relapse and/or progression in the conventional CNI-based and PTCy cohort, respectively. In both cohorts, the primary causes of NRM were infections and GVHD. In the conventional CNI-based cohort, these accounted for 8 cases (16%) and 25 cases (51%), respectively, whereas in the PTCy cohort, infections were responsible for 6 cases (35%), and GVHD contributed to 7 cases (41%). The cumulative incidence of NRM at 2 years was 17% (95% CI, 12-22) for the conventional CNI-based cohort and 11% (95% CI, 6-17) for the PTCy (P = .12; Table 2). In multivariable analysis (Table 3), increasing patient age was associated withincreased NRM (HR for 10 years increase 1.53; 95% CI, 1.2-1.9; P < .001).
The cumulative incidence of relapse at 2 years was 30% (95% CI, 23-36) for the conventional CNI-based and 17% (95% CI, 11-24) for PTCy (P = .007; Figure 2; Table 2). In the multivariable analysis (Table 3), the PTCy platform showed a reduced risk of relapse compared with the conventional CNI-based platform (HR, 0.58; 95% CI, 0.3-0.9; P = .03). Additionally, MUD was associated with a decreased risk of relapse (HR, 0.42; 95% CI, 0.2-0.7; P = .004), whereas refractory HL was linked to a higher relapse risk (HR, 2.36; 95% CI, 1.4-3.9; P < .001).
Survival outcomes
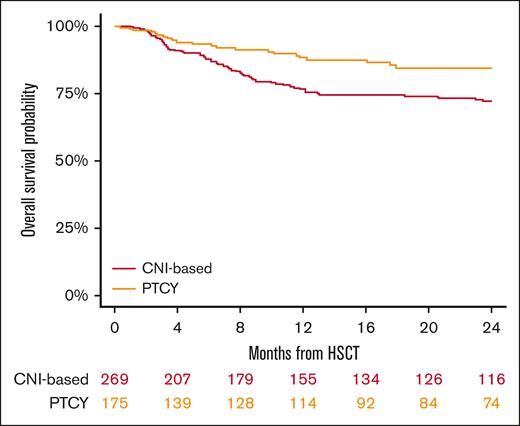
The median follow-up duration for surviving patients was 26 months (95% CI, 2-30), with 24 months for the conventional CNI-based group and 28 months for those receiving PTCy. The 2-year OS rates were 72% (95% CI, 66-77) for the conventional CNI-based cohort and 85% (95% CI, 77-90) for PTCy patients (P = .005; Figure 3; Table 2). In the multivariable analysis (Table 3), PTCy was associated with improved survival in comparison with the conventional CNI-based platform (HR, 0.57; 95% CI, 0.4-0.8; P = .005). Other factors associated with worse OS included patient age (HR for 10 years increase, 1.43; 95% CI, 1.2-1.7; P < .001) and refractory HL (HR, 2.01; 95% CI, 1.2-3.4; P = .007).
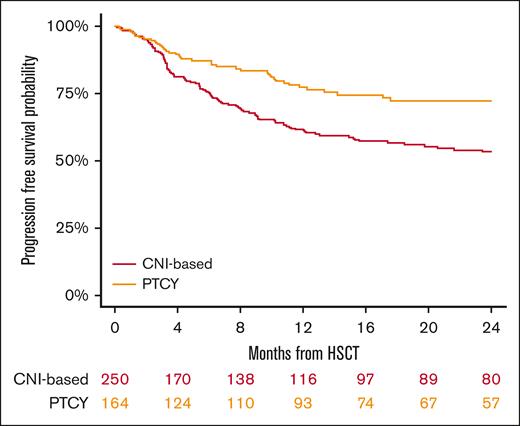
The 2-year PFS rates were 53% (95% CI, 46-60) for the conventional CNI-based group and 72% (95% CI, 64-79) for the PTCy cohort (P < .001; Figure 4; Table 2). In multivariable analysis (Table 3), PTCy was associated with improved PFS when compared with the conventional CNI-based platform (HR, 0.57; 95% CI, 0.4-0.8; P = .005). Another factor associated with worse PFS was refractory HL (HR, 1.96; 95% CI, 1.3-2.9; P < .001).
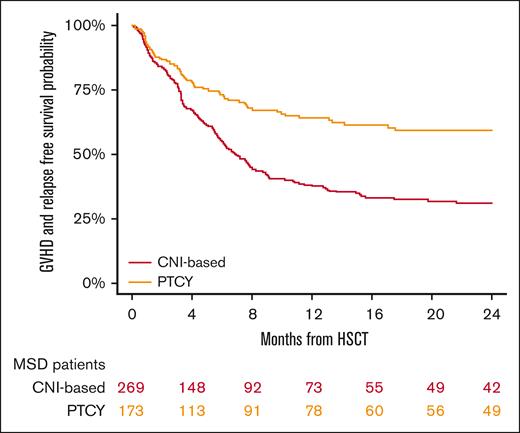
GRFS at 2 years was 31% (95% CI, 25-38) in the conventional CNI-based cohort and 59% (95% CI, 51-67) in the PTCy cohort (P < .001; Figure 5; Table 2). In the multivariable analysis (Table 3), PTCy was associated with improved GRFS compared with the conventional CNI-based platform (HR, 0.53; 95% CI, 0.4-0.7; P < .001), whereas refractory HL was associated with poorer GRFS (HR, 1.95; 95% CI, 1.4-2.7; P < .001) (Table 3).
Discussion
This study underscores that despite a delay in hematopoietic recovery associated with PTCy-based GVHD prophylaxis compared with conventional CNI-based regimens, it concurrently shows reduced rates of cGVHD and relapse, along with improved OS, PFS, and GRFS in patients with HL undergoing HSCT.
To our knowledge, this is the first study comparing the efficacy and safety of PTCy against conventional CNI-based GVHD prophylaxis in patients with HL undergoing HSCT from HLA-matched donors. Owing to the retrospective nature of a registry-based study, it is important to acknowledge that some potential bias cannot be entirely mitigated. Although all patients received either PTCy or conventional CNI-based GVHD prophylaxis, a variety of conditioning regimens were used on both platforms, particularly regarding the addition of other IS. Additionally, information regarding the duration of prophylaxis was not available. Furthermore, although the patient and disease characteristics were largely similar between the 2 cohorts, disparities were noted, including a higher proportion of patients with a history of autologous HSCT in the conventional CNI-based platform. The impact of pretransplant immune checkpoints, which have been associated with increased GVHD in multiple studies16 but with reduced relapse rates and significantly improved survival among patients with HL receiving PTCy,17 may be particularly relevant. Unfortunately, due to data unavailability, their potential impact was not explicitly addressed in our study, highlighting the need for future research. Transplant characteristics also exhibited differences, with a greater proportion of patients in the conventional CNI-based cohort undergoing transplantation from MSD. To address these limitations, adjustments for these variables were made using multivariable analyses.
Although the engraftment and primary graft failure rates were similar in both cohorts, the use of PTCy was associated with a prolonged time for neutrophil and platelet engraftment compared with conventional CNI-based regimens, as reported in previous studies.5,12,13 This represents a drawback of PTCy use, which has been associated with higher transfusion requirements and longer hospital admissions.13 Investigations on the use of reduced doses of PTCy are underway to mitigate this effect.18
In contrast to studies involving patients with other hematological malignancies, which reported significantly lower rates of acute GVHD with PTCy than with conventional CNI,5,11,19 our study, which focused on HL, did not demonstrate such differences. The limitations of our study, including the sample size and the potential influence of the specific disease analyzed, make it challenging to speculate on alternative explanations. Moreover, the presence of imbalances in certain potential confounding factors, such as donor type and heterogeneity in the additional immunosuppressive agents used in both platforms, adds complexity to the interpretation. However, the lower incidence of chronic and extensive cGVHD with PTCy found in our study aligns with previous reports including various hematological malignancies.5,8,20
Our study showed a trend toward a reduced incidence of NRM in the PTCy group compared with that in conventional CNI-based transplants. This observation aligns with findings from prior studies,19,21-23 suggesting that PTCy may be associated with a more favorable toxicity profile than that of alternative strategies. Additionally, our analysis identified patient age as a factor associated with higher NRM. It is widely acknowledged that age is a contributing factor to increased NRM across various lymphoma subtypes,24 including HL.25
Although the association between conditioning intensity and the incidence of cGVHD remains controversial in the literature,26-28 our analysis revealed a higher incidence of extensive cGVHD after reduced intensity conditioning (RIC). This finding aligns with those of other studies26 and may be attributed to association of RIC with older patient age and earlier withdrawal of immune suppression. The observed reduction in cGVHD with PTCy was not accompanied by an increased risk of disease relapse. In fact, our findings indicate a substantially lower incidence of relapse (17%) observed with PTCy-based GVHD prophylaxis, which contrasts with relapse rates in HLA-matched transplants (32%-63%) and Haplo transplants (24%-40%) reported in other series.25,29,30 Thus far, only 2 nonrandomized studies comparing Haplo, MSD, and MUD transplants in HL found lower relapse rates for Haplo recipients,25,29 traditionally attributed, at least in part, to the graft-versus-lymphoma effect induced by HLA disparity. However, we recently reported similar relapse rates between the Haplo and HLA-matched groups in the context of PTCy-based GVHD prophylaxis,31 suggesting PTCy's pivotal role. We can speculate that the antitumor effect may not solely be due to HLA disparity but rather attributable to PTCy. This could be due to 2 mechanisms: a direct antineoplastic effect of this alkylating agent on malignant disease and an indirect effect by inducing selective in vivo depletion of alloreactive T cells while preserving the graft-versus-tumor effect.19,21,22 This latter mechanism is particularly relevant in the setting of RIC regimens, which represented 75% of conditioning regimens in the PTCy cohort. Regarding other variables associated with relapse in our study, it was not surprising to find a correlation between a higher risk of relapse and refractory disease, which is a common trend across malignant diseases. Notably, a higher relapse risk was observed in MSD transplant recipients compared with MUD. This finding, previously documented in other hematological malignancies,32,33 may possibly be linked to older MSD donors with age-related T-cell exhaustion.
In conclusion, our study shows that in patients with HL undergoing HLA-matched HSCT, PTCy-based GVHD prophylaxis was associated with a reduced incidence of cGVHD, relapse and improved OS, PFS, and GRFS compared with conventional CNI-based regimens. These findings provide valuable insights for clinicians to consider when selecting the most suitable GVHD prophylaxis for transplantation and underscore the importance of further investigation and confirmation through prospective controlled trials.
Authorship
Contribution: J.M. contributed to the conception and design of the study; J.M. and M.N. contributed to data analysis and interpretation; and all authors wrote and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Juan Montoro, Department of Hematology, Hospital Universitario y Politécnico La Fe, Avenida Fernando Abril Martorell 106, 46026 Valencia, Spain; email: juanmontorogomez@gmail.com.
References
Author notes
Data are available on request from the corresponding author, Juan Montoro (juanmontorogomez@gmail.com).