Key Points
Linkage of CIBMTR with cancer registry data leads to more complete estimates of cumulative incidence of solid neoplasms following HCT.
This collaborative approach can improve risk factor evaluation to help inform follow-up care practice in the growing HCT survivor population.
Visual Abstract
Compared with the general population, hematopoietic cell transplantation (HCT) survivors are at elevated risk for developing solid subsequent neoplasms (SNs). The Center for International Blood and Marrow Transplant Research (CIBMTR) is a key resource for quantifying solid SN incidence following HCT, but the completeness of SN ascertainment is uncertain. Within a cohort of 18 450 CIBMTR patients linked to the California Cancer Registry (CCR), we evaluated the completeness of solid SN data reported to the CIBMTR from 1991 to 2018 to understand the implications of using CIBMTR data alone or combined with CCR data to quantify the burden of solid SNs after HCT. We estimated the cumulative incidence of developing a solid SN, accounting for the competing risk of death. Within the cohort, solid SNs were reported among 724 patients; 15.6% of these patients had an SN reported by CIBMTR only, 36.9% by CCR only, and 47.5% by both. The corresponding cumulative incidence of developing a solid SN at 10 years following a first HCT was 4.0% (95% confidence interval [CI], 3.5-4.4) according to CIBMTR data only, 5.3% (95% CI, 4.9-5.9) according to CCR data only, and 6.3% (95% CI, 5.7-6.8) according to both sources combined. The patterns were similar for allogeneic and autologous HCT recipients. Linking detailed HCT information from CIBMTR with comprehensive SN data from cancer registries provides an opportunity to optimize SN ascertainment for informing follow-up care practices and evaluating risk factors in the growing population of HCT survivors.
Introduction
Hematopoietic cell transplantation (HCT) plays an important role as a curative treatment for many malignant and nonmalignant hematologic disorders. Treatment advances and expanded eligibility to older populations have led to a growing population of HCT survivors,1 from ∼100 000 in 2009 to a projected 500 000 by 2030 in the United States.2 Compared with the general population, HCT survivors are at elevated risk for developing posttransplant complications, including solid subsequent neoplasms (SNs), with the risk for most solid SN types increasing with time since HCT.3-8
The Center for International Blood and Marrow Transplant Research (CIBMTR), a research collaboration between the National Marrow Donor Program and the Medical College of Wisconsin (https://www.cibmtr.org/pages/index.aspx), maintains a large, comprehensive HCT registry incorporating patient and donor demographics, clinical characteristics, HCT details, and long-term clinical outcomes including SNs, which are reported by HCT centers using standardized data collection systems. Reporting to the CIBMTR is mandatory for all US allogeneic (allo) HCTs (since 2006), and an estimated 85% of autologous (auto) HCTs are reported voluntarily.9 The CIBMTR is a key resource for evaluating risk factors and quantifying the SN burden after HCT. However, the completeness and accuracy of SN data reported to the CIBMTR are unknown because SNs, particularly those occurring years after transplantation, are often diagnosed outside of HCT centers.10 Additionally, unlike population-based cancer registries, the CIBMTR is not designed specifically to capture SNs. High-quality US population-based cancer registries collect data on ≥95% of new cancer diagnoses in their catchment area11 and have been used extensively to characterize SN incidence in cancer survivor populations.12,13 Cancer registry data, however, lack detailed information on HCT treatment approaches to examine risk factors for SNs after HCT,14 and are limited to cancers diagnosed within their catchment area. Thus, the linkage of CIBMTR data with population-based cancer registries may improve the ascertainment of SNs while leveraging CIBMTR’s detailed HCT data.
To our knowledge, no studies have compared the solid SN data reported to the CIBMTR with population-based cancer registry data. To address this gap, using a cohort of CIBMTR patients linked to the California Cancer Registry (CCR),14 we evaluated the completeness of the CIBMTR solid SN data to understand the implications of using CIBMTR data alone or in combination with CCR data for quantifying the burden of solid SNs following HCT.
Methods
Study design and population
From CIBMTR, we selected patients who underwent a first HCT for a hematologic malignancy diagnosed between 1991 and 2016, resided in California (per residential zip code in CIBMTR records) at the time of transplantation (or underwent HCT in California if residential zip code was unknown), and consented to research. From CCR, we selected all patients diagnosed with a hematologic malignancy between 1991 and 2016. We then linked the CIBMTR and CCR cohorts on date of birth, sex, social security number, residence zip code, date and type of hematologic malignancy diagnosis, transplant center, and the date and type of HCT, as described previously along with the completeness of linkage variables.14 From an initial population of 22 733 CIBMTR patients, 18 886 patients were linked to the CCR. After excluding patients with unknown date of last follow-up from either CCR or CIBMTR (n = 88), patients with last known follow-up from CCR or CIBMTR before the first HCT (n = 111), or patients who underwent an HCT before 1991 (n = 237), our final analytic cohort included 18 450 patients successfully linked to the CCR (supplemental Figure 1).
Ascertainment and classification of SNs
CIBMTR
SNs are captured in posttransplant data collection systems at 100 days, 6 months, and then annually until 6 years following HCT, after which reporting is biennial until death.9 SNs are collected for all patients, including those on the Transplant Essential Data (TED) or the more expansive Comprehensive Report Form (CRF) track, which collects additional disease and HCT information from a weighted random selection of patients. We classified solid SNs into 11 broad categories available from CIBMTR data collection forms over the entire study period (breast, brain/central nervous system [CNS], lung, gastrointestinal [GI], genitourinary [GU], oropharyngeal, thyroid, sarcoma, melanoma, nonmelanoma skin cancer [NMSC], and other/unknown; supplemental Table 1). For some records, additional text fields provided more granular information about SN type (ie, site, morphology, and/or behavior). Where available, we reclassified “other” SNs into more specific categories based on text fields. Unless otherwise specified in text fields, all SNs were presumed invasive (malignant).
CCR
Reporting to the registry is mandated statewide for all invasive and in situ malignancies (excluding cervix in situ cases diagnosed after 1995) and for benign brain and CNS tumors (since 2001) diagnosed in the state of California.15,16 Basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) NMSCs are not reportable. For each reported case, the CCR collects patient demographics, tumor, and some clinical characteristics. International Classification of Diseases for Oncology, third edition histology, topography, and behavior codes were used to classify solid tumors following HCT into the categories available for CIBMTR (supplemental Table 1).
From each source (CIBMTR and CCR), we captured the first report of each SN type (breast, brain/CNS, lung, GI, GU, oropharyngeal, thyroid, sarcoma, and melanoma), restricting to tumors reportable to CCR and excluding NMSC and other/unknown neoplasms. We captured multiple SNs of different types per patient from each source (where applicable), but only the first of each SN type. Additionally, we restricted the ascertainment of SNs to the overlapping (shared) follow-up period from the 2 sources, which began at the first HCT and continued until the earliest date of a second HCT, CIBMTR end of follow-up (date of death or last known alive), CCR end of follow-up (date of death or last known alive), or study end (31 December 2018). We excluded 9 CIBMTR SNs missing a year of diagnosis but retained the patients, some of whom had other SN diagnoses.
Statistical methods
Descriptive analyses
Among patients with at least 1 solid SN, we calculated the proportion of patients identified as having an SN by CIBMTR only, CCR only, or both sources. Each SN was also classified by ascertainment status as CIBMTR only, CCR only, or both (considered concordant if the diagnoses were in the same broad category and dates reported by CCR and CIBMTR were within 90 days). Concordance was based on broad category because CIBMTR did not systematically collect specific sites for GI, GU, and oropharyngeal SNs or sarcoma. We then evaluated the source distribution of cases, overall and by a priori selected characteristics, including HCT type, SN type, sex, age at HCT, race and ethnicity, center volume,14 CIBMTR reporting track, year of SN, and interval between HCT and SN. For cases diagnosed by both sources, the earliest reported diagnosis date was used for SN year and interval. We report the median years of person-time contributed by each patient during the shared follow-up and median interval from HCT until the date of the patient’s first SN from each source.
Additional descriptive analyses were undertaken to explore whether misclassification of cancer type or outmigration from California could explain SNs reported by CCR only or CIBMTR only. Specifically, we evaluated whether other SNs, including NMSC and other/unknown (excluded from primary analyses), were reported for these patients during the shared follow-up period. Using residential history information from a public-records database obtained through LexisNexis linkage with the CCR, we also examined whether cases reported by CIBMTR only occurred after outmigration from California. All descriptive analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Cumulative incidence
We estimated the overall cumulative incidence of developing a first solid SN within the full data set of 18 450 patients, accounting for competing risk of mortality, based on SNs reported in CCR data alone, CIBMTR data alone, and both sources combined using Stata Statistical Software: Release 18 (StataCorp LLC, College Station, TX).17 These analyses were done to illustrate the effect of using the different data sources and are not intended for comparison with previous studies of the CIBMTR data based on populations that differed in their distribution by age, HCT type and date, and other factors.5,6
Multivariable logistic regression models
We fitted polychotomous multivariable generalized estimating equations regression models18 accounting for within-person correlation (because individuals could have >1 SN type from each source) to investigate the association between the patient-, HCT-, and SN-related characteristics included in the descriptive analyses and reporting source (CCR only vs both, CIBMTR only vs both). Odds ratios (ORs) and 95% confidence intervals (CIs) of CCR only vs both and CIBMTR only vs both were estimated from a mutually adjusted model (SAS version 9.4).
P values <.05 were considered statistically significant. Analyses were conducted overall and by HCT type (alloHCT, autoHCT). Case counts and percentages were suppressed for cell sizes <5 to protect patient confidentiality.
The protocol for this study was approved by the institutional review boards of the University of California, Davis, the California Committee for the Protection of Human Subjects and the National Marrow Donor Program, and the study was determined not to be human subjects research by the National Cancer Institute.
Results
Within the study population of 18 450 patients, 8232 underwent alloHCT (44.6%), and 10 218 (55.4%) underwent autoHCT; 10 822 (58.7%) were male; and the median age at HCT was 50 years (range, 0.4-84; supplemental Table 2). For alloHCT, primarily performed for patients with acute myeloid leukemia and acute lymphoblastic leukemia/lymphoma, the median age at HCT was 42 years (range, 0.4-78.6). For autoHCT, the primary indications were multiple myeloma/plasma cell neoplasms followed by diffuse large B-cell lymphoma, and the median age at HCT was 55 years (range, 0.4-84). Only 2795 patients (15.1%) underwent HCT before 2000, whereas nearly half of patients (n = 8748; 47.4%) underwent HCT from 2010 to 2016. Overall, the median person-years from HCT to end of shared follow-up (as defined above) were 2.4 years (range, 0.003-27.4; alloHCT, 1.8 years; autoHCT, 2.9 years), with 28% of patients having at least 5 years and 9% having at least 10 person-years of follow-up.
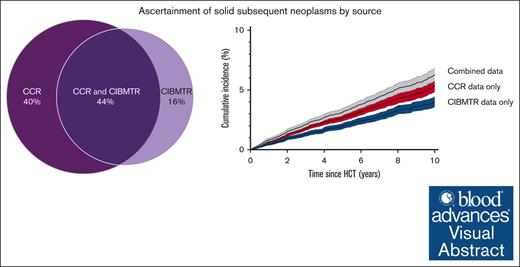
We first conducted analyses at the patient level. According to the CIBMTR data alone, 457 patients (2.5%) had an SN, of whom 13 (2.8%) had >1 type, yielding a cumulative incidence of 4.0% (95% CI, 3.5-4.4) of developing an SN at 10 years (Figure 1A). According to the CCR data alone, SNs were identified among 611 patients (3.3%), of whom 44 (7.2%) had >1 SN type, with a corresponding cumulative incidence of 5.3% (95% CI, 4.9-5.9) at 10 years. In the combined CIBMTR and CCR data, 724 patients had an SN, of whom 47 (6.5%) had >1 type. The corresponding cumulative incidence of developing an SN at 10 years was 6.3% (95% CI, 5.7-6.8; Figure 1A). Although the magnitude differed, the cumulative incidence patterns based on CIBMTR data only, CCR data only, and the combined data set were similar by HCT type (Figure 1B-C) and time since HCT (supplemental Table 3). Among these 724 patients, 113 (15.6%) had an SN reported by CIBMTR only, 267 (36.9%) by CCR only, and 344 (47.5%) by both (Figure 2), with similar patterns observed in the alloHCT and autoHCT groups.
Cumulative incidence of developing a subsequent solid neoplasm, by source, overall, and by HCT type. Cumulative incidence of developing a solid SN (excluding NMSC and other/unknown neoplasms) for all patients combined (A), following alloHCT (B), and following autoHCT (C). Includes SNs diagnosed after 1990, after first HCT and before the end of follow-up (defined as the earliest occurrence of a second HCT, death, last known alive status from either CCR or CIBMTR, or the study end date [31 December 2018]). Cumulative incidence at 10 years following HCT shown in text.
Cumulative incidence of developing a subsequent solid neoplasm, by source, overall, and by HCT type. Cumulative incidence of developing a solid SN (excluding NMSC and other/unknown neoplasms) for all patients combined (A), following alloHCT (B), and following autoHCT (C). Includes SNs diagnosed after 1990, after first HCT and before the end of follow-up (defined as the earliest occurrence of a second HCT, death, last known alive status from either CCR or CIBMTR, or the study end date [31 December 2018]). Cumulative incidence at 10 years following HCT shown in text.
SN source of ascertainment (percentage) among patients with at least 1 reported SN within the linked CIBMTR–CCR data set of 18 450 patients. Includes patients diagnosed with a solid SN in the breast, brain and CNS, lung, GI and GU tracts, oropharynx, or thyroid, or sarcoma or melanoma after the first HCT and before the end of follow-up (defined as the earliest occurrence of a second HCT, death, last known alive status from either CCR or CIBMTR, or the study end date [31 December 2018]). For this figure, patients with multiple SN types were counted once.
SN source of ascertainment (percentage) among patients with at least 1 reported SN within the linked CIBMTR–CCR data set of 18 450 patients. Includes patients diagnosed with a solid SN in the breast, brain and CNS, lung, GI and GU tracts, oropharynx, or thyroid, or sarcoma or melanoma after the first HCT and before the end of follow-up (defined as the earliest occurrence of a second HCT, death, last known alive status from either CCR or CIBMTR, or the study end date [31 December 2018]). For this figure, patients with multiple SN types were counted once.
We then conducted analyses at the SN level, counting multiple SN types per patient but only the patient’s first diagnosis of each SN type from each source. Collectively, 785 SNs were diagnosed among the 724 patients. Overall and by HCT type, SNs of the GU tract and melanoma were the most common types reported by both CIBMTR and CCR (Figure 3). The median interval from HCT to a first SN (any type) was 3.9 years for CIBMTR and 3.7 years for CCR. At the SN level, considering each SN type diagnosis separately, we evaluated whether any patient-, HCT- or SN-related characteristics were associated with the reporting source of SN (Figures 4 and 5; supplemental Figures 2-4). Overall, 16.4% of SNs were identified by CIBMTR only, 40.1% by CCR only, and 43.4% by both (40.4% with diagnosis dates within 90 days in the 2 sources and 3.0% with discordant dates) (Figure 4). Given the small number of SNs diagnosed by both sources with discordant dates, further analyses did not consider date discrepancy.
SN types (percentage) reported by each source, overall, and by HCT type. Percentages based on solid SNs excluding NMSC and other/unknown neoplasms diagnosed after 1990, after the first HCT and before the end of follow-up (defined as the earliest occurrence of a second HCT, death, last known alive status from either CCR or CIBMTR, or the study end date [31 December 2018]). For each patient, a maximum of 1 SN per type is counted, but patients with multiple SNs can contribute to >1 SN type. In CIBMTR, there were a total of 470 SNs diagnosed among 457 patients. In CCR, there were a total of 656 SNs among 611 patients.
SN types (percentage) reported by each source, overall, and by HCT type. Percentages based on solid SNs excluding NMSC and other/unknown neoplasms diagnosed after 1990, after the first HCT and before the end of follow-up (defined as the earliest occurrence of a second HCT, death, last known alive status from either CCR or CIBMTR, or the study end date [31 December 2018]). For each patient, a maximum of 1 SN per type is counted, but patients with multiple SNs can contribute to >1 SN type. In CIBMTR, there were a total of 470 SNs diagnosed among 457 patients. In CCR, there were a total of 656 SNs among 611 patients.
Distribution (percentage) of 785 SNs among 724 patients, by reporting source. Includes solid SNs excluding NMSC and other/unknown neoplasms diagnosed after the first HCT and before the end of follow-up (defined as the earliest occurrence of a second HCT, death, last known alive status from either CCR or CIBMTR, or the study end date [31 December 2018]). Other and unknown race includes Native Hawaiian and other Pacific Islander and American Indian/Alaska Native patients. For each patient, a maximum of 1 SN per type is counted, but patients with multiple SNs can contribute to >1 SN type. For SNs reported by both sources, date of SN and interval between HCT and SN are based on the earlier reported SN (if dates differed between CCR and CIBMTR). For confidentiality, row percentages were suppressed if any cells had <5 SNs. CA, California; NHB, non-Hispanic Black; NHW, non-Hispanic White.
Distribution (percentage) of 785 SNs among 724 patients, by reporting source. Includes solid SNs excluding NMSC and other/unknown neoplasms diagnosed after the first HCT and before the end of follow-up (defined as the earliest occurrence of a second HCT, death, last known alive status from either CCR or CIBMTR, or the study end date [31 December 2018]). Other and unknown race includes Native Hawaiian and other Pacific Islander and American Indian/Alaska Native patients. For each patient, a maximum of 1 SN per type is counted, but patients with multiple SNs can contribute to >1 SN type. For SNs reported by both sources, date of SN and interval between HCT and SN are based on the earlier reported SN (if dates differed between CCR and CIBMTR). For confidentiality, row percentages were suppressed if any cells had <5 SNs. CA, California; NHB, non-Hispanic Black; NHW, non-Hispanic White.
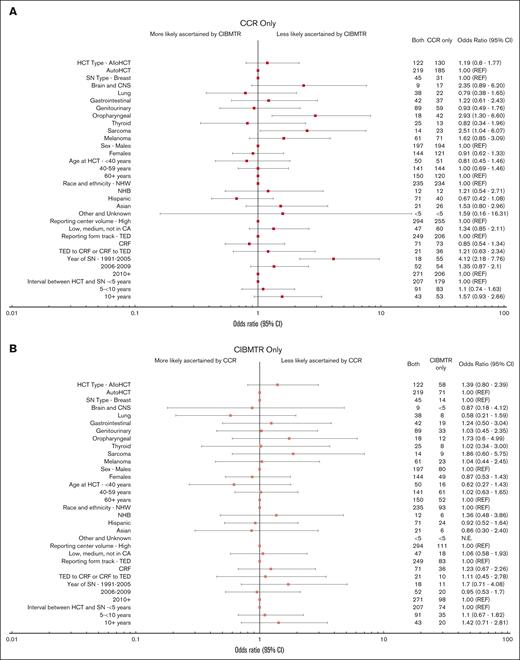
Forest plot of odds of SN ascertainment by source. ORs and 95% CIs derived from multivariable polychotomous generalized estimating equations models accounting for within-person correlation due to patients having >1 SN type. Other and unknown race includes Native Hawaiian and other Pacific Islander and American Indian/Alaska Native patients. Center volume is defined as the number of alloHCTs performed at each center using information in the CIBMTR database and grouped into tertiles (low, <140; medium, 140-459; high, ≥460).14 For SNs reported by both sources, date of SN and interval between HCT and SN are based on the earlier reported SN (if dates differed between CCR and CIBMTR).
Forest plot of odds of SN ascertainment by source. ORs and 95% CIs derived from multivariable polychotomous generalized estimating equations models accounting for within-person correlation due to patients having >1 SN type. Other and unknown race includes Native Hawaiian and other Pacific Islander and American Indian/Alaska Native patients. Center volume is defined as the number of alloHCTs performed at each center using information in the CIBMTR database and grouped into tertiles (low, <140; medium, 140-459; high, ≥460).14 For SNs reported by both sources, date of SN and interval between HCT and SN are based on the earlier reported SN (if dates differed between CCR and CIBMTR).
The primary factors associated with SN reporting source were SN type and calendar year of the SN diagnosis (Figures 4 and 5; supplemental Figures 2-4). For all patients combined, oropharyngeal (58.3% [n = 42]; OR, 2.93; 95% CI, 1.30-6.60) and sarcoma (50.0% [n = 23]; OR, 2.51; 95% CI, 1.04-6.07) SNs were more likely to be reported by CCR only than by both sources compared with the referent category of breast cancer (34.4%; n = 31) (Figures 4 and 5). The oropharyngeal finding was only statistically significant within the alloHCT group (supplemental Figures 2 and 3), whereas the sarcoma association was only statistically significant in the autoHCT group (supplemental Figures 2 and 4). Melanoma was more likely to be ascertained by CCR only than by both sources (supplemental Figures 2 and 4) after autoHCT. Compared with the most recent calendar-year period, SNs diagnosed before 2005 were more likely to be ascertained by CCR only (35.8% [n = 206]) than by both sources (OR, 4.12; 95% CI, 2.18-7.76), overall (Figures 4 and 5) and by HCT type (supplemental Figures 2-4).
For all patients combined, other analyzed factors were not significantly associated with ascertainment source (Figures 4 and 5). After autoHCT, however, the ascertainment by CCR only vs both sources further varied by time since HCT and center volume. SNs diagnosed at ≥10 years since HCT (vs <5 years) were more likely to be CCR only, as were SNs among patients who underwent HCT outside of California high-volume centers (supplemental Figures 2 and 4). Lastly, although most autoHCT survivors were on the TED track (86.7%), the odds of ascertainment by CIBMTR only vs both sources were more than twofold higher for patients on the CRF vs TED track.
Cases reported by CCR only
Among the 315 SNs reported by CCR only (Table 1), 29% (n = 90) occurred in patients for whom a different SN type (including NMSC or other/unknown, which were excluded from the main analyses) was reported by CIBMTR. Specifically, 14% had a CIBMTR SN diagnosed within 90 days of the CCR-only SN. Notably, 67% of patients with an oropharyngeal SN ascertained by CCR only had a different SN type reported by CIBMTR. A number of these CCR-only oropharyngeal cases, primarily SCC, had a CIBMTR report for NMSC (some specifying “mouth” as the site) within 90 days of the CCR diagnosis. Additionally, some of the sarcomas reported by CCR only were in the GU tract and had a concurrent SN of the GU reported by CIBMTR. Lastly, 25% of the SNs reported by CCR only were benign brain tumors or in situ tumors, predominantly melanomas and breast tumors.
Further evaluation of SNs identified by CCR only or CIBMTR only
| CCR only . | Number of CCR-only SNs . | % of CCR-only cases with a different SN in CIBMTR in the same patient . | % of CCR-only cases with a different SN in CIBMTR within 90 d of the CCR SN in the same patient . | % of CCR-only SNs that are malignant . |
|---|---|---|---|---|
| SN type . | ||||
| All | 315 | 29 | 14 | 75 |
| Breast | 31 | 10 to <15 | None | 74 |
| Brain/CNS | 17 | 29 | None | 5 to <10 |
| Lung | 22 | 23 | 10 to <15 | 100 |
| GI | 37 | 22 | 5 to <10 | 78 |
| GU | 59 | 15 | 5 to <10 | 78 |
| Oropharyngeal | 42 | 67 | 38 | 95 |
| Thyroid | 13 | 5 to <10 | 5 to <10 | 100 |
| Sarcoma | 23 | 43 | 30 | 100 |
| Melanoma | 71 | 28 | 13 | 55 |
| CCR only . | Number of CCR-only SNs . | % of CCR-only cases with a different SN in CIBMTR in the same patient . | % of CCR-only cases with a different SN in CIBMTR within 90 d of the CCR SN in the same patient . | % of CCR-only SNs that are malignant . |
|---|---|---|---|---|
| SN type . | ||||
| All | 315 | 29 | 14 | 75 |
| Breast | 31 | 10 to <15 | None | 74 |
| Brain/CNS | 17 | 29 | None | 5 to <10 |
| Lung | 22 | 23 | 10 to <15 | 100 |
| GI | 37 | 22 | 5 to <10 | 78 |
| GU | 59 | 15 | 5 to <10 | 78 |
| Oropharyngeal | 42 | 67 | 38 | 95 |
| Thyroid | 13 | 5 to <10 | 5 to <10 | 100 |
| Sarcoma | 23 | 43 | 30 | 100 |
| Melanoma | 71 | 28 | 13 | 55 |
| CIBMTR only . | Number of CIBMTR-only SNs . | % of CIBMTR-only cases with a different SN in CCR in the same patient . | % of cases with a different SN in CCR within 90 d of the CIBMTR SN in the same patient . | % of patients who migrated out of CA before the date of the first SN (of this type) . |
|---|---|---|---|---|
| SN type . | ||||
| All | 129 | 19 | 12 | 16 |
| Breast | 14 | 5 to <10 | 5 to <10 | 20 to <25 |
| Brain/CNS | <5 | None | None | NR |
| Lung | 8 | 25 to <30 | 10 to <15 | None |
| GI | 19 | 15 to <20 | 10 to <15 | 20 to <25 |
| GU | 33 | 15 to <20 | 10 to <15 | 10 to <15 |
| Oropharyngeal | 12 | 67 | 50 | 25 to <30 |
| Thyroid | 8 | None | None | 25 to <30 |
| Sarcoma | 9 | 20 to <25 | 10 to <15 | 10 to <15 |
| Melanoma | 23 | 5 to <10 | None | 5 to <10 |
| CIBMTR only . | Number of CIBMTR-only SNs . | % of CIBMTR-only cases with a different SN in CCR in the same patient . | % of cases with a different SN in CCR within 90 d of the CIBMTR SN in the same patient . | % of patients who migrated out of CA before the date of the first SN (of this type) . |
|---|---|---|---|---|
| SN type . | ||||
| All | 129 | 19 | 12 | 16 |
| Breast | 14 | 5 to <10 | 5 to <10 | 20 to <25 |
| Brain/CNS | <5 | None | None | NR |
| Lung | 8 | 25 to <30 | 10 to <15 | None |
| GI | 19 | 15 to <20 | 10 to <15 | 20 to <25 |
| GU | 33 | 15 to <20 | 10 to <15 | 10 to <15 |
| Oropharyngeal | 12 | 67 | 50 | 25 to <30 |
| Thyroid | 8 | None | None | 25 to <30 |
| Sarcoma | 9 | 20 to <25 | 10 to <15 | 10 to <15 |
| Melanoma | 23 | 5 to <10 | None | 5 to <10 |
Different SNs include all of the SN types listed in the table and the categories of NMSC and other and unknown. Percentages are presented in categories to suppress actual cell sizes <5.
NR, not reported because of small sample size.
Cases reported by CIBTMR only
There were 129 SNs reported by CIBMTR only (Table 1). Nearly 20% occurred in patients for whom CCR reported a different SN type. For some GU SNs reported by CIBMTR only, the patients had sarcoma of the GU tract, which we captured as sarcoma in the CCR data (as noted above). A few cases were reported to CIBMTR as lung and oropharyngeal SNs with a concurrent CCR report classified as other/unknown on the basis of histology and topography codes indicative of mesothelioma and cancer of the larynx, respectively. Approximately 16% of cases diagnosed by CIBMTR only occurred after migration from California, as determined through data linkage with LexisNexis.
Discussion
Our results suggest that linking CIBMTR and CCR data leads to a more complete assessment of the burden of subsequent solid SNs (excluding BCC and SCC) after HCT than either source alone. The 10-year cumulative incidence of developing a solid SN after HCT increased from 4.0% using CIBMTR data only to 5.3% using CCR data only to 6.3% using the combined data set. Importantly, at both the patient and SN level, each source ascertained cases not captured by the other, although more cases were identified from CCR only (40.1%) than from CIBMTR only (16.4%). Oropharyngeal and sarcoma SNs were more likely to be reported by CCR only compared with other cancer types, as were SNs diagnosed before 2005 compared with the 2010 to 2016 period. Ascertainment was not significantly associated with other patient- or HCT-related factors overall. Given that all analyses were conducted during the shared follow-up time available from both CIBMTR and CCR, longer follow-up in CCR19 does not explain our findings. Below, we consider the implications of these findings for surveillance and risk factor analyses of SNs following HCT and opportunities to improve data collection for long-term outcomes among HCT recipients.
Further investigation of SNs reported by CCR only or CIBMTR only suggested that some SNs were indeed reported to the other source but had been classified as a different SN type, emphasizing the importance of SN pathology report collection by HCT centers. For CCR, we used histology and topography codes to classify SNs, whereas for CIBMTR, we relied on the SN categories in the reporting form. Upon further evaluation of the oropharyngeal (primarily SCCs) SNs reported by CCR only, 38% had a different SN reported to CIBMTR within 90 days of the CCR SN, most commonly NMSC (including some that specified “mouth” as the site). One strength of CIBMTR is the inclusion of BCC and SCC of the skin, which are not reported to CCR or most US cancer registries. Our study suggests, however, that some noncutaneous SCCs may inadvertently be reported to CIBMTR as NMSC, although we cannot confirm this without pathology reports. Notably, oropharyngeal SNs accounted for a greater proportion of SNs after allo vs auto HCT, consistent with the previously reported SN patterns20 after alloHCT and autoHCT, which may explain why the association was only statistically significant in the alloHCT group. Although based on much smaller numbers, we also identified sarcomas of the GU tract (based on CCR histology codes) reported by CCR only among patients who had a GU tumor reported by CIBMTR only with the same date of diagnosis. Importantly, CIBMTR recently introduced a more detailed reporting form for SNs specifying that soft tissue sarcomas of all sites are to be reported under the category of sarcoma, and thus our findings may not apply to more recently collected CIBMTR data. Nevertheless, these findings highlight the importance of reviewing pathology reports to confirm diagnoses reported to the CIBMTR, as typically done in CIBMTR SN studies.5,6 Pathology report submission is highly recommended for subsequent malignancies but is not required; reports are only available for a subset of SN reports to CIBMTR and thus require separate requests for individual studies. Increased collection of SN pathology reports by HCT centers will likely continue to improve SN classification.
Classification differences alone, however, cannot fully explain our findings given that most SNs ascertained by CCR only were among patients for whom no SN (including NMSC and other/unknown) was reported to CIBMTR and vice versa. Reassuringly, the proportion of tumors reported by CCR only decreased over calendar time (year of SN diagnosis), most notably in the autoHCT group, suggesting improved ascertainment by CIBMTR. Although we restricted the comparison to the follow-up time shared between CCR and CIBMTR, a previous study of mortality outcomes within the linked CIBMTR-CCR cohort suggested that the duration of follow-up has also improved in CIBMTR over time.19 Nonetheless, variation in the completeness of SNs reported to CIBMTR by calendar time should be considered when investigating potential HCT risk factors that may also vary by time, such as alloHCT conditioning regimens.21 Notably, ∼25% of SN cases that were reported by CCR only were benign brain tumors or in situ tumors, primarily of the breast or melanomas. The CIBMTR may consider specifically requesting that these tumors be reported in future revisions of the SN data collection form.
CIBMTR reporting track and center volume were associated with ascertainment among autoHCT but not among alloHCT recipients. SNs occurring among recipients who received their HCT from lower-volume and out-of-state facilities were more likely to be ascertained by CCR only than were SNs from high-volume centers. High-volume centers may have more resources and infrastructure to identify and report events after autoHCT compared with low-volume centers, whereas patients who go out of state for their HCT are potentially less likely to have their subsequent malignancies reported to the HCT center. In contrast, SNs diagnosed among patients on the CRF track were more likely to be ascertained by CIBMTR only than by both sources, compared with those on the TED track. This finding should be interpreted cautiously given the small number of autoHCT recipients in the CRF group.
Linkage between the CIBMTR and cancer registry data increases the ascertainment of SNs and provides additional details regarding tumor histology, topography, and behavior that are not routinely collected by the CIBMTR. Incorporating the collection of these tumor characteristics into the CIBMTR reporting forms could enable more precise classification while reducing the need for conducting pathology report reviews, as has been done previously.5,6 Importantly, the new SN form noted above captures subtypes within broad categories of GI, GU, and CNS, whereas these subtypes were not systematically collected in prior years and thus could not be evaluated herein. Comparisons at these more detailed levels might have yielded different estimates of the source distribution. Additional SN details, however, are only useful for cases that are reported to the CIBMTR. Our findings suggest that linkage with cancer registry data can improve the completeness of SN ascertainment and potentially reduce the burden of reporting by transplant centers. However, our findings indicate that ∼16% of SNs occurred after the patient migrated out of California and thus out of the registry’s catchment area. Linkage to the Virtual Pooled Registry Cancer Linkage System, a mechanism coordinated by the North American Association of Cancer Registries to facilitate linkage with population-based cancer registries across the United States, would largely reduce the impact of migration. Continued ascertainment of solid SNs by CIBMTR, however, is also important because it collects BCC and SCC of the skin, which are not reportable to the cancer registries and have been associated with prior radiation exposure and lead to substantial patient morbidity.6 Additionally, registry linkage is complex and resource-intensive.
We interpret our findings within the context of several additional limitations. We present cumulative incidence estimates of solid SNs based on the 9 specific SN types evaluated herein, for all patients combined, and by HCT type, but recognize that cumulative incidence varies by other factors such as sex, age, and conditioning regimen, which were not accounted for. Our estimates are intended to illustrate the impact of using the different data sources (CIBMTR data alone, CCR data alone, or combined) and are not intended for comparison with previous studies of the CIBMTR data based on populations that differed in their distribution by age, HCT type and date, and other factors.5,6 For the same reasons, we cannot compare the estimates between the alloHCT and autoHCT groups. The association between patient- and HCT-related factors and the risk of solid SNs will be explored in future studies, and cumulative incidence estimates will be presented accordingly. These analyses will leverage the detailed cancer (prior malignancies, tumor characteristics, initial course of treatment) and patient demographic information available from the CCR with the detailed HCT-related information (conditioning regimens, graft-versus-host disease, graft-versus-host disease prophylaxis, Karnofsky score, and donor source and type). Although this study was based on a large cohort, small sample sizes for some variables were a limitation, particularly for the ORs by HCT type. Thus, these results should be interpreted cautiously. Given that our study was restricted to California, ascertainment estimates could vary in other geographic locations, depending on the quality of the cancer registry and the capacity of local HCT centers to follow-up patients and report this follow-up to the CIBMTR (or another transplant registry). However, our analyses did not suggest that transplant center characteristics were major determinants of ascertainment. Strengths of this study include the broad calendar period and the inclusion of all age groups and both alloHCT and autoHCT recipients who underwent transplantation for all types of hematologic malignancies, thereby allowing for a comprehensive evaluation of factors associated with SN ascertainment.
In conclusion, this study suggests that using either CCR or CIBMTR data alone leads to incomplete ascertainment of SNs following HCT for hematologic malignancies. Although there are inherent limitations to conducting SN analyses in CIBMTR given that this registry was not designed specifically to capture SNs, linking detailed HCT treatment information from CIBMTR with comprehensive SN data from cancer registries provides a unique opportunity to optimize SN ascertainment. This collaborative approach can improve risk factor evaluation to help inform follow-up care practices in the growing population of HCT survivors and recipients of novel cellular therapies while leveraging the CIBMTR’s detailed treatment data.
Acknowledgments
This work was supported in part by the Intramural Program of the National Cancer Institute (NCI) and by a contract from the NCI (grant 75N91019Q0116). T.H.M.K. is supported by the University of California Davis Comprehensive Cancer Center (grant P30CA093373). T.W. is supported by the National Center for Advancing Translational Sciences (grant UL1 0000860). The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to the California Health and Safety Code Section 103885; the Centers for Disease Control and Prevention (CDC) National Program of Cancer Registries (cooperative agreement 5NU58DP006344); and the NCI’s Surveillance, Epidemiology, and End Results Program under contract HHSN261201800032I awarded to the University of California San Francisco, contract HHSN261201800015I awarded to the University of Southern California, and contract HHSN261201800009I awarded to the Public Health Institute. The Center for International Blood and Marrow Transplant Research is supported primarily by the Public Health Service Grant (grant U24CA076518) from the NCI, the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases; the Health Resources and Services Administration (grant 75R60222C0001); and the Office of Naval Research (awards N00014-23-1-2057 and N00014-24-1-205). Support is also provided by the Medical College of Wisconsin, the National Marrow Donor Program, Gateway for Cancer Research, Pediatric Transplantation and Cellular Therapy Consortium and the following commercial entities: AbbVie, Actinium Pharmaceuticals, Adaptive Biotechnologies Corporation; ADC Therapeutics, Adienne SA, Alexion, AlloVir, Amgen, Anthem, Astellas Pharma US, AstraZeneca, Atara Biotherapeutics, BeiGene, BioLineRX, Blue Spark Technologies, bluebird bio, Blueprint Medicines, Bristol Myers Squibb, CareDx, CSL Behring, CytoSen Therapeutics, Eurofins Viracor (doing business as Eurofins Transplant Diagnostics), Gamida Cell, Gilead, Gift of Life Biologics, Gift of Life Marrow Registry, GlaxoSmithKline, HistoGenetics, Incyte Corporation, Iovance, Janssen Research & Development, Janssen/Johnson & Johnson, Jasper Therapeutics, Jazz Pharmaceuticals, Karius, Kashi Clinical Laboratories, Kiadis Pharma, Kite (a Gilead Company), Kyowa Kirin, Labcorp, Legend Biotech, Mallinckrodt Pharmaceuticals, Med Learning Group, Medac, Merck, Mesoblast, Millennium (the Takeda Oncology Company), Miller Pharmacal Group, Miltenyi Biotec, MorphoSys, MSA-EDITLife, Neovii Pharmaceuticals, Novartis Pharmaceuticals Corp, Omeros, OptumHealth, Orca Biosystems, OriGen BioMedical, Ossium Health, Pfizer, Pharmacyclics, PPD Development, REGiMMUNE, Registry Partners, Rigel Pharmaceuticals, Sanofi, Sarah Cannon, Seagen, Sobi, Stemcell Technologies, Stemline Technologies, STEMSOFT, Takeda Pharmaceuticals, Talaris Therapeutics, Vertex Pharmaceuticals, Vor Biopharma, and Xenikos BV.
The ideas and opinions expressed herein are those of the authors and do not necessarily reflect the opinions of the California Department of Public Health, NCI, or CDC, or their contractors and subcontractors.
Authorship
Contribution: S.J.S., B.V., C.L.M., A.B., J.J.P.C., R.A., T.W., J.J.A., L.M., T.H.M.K., and L.M.M. conceptualized and designed the study; S.J.S., B.V., C.L.M., B.E.S., R.P., J.D.R., A.B., J.J.P.C., T.W., R.Y., S.R.S., J.J.A., L.M., T.H.M.K., and L.M.M. contributed to collection and/or curation of data; S.J.S. performed statistical analysis; all authors interpreted the data; S.J.S. and L.M.M. drafted the manuscript; and all authors provided substantive comments to the manuscript drafts and approved the final version.
Conflict-of-interest disclosure: C.L.M. reports research funding from Sanofi. R.P. reports research funding from Amgen and serving on the advisory board of bluebird bio. The remaining authors declare no competing financial interests.
Correspondence: Sara J. Schonfeld, Division of Cancer Epidemiology and Genetics, National Cancer Institute, 9609 Medical Center Dr, MSC 9778, Rockville, MD 20892; email: schonfes@mail.nih.gov.
References
Author notes
The data that support the findings of this study are available from the California Cancer Registry and the Center for International Blood and Marrow Transplant Research. Access to data is granted through an application process by the management or data custodians for each data resource.
The full-text version of this article contains a data supplement.

![Cumulative incidence of developing a subsequent solid neoplasm, by source, overall, and by HCT type. Cumulative incidence of developing a solid SN (excluding NMSC and other/unknown neoplasms) for all patients combined (A), following alloHCT (B), and following autoHCT (C). Includes SNs diagnosed after 1990, after first HCT and before the end of follow-up (defined as the earliest occurrence of a second HCT, death, last known alive status from either CCR or CIBMTR, or the study end date [31 December 2018]). Cumulative incidence at 10 years following HCT shown in text.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/15/10.1182_bloodadvances.2024012693/2/m_blooda_adv-2024-012693-gr1.jpeg?Expires=1767719404&Signature=Zxw9DbfvKU8sRk~BCQ4gnko6pbF8kLiFm-CyiVCKFjGqVs7rJN0jHeCg9bhD334fps1si9bc6FlR~ZS5SgAbixKeglLiy3NjHGmW94RFS~iZAZdDRavmnC3jf8EEJE5wHI5MTJPe5W2FPXbP2YjELp2SwFihheVlamkKStcmYSLZLS0hgcbP6SPmEcQDhsFwr~N2ozsYPvxdGGqcrh~4sQharj55~zsungGmdyTnBPvS5G1HbjDfTO28BhRLrwKkru~XMlddX-nWantV~uhMWsrU8Dveu73yINGNK1d7c9MAuOKFnrfU8GdUj7aUdSXaMx9Q26HLgynt3vnudmRJmw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![SN source of ascertainment (percentage) among patients with at least 1 reported SN within the linked CIBMTR–CCR data set of 18 450 patients. Includes patients diagnosed with a solid SN in the breast, brain and CNS, lung, GI and GU tracts, oropharynx, or thyroid, or sarcoma or melanoma after the first HCT and before the end of follow-up (defined as the earliest occurrence of a second HCT, death, last known alive status from either CCR or CIBMTR, or the study end date [31 December 2018]). For this figure, patients with multiple SN types were counted once.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/15/10.1182_bloodadvances.2024012693/2/m_blooda_adv-2024-012693-gr2.jpeg?Expires=1767719404&Signature=ujMnM5aMt5cb-HIODb1BZ2L~dv0Qqe7w~KMtwKcZHUQZaghWqBWSucmtgU1DmDMlrV9vHawsTUhblzd5QsZAbPl4kzwkHtVMLplmHdfZxuHck7JGFiu49cHdi0iF3WWXsdx7yRQWJVmXUeo8Pu9Er75kh04fZZ7jnPYhHs-zjabkppE3f4B1ypLcO2RRNQxq4pzVcH7BHqX-o045Gvtlb8M2nnscn0qcZ358ahzoZBJDVeaPEP2JBDo5FW4JUSj7I6IrsoW1bpzbYFqQJOQJFS6aXV8ghXQqMO1yKk18ysBeZ9qYfLt1OZrN9DrANp5OLQNm1uVtRrF-zVV-YOUR-w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![SN types (percentage) reported by each source, overall, and by HCT type. Percentages based on solid SNs excluding NMSC and other/unknown neoplasms diagnosed after 1990, after the first HCT and before the end of follow-up (defined as the earliest occurrence of a second HCT, death, last known alive status from either CCR or CIBMTR, or the study end date [31 December 2018]). For each patient, a maximum of 1 SN per type is counted, but patients with multiple SNs can contribute to >1 SN type. In CIBMTR, there were a total of 470 SNs diagnosed among 457 patients. In CCR, there were a total of 656 SNs among 611 patients.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/15/10.1182_bloodadvances.2024012693/2/m_blooda_adv-2024-012693-gr3.jpeg?Expires=1767719404&Signature=LbXolMbSmWEwy5dp7OFVN97Ft7GcJ7YeM9WgqsUejg4qbyRhESXBd9wT-fJmTMz7daM9XNraJXXG4G~jYdrzqQFDGgiUoDvCLA0d~DPxUZCWS5xaOxYupjD5O1i610ibb2KgKThRQSvpClnaRLcCDm7YTWzMB~clwzp~wRss9xPcccq7QvgDo-FqZa~bA71F-IWqyp3Ey8YTAR8Xu8EUbCXTDr9PImqB4Awyj~DgmxA32hCQuAd8sHzayaAyVlZSXHim3kK-6zMNvwtBaTAy1W6OlKbOS726MWzSbjUbkCczcQKPnVWuECPo3FhRn3dM9JWZ~RB4SdNQxJE-vGKUtg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Distribution (percentage) of 785 SNs among 724 patients, by reporting source. Includes solid SNs excluding NMSC and other/unknown neoplasms diagnosed after the first HCT and before the end of follow-up (defined as the earliest occurrence of a second HCT, death, last known alive status from either CCR or CIBMTR, or the study end date [31 December 2018]). Other and unknown race includes Native Hawaiian and other Pacific Islander and American Indian/Alaska Native patients. For each patient, a maximum of 1 SN per type is counted, but patients with multiple SNs can contribute to >1 SN type. For SNs reported by both sources, date of SN and interval between HCT and SN are based on the earlier reported SN (if dates differed between CCR and CIBMTR). For confidentiality, row percentages were suppressed if any cells had <5 SNs. CA, California; NHB, non-Hispanic Black; NHW, non-Hispanic White.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/15/10.1182_bloodadvances.2024012693/2/m_blooda_adv-2024-012693-gr4.jpeg?Expires=1767719404&Signature=pUjaRMgS1NkxO4h4ZuXl73hejnTW8KGTUonoLjgGBOzC8pY4VXE59NhYbJBsqyghPuuJH0xzIpBzWeW31H73Ox-q6Pc42~8VamPRsinfNCBwk9PfQ9-TsoBAUyovuaM5-HEMmMZrCeuzKjpQTW1mcGgo0lUEGPC83yiG~JCeF6ppKSfwKQpQJkejJEXKn~3xGnUp24QmIvHuTTUxw3QpJL~Rr7S7a0TEEVtRqUdwseNzfbh1aE9-SLjCvo6MRye6dzkfK2QRa6ONybC2NYOOAfLCaI6kRRwOvZEIAb9tiqojSNQZW5jEgdCzes3pXvX1O53rQIpqObcIWS3bUAtoMg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)