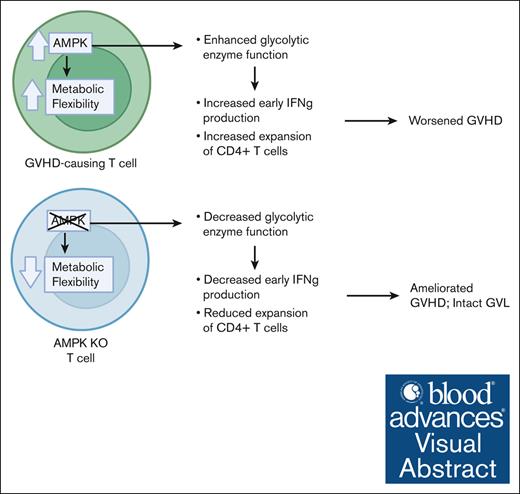
Key Points
AMPK plays a key role in driving both and oxidative and glycolytic metabolism in T cells during GVHD.
Absence of AMPK simultaneously impairs both glycolytic enzyme activity, most notably by aldolase, and interferon gamma production.
Visual Abstract
Allogeneic T cells reprogram their metabolism during acute graft-versus-host disease (GVHD) in a process involving the cellular energy sensor adenosine monophosphate (AMP)–activated protein kinase (AMPK). Deletion of AMPK in donor T cells limits GVHD but still preserves homeostatic reconstitution and graft-versus-leukemia effects. In the current studies, murine AMPK knock-out (KO) T cells decreased oxidative metabolism at early time points posttransplant and lacked a compensatory increase in glycolysis after inhibition of the electron transport chain. Immunoprecipitation using an antibody specific to phosphorylated targets of AMPK determined that AMPK modified interactions of several glycolytic enzymes including aldolase, enolase, pyruvate kinase M, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), with enzyme assays confirming impaired aldolase and GAPDH activity in AMPK KO T cells. Importantly, these changes in glycolysis correlated with both an impaired ability of AMPK KO T cells to produce significant amounts of interferon gamma upon antigenic restimulation and a decrease in the total number of donor CD4 T cells recovered at later times posttransplant. Human T cells lacking AMPK gave similar results, with glycolytic compensation impaired both in vitro and after expansion in vivo. Xenogeneic GVHD results also mirrored those of the murine model, with reduced CD4/CD8 ratios and a significant improvement in disease severity. Together these data highlight a significant role for AMPK in controlling oxidative and glycolytic metabolism in both murine and human T cells and endorse further study of AMPK inhibition as a potential clinical target for future GVHD therapies.
Introduction
Allogeneic stem cell transplantation is a curative treatment for a variety of hematological disorders including cancer, where it provides the added benefit of a graft-versus-leukemia (GVL) response. Although allogeneic stem cell transplantation can be life-saving procedure, it carries the risk of acute graft-versus-host disease (aGVHD), in which donor T cells attack tissues in the liver, gastrointestinal tract, and skin.1 First-line treatment for aGVHD continues to be corticosteroids, although recent additions include agents such as the janus kinase inhibitor ruxolitinib.2 In addition to reduced GVL efficacy and increased risks of infection,3 many agents carry drug-specific side effects including hypertension, hyperglycemia, psychiatric concerns, nephrotoxicity, and marrow suppression.4-7 Furthermore, corticosteroids continue to show limited long-term utility, with remission rates approximating 55%.8 Thus, treatments to specifically target GVHD-causing T cells, while maintaining physiologic immunity, are urgently needed.9,10
The recent literature has elucidated a strong connection between metabolism and subsequent T-cell function,11-14 with alloreactive T cells experiencing significant metabolic demands after transplant, including increased oxygen consumption and heightened glycolytic capacity,15 GLUT1 expression, fat oxidation, and glutaminolysis.16 In contrast, lymphopenia-driven reconstitution is much less energy intensive for donor T cells,17 requiring fewer metabolic adaptations, thus creating an opportunity for selective targeting of metabolic programs only in alloreactive T cells. One target that plays a significant role in T cell metabolic adaptation is the energy sensor adenosine monophosphate (AMP)–activated protein kinase (AMPK).
When intracellular energy stores are low, a rise in the AMP/adenosine triphosphate ratios activates AMPK through phosphorylation of Thr172 on the α subunit, driving AMPK-mediated phosphorylation of downstream targets.18,19 AMPK is important in mediating T-cell adaptations to stressful in vivo environments,20-22 and we have previously shown that deletion of AMPK in donor T cells reduces aGVHD severity while still preserving GVL effects.8 However, the in vivo metabolic consequences of deleting AMPK have not been well characterized, and it is unknown whether human T cells depend on AMPK to a similar extent during GVHD and GVL responses.
In these studies, we demonstrate that AMPK-deficient murine and human T cells lack a compensatory increase in glycolysis, particularly following inhibition of the electron transport chain (ETC). This decreased glycolytic response occurs concomitant with an early decrease in cell intrinsic expression of interferon gamma (IFN-γ), a critical player in GVHD pathogenesis.23 Together, these data suggest a paradigm in which AMPK is necessary to augment the glycolytic capacity and effector function of alloreactive T cells during the metabolic stress of GVHD pathogenesis. Consistent with this hypothesis, deletion of AMPK in human T cells significantly ameliorates xenogeneic GVHD, highlighting the potential clinical utility of targeting AMPK for the prevention and treatment of aGVHD.
Methods
Mice
C57BL/6 (B6, H2b), B6 x DBA2 F1 (B6D2F1), CD45.1 (B6.SJL-PtprcaPepcb/BoyJ), CD4cre (B6.Cg-Tg(Cd4-cre)1Cwi/BfluJ), NSG (NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ), and Thy1.1 (B6.PL-Thy1a/CyJ) mice were purchased from Jackson Laboratories. AMPKα1fl/flα2fl/fl mice were a kind gift from Sean Morrison.24 Male and female mice were used interchangeably. Recipient (8-12 weeks) and donor animals (8-16 weeks) were housed in a specific pathogen-free facility.
Bone marrow transplantation
B6D2F1 mice were conditioned with 1100 cGy total body irradiation in a split dose from an X-ray source (X-rad 320; Precision X-ray, North Branford, CT) and received transplantations as previously described.8 In xenogeneic GVHD experiments, NSG mice were irradiated with 160 cGy followed by administration of variable numbers of expanded human T cells and 1 × 106 recently thawed autologous non–T-cell antigen presenting cells (APCs).
Seahorse analysis
The Seahorse XF Cell Mito Stress Test Kit (Agilent, Santa Clara, CA) was run on a Seahorse XFe96 Bioanalyzer (Agilent) to determine basal and maximal rates of oxygen consumption (OCR), spare respiratory capacity (SRC), and extracellular acidification (ECAR). T cells were plated at 1 × 105 cells per well. The XF Mito Stress Test report generator and Agilent Seahorse analytics were used to calculate parameters from Wave software (Agilent, version 2.6.1.53).
CRISPR/Cas9 gene editing
Primary human T cells were magnetically isolated from a healthy human buffy coat, stimulated with CD3/CD28 dynabeads for 48 to 72 hours, and rested for an additional 2 to 24 hours. Between 4 × 106 and 5 × 106 cells were electroporated with a single guide RNA targeting AMPKα1 (ATGTGATGGGATCTTCTATA) following the IDT-published protocol and Neon Transfection System (Thermo Fisher). Electroporator settings were 3× 1600V pulses with a 10 millisecond pulse width using either the 10 or 100 uL pipette. After electroporation, cells were rested for 4 hours at 37°C in antibiotic-free media and plated in a 96-well plate in AIM V media with 5% immune cell serum replacement (SR) and 100 IU of human interleukin-2 (IL-2).
Protein isolation and immunoblot
A total of 1.5 × 105 human or murine T cells were washed in phosphate buffered saline (PBS) and resuspended in 10% trichloroacetic acid as previously described.8,25 Electrophoresis was performed on NuPAGE 4%-12% Bis-Tris Protein Gels (Invitrogen) with transfer to Invitrolon 0.45 μm polyvinylidene difluoride membranes (Invitrogen). Immunoblotting was performed according to the Cell Signaling Technology western blot protocol using antibodies listed in supplemental Table 5. Blots were stripped for 30 minutes (1% sodium dodecyl sulfate, 25 mM glycine, pH 2.0) before reprobing and developed with Super Signal West Femto chemiluminescence reagents (Thermo Fisher Scientific), detected by CL-X Posure Film (Thermo Scientific), and scanned in grayscale using an Epson V600 scanner.
Flow cytometry
Cells were stained in PBS with 2% fetal bovine serum at 1:100 dilution for 30 minutes. For detection of intracellular cytokines, 3 × 105 cells were recovered from spleens of recipient mice and cultured with 3 × 105 fresh F1 splenocytes for 6 hours in the presence of either 1 mM brefeldin A or monensin (BioLegend) according to manufacturers instructions. Further details are provided in supplemental Methods. Flow data were captured on a BD Fortessa analyzer (BD Biosciences) and evaluated using FlowJo software (version 10.1, Tree Star).
Immunoprecipitation of AMPK substrates and mass spectrometric analysis
A total of 1 × 106 T cells were lysed, lysates sonicated and centrifuged to remove insoluble debris, and the remaining lysate incubated with phospho-AMPK Substrate Motif mAb mix (Cell Signaling Technologies; catalog no. 5759) for 12 hours at 4°C. Antibody complexes were incubated with protein A beads (Cell Signaling Technologies; catalog no. 9863) for 1 hour at room temperature, washed twice with lysis buffer, and proteins eluted with 8 M urea (U5128; Sigma) and 0.1 M Tris–HCl at pH 8.5. Resulting lysates were then assayed by mass spectrometry as detailed in supplemental Methods. Data were collected in positive ionization mode. PEAKS9 software was used to sequence and identify peptides using a decoy search at a 1% false discovery rate using the UniProt murine database. Label-free quantitation was performed using the quantitative module in the PEAKS9 software.
Glycolytic enzyme assays
Aldolase A and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) enzymatic assays were run according to manufacturer’s instructions (Biovision-Abcam) on lysates from 2.5 × 105 T cells. For aldolase activity, T cells were collected after 3 days of anti-CD3/CD28 stimulation. GAPDH activity was measured on T cells positively selected from day 7 recipient spleens.
Statistics
Graphing and statistical analysis was performed using GraphPad Prism for Windows (version 9.3.0, San Diego, CA; www.graphpad.com). Unpaired 2-tailed Student t test and 2-way analysis of variance analysis were used to determine statistical significance. Kaplan-Meier analysis defined statistically significant differences in survival curves. Outlier analysis was performed using the ROUT method, and identified values excluded from further statistical consideration. Any exclusions were demarcated in the legends of the accompanying figure. Unless noted otherwise, data are displayed as mean ± standard deviation. In all cases, ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001.
Study approval
All animal studies were approved and carried out according to Institutional Animal Care and Use Committee guidelines from the University of Pittsburgh. All studies on human cells were designated Exempt status by the University of Pittsburgh Institutional Review Board.
Results
AMPK KO T cells reduce both oxidative and glycolytic metabolism after transplant
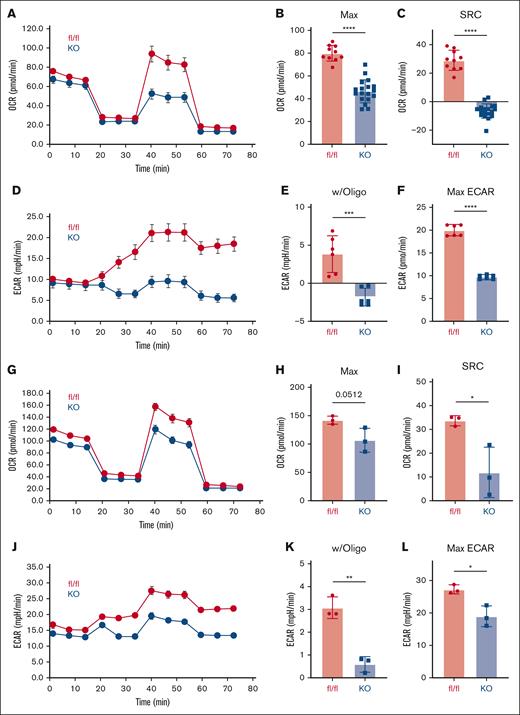
Previous work has demonstrated that AMPK-deficient murine T cells mediate less severe GVHD, but the metabolic underpinnings of this reliance were not previously explained. Given the known role of AMPK as a master metabolic regulator,15,26 we investigated the metabolic capacity of posttransplant AMPK KO T cells. Wild-type (WT) or AMPK KO T cells were transplanted into B6D2F1 recipients, recovered on day 7 from recipient spleens, and analyzed on the Seahorse metabolic analyzer. Baseline OCR were equivalent between WT and AMPK KO cells (Figure 1A), but AMPK KO T cells decreased both their maximal OCR and SRC, suggesting that AMPK KO T cells operate near their maximal oxidative capacity at baseline (Figure 1B-C). Baseline glycolysis (as measured by ECAR) was unchanged between WT and AMPK KO T cells (Figure 1D), but AMPK KO T cells failed to increase glycolysis upon ETC inhibition with oligomycin (Figure 1D-E), resulting in diminished maximal ECAR values (Figure 1F). Importantly, these metabolic perturbations persisted in AMPK KO cells on day 21 posttransplant (Figure 1G-L) and together demonstrate that AMPK regulates both oxidative and glycolytic metabolism in GVHD T cells, with an important function for AMPK in facilitating both early and ongoing metabolic adaptations.
AMPK KO T cells reduce both oxidative and glycolytic metabolism. A total of 2 × 106 CD45.1+ WT (fl/fl) or AMPK KO (blue) T cells and 5 × 106 T-cell depleted (TCD) B6 bone marrow cells were transplanted into irradiated allogeneic (B6D2F1) recipients. On day 7 after transplant, donor T cells were purified by negative selection over a magnetic column, placed into the Seahorse metabolic analyzer, and metabolism interrogated using the mitochondrial stress kit. OCRs (A), including both maximal OCR (B) and SRC (C), were measured simultaneously with ECARs, as shown in panel D. Response to oligomycin (w/oligo) was calculated by subtracting individual values from the averaged baseline values prior to oligomycin administration (E). Maximal ECAR values were simply the highest ECAR values obtained over the course of the analysis (F). (G-L) Donor T cells were recovered from a separate cohort of B6D2F1 recipients on day 21 posttransplant and measured for OCR (G-H), SRC (I), ECAR (J), response to oligomycin (K), and maximal ECAR values (L). Panels A-F, n = 2 pooled samples per group (3-4 mice in each pool), with plots representative of 2 independent experiments. In panels G-L, n = 3 pooled samples, with 3 to 4 mice per pool. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. fl/fl, floxed/floxed.
AMPK KO T cells reduce both oxidative and glycolytic metabolism. A total of 2 × 106 CD45.1+ WT (fl/fl) or AMPK KO (blue) T cells and 5 × 106 T-cell depleted (TCD) B6 bone marrow cells were transplanted into irradiated allogeneic (B6D2F1) recipients. On day 7 after transplant, donor T cells were purified by negative selection over a magnetic column, placed into the Seahorse metabolic analyzer, and metabolism interrogated using the mitochondrial stress kit. OCRs (A), including both maximal OCR (B) and SRC (C), were measured simultaneously with ECARs, as shown in panel D. Response to oligomycin (w/oligo) was calculated by subtracting individual values from the averaged baseline values prior to oligomycin administration (E). Maximal ECAR values were simply the highest ECAR values obtained over the course of the analysis (F). (G-L) Donor T cells were recovered from a separate cohort of B6D2F1 recipients on day 21 posttransplant and measured for OCR (G-H), SRC (I), ECAR (J), response to oligomycin (K), and maximal ECAR values (L). Panels A-F, n = 2 pooled samples per group (3-4 mice in each pool), with plots representative of 2 independent experiments. In panels G-L, n = 3 pooled samples, with 3 to 4 mice per pool. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. fl/fl, floxed/floxed.
Decreased glycolytic enzyme activity in AMPK KO T cells
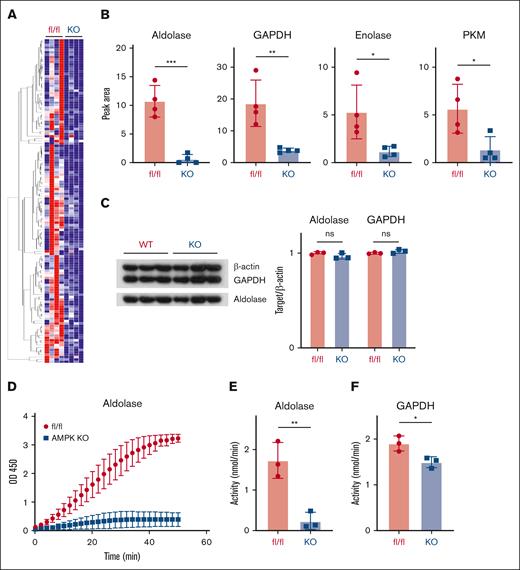
The mechanistic role for AMPK in facilitating glycolysis in GVHD T cells has not been well studied. To understand potential mechanisms, we identified protein targets phosphorylated by AMPK in GVHD T cells using an antibody that detects the phosphorylated AMPK-specific motif LxRxx(pS/pT). In this way, we aimed to pull down targets directly phosphorylated by AMPK, as well as any proteins in complex with them. Liquid chromatography–mass spectrometry (LC-MS) was then used to analyze phosphorylated proteins and their associated protein complexes to discriminate targets represented at levels threefold or greater in WT vs AMPK KO donor cells. This method identified 134 proteins differentially recovered from WT vs AMPK KO T cells on day 7 after transplant (Figure 2A; supplemental Table 1). Four of the top candidates represented glycolytic enzymes including aldolase A, GAPDH, pyruvate kinase M1, and enolase (Figure 2B). Importantly, there was no difference in total levels of these enzymes on day 7 after transplant (Figure 3C), suggesting that differential recovery could not be explained by variable levels of total protein. To evaluate whether AMPK affected enzyme function, we quantitated the specific activity of aldolase and GAPDH, the 2 more differentially recovered candidates. Enzyme analysis showed a marked reduction in aldolase activity in AMPK KO T cells after in vitro anti-CD3/CD28 stimulation (Figure 2D-E), and GAPDH activity consistently decreased >20% in AMPK KO T cells recovered on day 7 after transplant (Figure 2F). Together, these data demonstrate that multiple glycolytic enzymes were immunoprecipitated at higher levels from WT T cells using a phospho-motif–specific antibody, suggesting that AMPK modifies association of these enzymes and likely potentiates glycolytic function, as AMPK KO T cells reduced function of both aldolase and GADPH, 2 of the more differentially recovered enzymes.
Decreased aldolase activity in AMPK KO T cells. WT vs AMPK KO T cells were transplanted into B6D2F1 recipients, recovered on day 7, and proteins immunoprecipitated from cell lysates using an antibody detecting the phosphorylated AMPK-specific motif LxRxx(pS/pT). Precipitated candidate proteins were subsequently identified via LC-MS and a heat map generated of those recovered at threefold or higher levels in WT vs AMPK KO T cells (A). Representative LC-MS data from the heat map in panel A is shown for the 4 glycolytic enzymes of interest (B). Control protein samples were recovered from day 7 samples before immunoprecipitation and blotted for total levels of candidate proteins aldolase and GAPDH (C). (D-E) WT or AMPK KO T cells were stimulated on CD3/CD28 coated plates for 72 hours, followed by T-cell recovery and measurement of aldolase activity in cell lysates. GAPDH was measured in a similar fashion from T cells recovered on day 7 posttransplant (F). For data in panels A-B, 12× WT and 12× AMPK KO T cells were recovered on day 7 after transplant and divided into 4 groups of 3 recipients each. These 4 groups were then processed as individual replicates through cell lysis, IP, and LC-MS analysis. In panels C,F, n = 3 replicates pooled from ≥9 individual recipients (eg, 3 groups of 3 recipients each). Graphs in panels D-E represent data from 3 separate biological donors. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. PKM, pyruvate kinase M.
Decreased aldolase activity in AMPK KO T cells. WT vs AMPK KO T cells were transplanted into B6D2F1 recipients, recovered on day 7, and proteins immunoprecipitated from cell lysates using an antibody detecting the phosphorylated AMPK-specific motif LxRxx(pS/pT). Precipitated candidate proteins were subsequently identified via LC-MS and a heat map generated of those recovered at threefold or higher levels in WT vs AMPK KO T cells (A). Representative LC-MS data from the heat map in panel A is shown for the 4 glycolytic enzymes of interest (B). Control protein samples were recovered from day 7 samples before immunoprecipitation and blotted for total levels of candidate proteins aldolase and GAPDH (C). (D-E) WT or AMPK KO T cells were stimulated on CD3/CD28 coated plates for 72 hours, followed by T-cell recovery and measurement of aldolase activity in cell lysates. GAPDH was measured in a similar fashion from T cells recovered on day 7 posttransplant (F). For data in panels A-B, 12× WT and 12× AMPK KO T cells were recovered on day 7 after transplant and divided into 4 groups of 3 recipients each. These 4 groups were then processed as individual replicates through cell lysis, IP, and LC-MS analysis. In panels C,F, n = 3 replicates pooled from ≥9 individual recipients (eg, 3 groups of 3 recipients each). Graphs in panels D-E represent data from 3 separate biological donors. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. PKM, pyruvate kinase M.
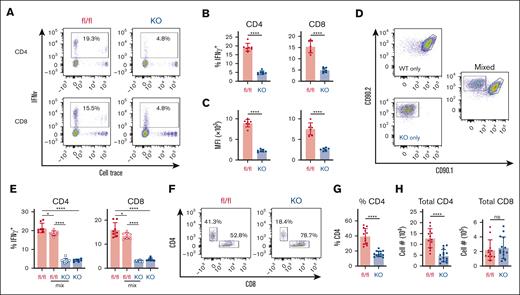
Decreased IFN-γ production in AMPK KO T cells is cell-intrinsic. (A-C) WT or AMPK KO T cells were transplanted individually into irradiated B6D2F1 recipients, recovered on day 7, stimulated for 6 hours with fresh B6D2F1 splenocytes in the presence of monensin, and analyzed for intracellular cytokine production. Representative flow plots are shown in panel A, with the percentage of IFN-γ+ cells (B) and the median fluorescence intensity (MFI) of cells falling within the IFN-γ+ gate shown in panel C, respectively. (D-E) WT (CD90.1/2) and AMPK KO (CD90.2) T cells were transplanted separately or in a 1:1 combination (mixed) into irradiated B6D2F1 recipients and intracellular IFN-γ detected as outlined in panels A-C. Representative flow plots for individual and mixed samples are shown in panel D, whereas panel E represents the percentage of IFN-γ+ cells in CD4 vs CD8 T cells from multiple samples. (F-H) T cells were recovered from a separate cohort of B6D2F1 recipients on day 21 and analyzed by flow cytometry for CD4 vs CD8 expression (F), with CD4 percentages plotted for individual mice (G). CD4 and CD8 percentages were then multiplied by the total number of lymphocytes recovered to calculate the total number of CD4+ (left) and CD8+ (right) T cells on day 21 (H). n = 7 to 8 recipients per group for panels A-E, and n = 12 to 14 recipients per group for panels F-H. ∗P < .05; ∗∗∗∗P < .0001.
Decreased IFN-γ production in AMPK KO T cells is cell-intrinsic. (A-C) WT or AMPK KO T cells were transplanted individually into irradiated B6D2F1 recipients, recovered on day 7, stimulated for 6 hours with fresh B6D2F1 splenocytes in the presence of monensin, and analyzed for intracellular cytokine production. Representative flow plots are shown in panel A, with the percentage of IFN-γ+ cells (B) and the median fluorescence intensity (MFI) of cells falling within the IFN-γ+ gate shown in panel C, respectively. (D-E) WT (CD90.1/2) and AMPK KO (CD90.2) T cells were transplanted separately or in a 1:1 combination (mixed) into irradiated B6D2F1 recipients and intracellular IFN-γ detected as outlined in panels A-C. Representative flow plots for individual and mixed samples are shown in panel D, whereas panel E represents the percentage of IFN-γ+ cells in CD4 vs CD8 T cells from multiple samples. (F-H) T cells were recovered from a separate cohort of B6D2F1 recipients on day 21 and analyzed by flow cytometry for CD4 vs CD8 expression (F), with CD4 percentages plotted for individual mice (G). CD4 and CD8 percentages were then multiplied by the total number of lymphocytes recovered to calculate the total number of CD4+ (left) and CD8+ (right) T cells on day 21 (H). n = 7 to 8 recipients per group for panels A-E, and n = 12 to 14 recipients per group for panels F-H. ∗P < .05; ∗∗∗∗P < .0001.
Decreased glycolysis correlates with an early decrease in IFN-γ production in AMPK KO T cells
Decreased glycolysis has been previously linked with decreased IFN-γ levels in activated T cells, where diminished IFN-γ production results from either decreased levels of acetylation2 or a moonlighting role of GAPDH in sequestering IFN-γ transcripts.3 Given the importance of IFN-γ in GVHD pathogenesis, we assessed whether a similar association between GAPDH activity and IFN-γ levels was operational in AMPK KO T cells. T cells preloaded with CellTrace Violet were recovered from spleens of recipient animals on day 7, restimulated with F1 splenocytes in the presence of monensin, and intracellular cytokine production measured 6 hours later. Significantly fewer AMPK KO cells expressed IFN-γ after this restimulation (Figure 3A-B), along with a marked reduction in IFN-γ median fluorescence intensity, indicating less IFN-γ per cell (Figure 3C). Reduced IFN-γ production was also seen after restimulation of AMPK KO T cells in a mixed leukocyte reaction in vitro (supplemental Figure 1A-C). This reduction in intracellular IFN-γ contrasted with what is seen after phorbol 12-myristate 13-acetate/ionomycin stimulation of day 7 T cells8 but likely reflects a more physiologic response after re-exposure to F1-derived antigens. These results are also consistent with studies by Lepez et al, who documented decreased IFN-γ production by AMPK KO T cells both in vitro and in vivo.1 Importantly, absence of AMPK did not globally reduce proinflammatory cytokine production, because AMPK KO T cells generated equivalent amounts of tumor necrosis factor (TNF) in vitro (supplemental Figure 1D-F), and day 7 KO T cells had low but equal levels of both TNF and IL-17 (supplemental Figure 1G and data not shown). To understand this IFN-γ reduction mechanistically, we recovered RNA from day 7 CD4 and CD8 donor T cells and performed quantitative reverse transcription-polymerase chain reaction (RT-PCR). Tellingly, IFN-γ transcript levels were equivalent by RT-PCR between WT and AMPK KO cells (supplemental Figure 1H), suggesting that the lesion in IFN-γ production occurs after transcription.3
To test our hypothesis that intracellular changes drive decreased IFN-γ production, we determined whether changes in IFN-γ were cell intrinsic. WT and AMPK KO T cells, congenically marked with CD90.1/2 or CD90.2, respectively, were combined in a 1:1 ratio and transplanted into irradiated F1 recipients (Figure 3D). Donor T cells were recovered on day 7 and assessed for intracellular production of IFN-γ. T cells from recipients that received transplantation with only AMPK KO cells again demonstrated a marked defect in IFN-γ production (Figure 3D, left panels). Importantly, this sharp decrease was maintained when AMPK KO cells were cotransplanted with WT cells (Figure 3E), demonstrating that IFN-γ production decreases in a cell-intrinsic manner. WT T cells transplanted 1:1 with AMPK KO T cells had a slight decrease in IFN-γ positivity, perhaps because of decreased local cytokine production by neighboring AMPK KO cells.
To determine whether decreased IFN-γ production was maintained over time, we harvested AMPK KO and WT donor T cells on day 21 after transplant, restimulated them with F1 splenocytes, and measured intracellular cytokine production. In these studies, IFN-γ and TNF were low but equivalent between WT and AMPK KO T cells (supplemental Figure 2A-D). Because metabolic perturbations may have other subset-specific effects beyond cytokine production,27,28 we evaluated donor CD4 and CD8 percentages on day 21 after transplant, which revealed a marked reduction in both the percentage (Figure 3F-G) and total number (Figure 3H) of CD4+ AMPK KO donor T cells. Together, these data suggest that AMPK deficiency impairs intrinsic intracellular IFN-γ production early after transplant, a difference which dissipates as IFN-γ production wanes over time. In contrast, a decrease in AMPK KO CD4 T-cell numbers is maintained after transplant and likely attenuates GVHD severity, given the prominent role for CD4 T cells in driving GVHD pathogenesis.29,30 This result is also consistent with the importance of glycolysis in supporting CD4 T-cell responses both in vitro and in vivo.31,32
Human T cells lacking AMPK reduce oxidative and glycolytic metabolism in vitro
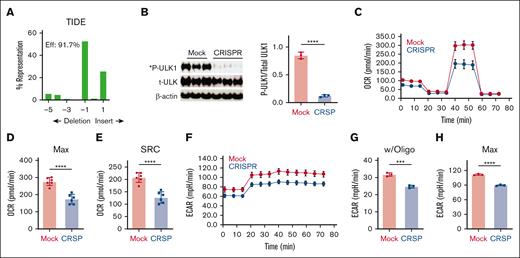
Given the impaired metabolism of murine T cells lacking AMPK, we determined whether similar changes occurred in human T cells, a result which could be leveraged therapeutically. To generate cells lacking AMPK, CD3+ human T cells were purified from the peripheral blood of healthy human donors, electroporated with ribonucleoprotein complexes containing the Cas 9 protein and a guide RNA targeting human AMPKα1, and expanded up to 10 days in media containing recombinant human IL-2. Targeting human AMPKα1 consistently achieved insertion-deletion efficiencies >90% as quantified by TIDE analysis (Figure 4A), with loss of AMPK function further confirmed by a >85% reduction in AMPK-specific phosphorylation of the target protein Unc51-Like Kinase-1 (ULK-1) (Figure 4B). Notably, although human T cells predominantly express AMPKα1,33 genetic elimination of AMPKα1 did not drive compensatory increases in AMPKα2 expression (supplemental Figure 3A-B), consistent with the continued lack of ULK-1 phosphorylation observed (Figure 4B). To evaluate the effects of AMPK deletion on metabolic potential, human T cells lacking AMPK were restimulated overnight in physiological glucose (5.5 mM) and analyzed the following day on the Seahorse metabolic analyzer. As seen in murine T cells, human T cells lacking AMPK decreased both maximal OCR (Figure 4C-D) and SRC (Figure 4E), compared with T cells electroporated without the guide RNA (mock). AMPK-deficient T cells also decreased ECAR values at baseline, which separated further upon inhibition of the ETC, highlighting their reduced glycolytic capacity (Figure 4F-H).
AMPK-deficient human T cells decrease oxidative and glycolytic metabolism in vitro. CD3+ human T cells were purified from the peripheral blood of healthy human donors, electroporated with ribonucleoprotein (RNP) complexes containing the Cas9 protein and a guide RNA (gRNA) targeting human AMPKα1 locus and expanded until day 10 in recombinant human IL-2. (A) Genomic DNA purified from day 10 T cells was used to amplify a 700 bp fragment covering the gRNA target site, followed by Sanger sequencing and decomposition analysis.19 (B) Protein from 1 × 105 day 10 T cells was precipitated with trichloroacetic acid (TCA) followed by immunoblot analysis for specific phosphorylation of ULK-1 on Ser555 by AMPK. (C-H) Day 10 T cells were stimulated overnight with CD3/CD28 dynabeads in physiologic glucose (5.5 mM) and placed on the Seahorse metabolic analyzer (C). Both maximal OCR (D) and SRC (E) were measured simultaneously with ECAR (F-H), including both the response to oligomycin (G) and the maximal ECAR values (H). Plots in panels C,F are representative of 2 independent donors, whereas data in panels D-G are the composite analysis from these 2 donors. ∗∗∗P < .001; ∗∗∗∗P < .0001.
AMPK-deficient human T cells decrease oxidative and glycolytic metabolism in vitro. CD3+ human T cells were purified from the peripheral blood of healthy human donors, electroporated with ribonucleoprotein (RNP) complexes containing the Cas9 protein and a guide RNA (gRNA) targeting human AMPKα1 locus and expanded until day 10 in recombinant human IL-2. (A) Genomic DNA purified from day 10 T cells was used to amplify a 700 bp fragment covering the gRNA target site, followed by Sanger sequencing and decomposition analysis.19 (B) Protein from 1 × 105 day 10 T cells was precipitated with trichloroacetic acid (TCA) followed by immunoblot analysis for specific phosphorylation of ULK-1 on Ser555 by AMPK. (C-H) Day 10 T cells were stimulated overnight with CD3/CD28 dynabeads in physiologic glucose (5.5 mM) and placed on the Seahorse metabolic analyzer (C). Both maximal OCR (D) and SRC (E) were measured simultaneously with ECAR (F-H), including both the response to oligomycin (G) and the maximal ECAR values (H). Plots in panels C,F are representative of 2 independent donors, whereas data in panels D-G are the composite analysis from these 2 donors. ∗∗∗P < .001; ∗∗∗∗P < .0001.
Creating a modified model of xenogeneic GVHD
We next evaluated the GVHD potential of AMPK-deficient T cells in a xenogeneic model by transplanting 6 × 106 CRISPR-treated human T cells into NOD-scid IL2Rgammanull (NSG) mice irradiated with 160 cGy. However, our initial approach yielded few human T cells, negligible weight loss, and minimal cytokine production by day 25 after transplant. Noting that previous xenogeneic models injected replete populations of peripheral blood mononuclear cells (PBMCs),8,34 we reasoned that maximal GVHD severity may require non–T-cell APCs in the donor inoculum.35 Based on this hypothesis, we added 1 × 106 autologous non–T-cell APCs to day 10 in vitro–expanded T cells before injection into NSG recipients. These APCs consisted largely of CD14+ monocytes (37%), CD15+ neutrophils (9%), and a consistent population of non-B/non-T/non-natural killer cell lymphocytes (supplemental Figure 4; supplemental Table 2). Inclusion of 1 × 106 APCs increased the percentage and total number of human CD45+CD3+ T cells recovered on day 25 after transplant (supplemental Figure 5A), with readily detectable levels of human IFN-γ in the serum of transplanted mice (supplemental Figure 5B). We next determined whether the number of T cells injected correlated with the quantity of cells recovered on day 25, to identify an optimal T-cell dose for further studies. T cells were diluted in threefold dilutions (6 × 106 cells to 0.67 × 106 T cells per recipient) and transplanted with 1 × 106 APCs into irradiated recipients. Lower starting doses of T cells yielded proportionally fewer human T cells on day 25 after transplant (supplemental Figure 5C-D), which further correlated with reduced serum levels of human IFN-γ (supplemental Figure 5E). Changes in donor T-cell number also correlated with serum levels of murine CCL-2, an accessory biomarker of disease severity in our modified GVHD model (supplemental Figure 5F). Thus, addition of APCs yielded both consistent cell numbers and reliable serum biomarkers by day 25 after transplant, and we ultimately chose to transplant 6 × 106 T cells and 1 × 106 autologous APCs in subsequent studies.
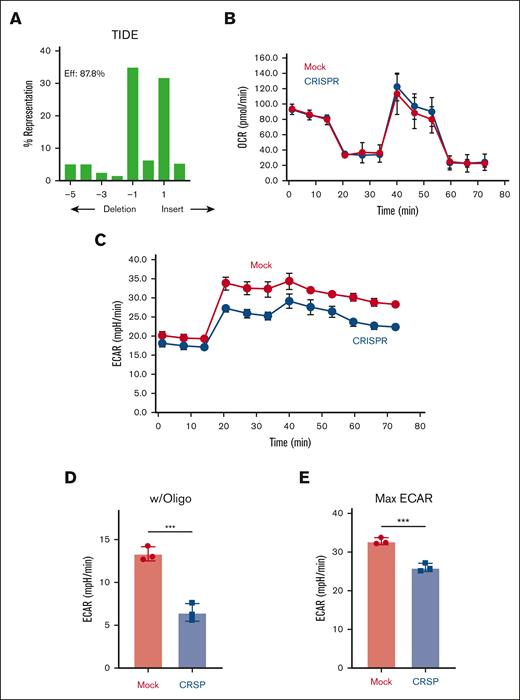
Human T cells lacking AMPK decrease glycolytic capacity after transplant
We next characterized AMPK’s role in human T cells in vivo using this modified xenogeneic model. Human T cells were electroporated with AMPKα1 targeting ribonucleoproteins, expanded in IL-2 until day 10, and cotransplanted with 1 × 106 autologous APCs into NSG recipients irradiated with 160 cGy. Importantly, human T cells recovered on day 25 after transplant continued to remain highly edited, with indel efficiencies >87% (Figure 5A). Intriguingly, human T cells lacking AMPK did not decrease oxidative capacity on day 25 after transplant (Figure 5B) and exhibited minimal changes in baseline ECAR values (Figure 5C). However, human T cells lacking AMPK still manifested an impaired compensatory response to oligomycin, increasing only 50% compared with mock T cells (Figure 5D) and with maximal ECAR values <80% of those for mock cells (Figure 5E). Thus, although lacking AMPK did not persistently affect oxidative metabolism, a reduction in glycolytic capacity remained consistent between mouse and human cells.
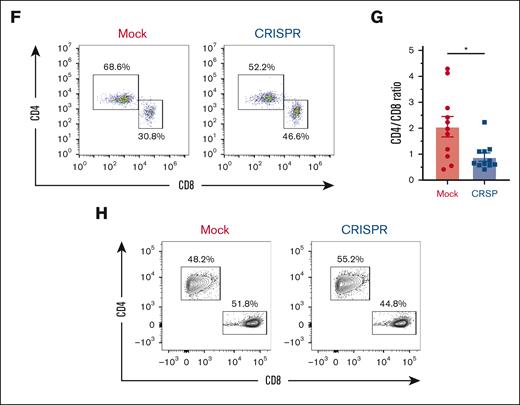
Decreased glycolytic compensation in human T cells lacking AMPK. A total of 6 × 106 day 10 human T cells were transplanted with 1 × 106 autologous non–T cells into irradiated NSG recipients. Human CD3+ T cells were recovered from spleens of NSG recipients on day 25 after transplant and purified via magnetic selection. (A) Genomic DNA was purified from day 25 T cells followed by PCR amplification, Sanger sequencing, and decomposition analysis. (B-E) T cells recovered on day 25 were placed into the Seahorse metabolic analyzer, where the OCR (B) was measured simultaneously with ECAR (C). ECAR response to oligomycin (D) and maximal ECAR values (E) were calculated as in Figure 1. (F-H) Similar to panels A-E, 6 × 106 day-10 human T cells were transplanted with 1 × 106 autologous non–T cells into irradiated NSG recipients, recovered on day 28, and CD4 vs CD8 percentages quantitated by flow cytometry (F). These values were then used to calculate the posttransplant CD4/CD8 ratio (G). Pretransplant (post-CRISPR) CD4 and CD8 percentages are shown for comparison (H). Plots in panels B-E represent data from 2 pooled samples per group (3-4 mice in each pool; 6-8 mice total). Eleven mice per group in panels F-G. Each experiment was performed at least twice. Data in panel G represent the mean ± standard error of the mean. ∗P < .05; ∗∗∗P < .001.
Decreased glycolytic compensation in human T cells lacking AMPK. A total of 6 × 106 day 10 human T cells were transplanted with 1 × 106 autologous non–T cells into irradiated NSG recipients. Human CD3+ T cells were recovered from spleens of NSG recipients on day 25 after transplant and purified via magnetic selection. (A) Genomic DNA was purified from day 25 T cells followed by PCR amplification, Sanger sequencing, and decomposition analysis. (B-E) T cells recovered on day 25 were placed into the Seahorse metabolic analyzer, where the OCR (B) was measured simultaneously with ECAR (C). ECAR response to oligomycin (D) and maximal ECAR values (E) were calculated as in Figure 1. (F-H) Similar to panels A-E, 6 × 106 day-10 human T cells were transplanted with 1 × 106 autologous non–T cells into irradiated NSG recipients, recovered on day 28, and CD4 vs CD8 percentages quantitated by flow cytometry (F). These values were then used to calculate the posttransplant CD4/CD8 ratio (G). Pretransplant (post-CRISPR) CD4 and CD8 percentages are shown for comparison (H). Plots in panels B-E represent data from 2 pooled samples per group (3-4 mice in each pool; 6-8 mice total). Eleven mice per group in panels F-G. Each experiment was performed at least twice. Data in panel G represent the mean ± standard error of the mean. ∗P < .05; ∗∗∗P < .001.
To evaluate the functional consequences of AMPK’s absence, donor T cells were recovered from NSG recipient mice on day 28 after transplant and analyzed for CD4/CD8 percentages and production of proinflammatory cytokines. Mirroring the murine data, CD4/CD8 ratios significantly decreased in recipients of AMPK-deficient human T cells (Figure 5F-G). Importantly, these differences were not present before transplant, with a notably higher CD4/CD8 ratio in AMPK-deficient cells (Figure 5H). Consistent with the day 21 murine studies, there were no differences in IFN-γ, TNF, or IL-2 in day 28 AMPK-deficient human donor T cells (supplemental Figure 6A-D). Together, these results demonstrate human T-cell deficiencies were similar to those seen in the murine model, including a defect in glycolytic compensation, a reduction in the CD4/CD8 ratio, and low but equivalent proinflammatory cytokine production.
Deletion of AMPK in human T cells decreases aGVHD severity without affecting antileukemia potential
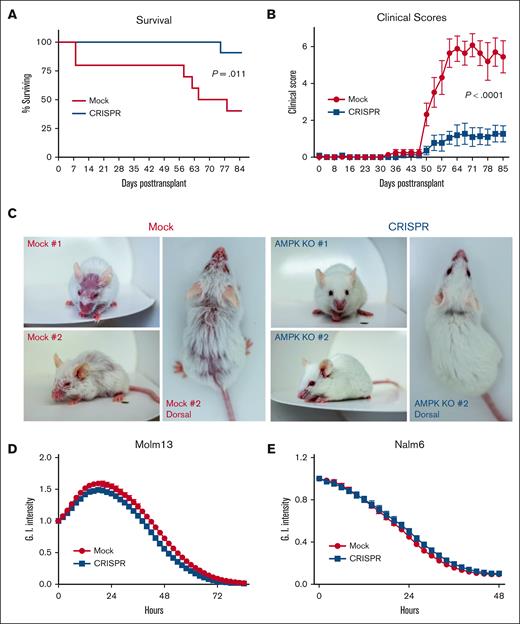
We hypothesized that limitations in glycolytic capacity and the lower CD4/CD8 ratio might limit the aGVHD potential of AMPK-deficient human cells. To investigate this idea, irradiated NSG recipients were transplanted with human T cells lacking AMPK and followed for survival and disease severity. Survival improved significantly in recipients of AMPK KO T cells (Figure 6A), with notably lower GVHD clinical scores beginning on day 50 (Figure 6B). Differences in clinical score were driven by increased skin manifestations, exaggerated fur ruffling, and more dramatic hunched posture in animals receiving mock-treated cells (Figure 6C). Weights were largely unchanged and did not differ between recipient groups (supplemental Figure 6E), which aligns with published reports using this NSG model, where minimal weight loss is thought to be related to a lack of gastrointestinal pathology.16 Our previous murine work demonstrated increased regulatory T-cell (Treg) percentages after transplantation of AMPK KO T cells, although Tregs were not responsible for improved GHVD severity in those studies.8 Here, human Treg percentages were equivalent between mock and KO groups throughout the CRISPR process, with minimal Treg percentages recovered in either group at 25 to 28 days after transplant (supplemental Figure 7A-D), consistent with a minimal role for human Treg in this model.
Deletion of AMPK decreases xenogeneic GVHD severity without affecting antileukemia potential. (A-C) A total of 6 × 106 mock or CRISPR-treated human T cells were transplanted with 1 × 106 autologous non–T cells into irradiated NSG recipients, and recipients followed for survival (A) and clinical score (B) to 12 weeks posttransplant. Increased clinical scores were driven by significant skin manifestations, exaggerated fur ruffling, and dramatically hunched posture (representative photographs shown in panel C). To assess antileukemic potential, mock vs CRISPR–treated human T cells were plated at an effector-to-target ratio of 2:1 with GFP+ Molm13 leukemia cells and cytotoxicity measured using an Incucyte analyzer and the loss of Green integrated (G.I.) intensity over time (D). A similar approach was used to measure cytotoxicity of mock vs CRISPR–treated, CD19–targeting CAR T cells placed into Incucyte incubator with GFP+ Nalm6 leukemia cells (E). Ten to 11 mice per group in panels A-B, with clinical manifestations shown for 2 representative animals in panel C. Experiments in panels D-E are representative of similar results using T cells from 2 independent donors.
Deletion of AMPK decreases xenogeneic GVHD severity without affecting antileukemia potential. (A-C) A total of 6 × 106 mock or CRISPR-treated human T cells were transplanted with 1 × 106 autologous non–T cells into irradiated NSG recipients, and recipients followed for survival (A) and clinical score (B) to 12 weeks posttransplant. Increased clinical scores were driven by significant skin manifestations, exaggerated fur ruffling, and dramatically hunched posture (representative photographs shown in panel C). To assess antileukemic potential, mock vs CRISPR–treated human T cells were plated at an effector-to-target ratio of 2:1 with GFP+ Molm13 leukemia cells and cytotoxicity measured using an Incucyte analyzer and the loss of Green integrated (G.I.) intensity over time (D). A similar approach was used to measure cytotoxicity of mock vs CRISPR–treated, CD19–targeting CAR T cells placed into Incucyte incubator with GFP+ Nalm6 leukemia cells (E). Ten to 11 mice per group in panels A-B, with clinical manifestations shown for 2 representative animals in panel C. Experiments in panels D-E are representative of similar results using T cells from 2 independent donors.
Finally, we sought to understand whether AMPK deletion negatively affects GVL potential in human T cells using 2 in vitro antileukemia models. In the first, day 10 mock vs AMPK KO T cells were restimulated for 48 hours with CD3/CD28 dynabeads, then cocultured with green fluorescent protein (GFP)+ MOLM13 acute myeloid leukemia cells. Loss of green fluorescence, using the Incucyte cell analyzer, monitored for leukemia killing. At effector-to-target ratios of both 2:1 and 3:1, AMPK-deficient T cells killed MOLM13 leukemia cells equivalent to mock-treated controls (Figure 6D). To ensure reproducibility, we generated CD19–targeting chimeric antigen receptor (CAR) T cells from healthy human donors, deleted AMPK via CRISPR, and cocultured CAR T cells (±AMPK) with CD19+ Zs-Green+ NALM6 leukemia cells for 72 hours. Again, human CAR T cells lacking AMPK equivalently killed Nalm6 leukemia targets (Figure 6E), suggesting that although AMPK deficiency definitively impairs GVHD pathogenesis, GVL capacity remains intact.
Discussion
Intracellular metabolism is central to T-cell biology, with reports suggesting its control could be key to ameliorating T-cell–mediated disease. We have previously shown that elimination of the cellular energy sensor AMPK mitigates GVHD while still maintaining homeostatic immune reconstitution and beneficial GVL responses. Here, we extend these studies by documenting specific metabolic changes that occur in T cells lacking AMPK, uncover potential mechanisms that make these changes possible, and correlate metabolic alterations with downstream function. To facilitate potential clinical application, we also delete AMPK in primary human T cells to demonstrate that AMPK affects metabolic capacity and impairs GVHD severity in both murine and human T cells.
Numerous xenogeneic models have been developed over the last 30 years,36,37 and we began by transplanting in vitro–expanded human T cells alone into NSG mice. However, the low number of donor T cells recovered at early time points was not conducive to studying early changes in metabolic reprogramming. Knowing that most NSG models rely on a full complement of human PBMCs, we reasoned that absence of APCs in our original inoculum was likely responsible for reduced T-cell recovery and limited GVHD severity. Indeed, simple reintroduction of 1 × 106 autologous APCs markedly increased disease severity, supported by elevated serum IFN-γ levels on day 25, and consistent with other xenogeneic models in which human T cells develop a T helper type 1 (Th1) effector phenotype.38 Multiple studies have highlighted a role for class I and class II molecules in driving xenogeneic GVHD,34,39-41 and we suspect that adding autologous APCs increased GVHD severity via presentation of murine antigens by human APCs,35,42,43 with an attendant increase in T-cell activation.
Metabolically, alloreactive T cells upregulate both oxidative and glycolytic metabolism early after transplant.44 We predicted AMPK would control oxidative metabolism, based on its role in other cell types,45,46 but our data suggest that AMPK affects both oxidative and glycolytic capacity after transplant, with a more lasting impact on glycolysis. Regarding AMPK’s role in glycolysis, significant differences existed in the precipitation of glycolytic enzymes from WT vs AMPK KO T cells, without discernible variation in total protein levels. Furthermore, short of aldolase, none of the preferentially precipitated glycolytic enzymes contained a canonical LxRxx(pS/pT) AMPK phosphorylation motif. One possible explanation, which we favor, is the generation of a supramolecular complex of glycolytic enzymes, one whose association is facilitated by an AMPK phosphorylated target. Indeed, supramolecular organization of glycolytic enzymes has previously been shown in CD3+ T cells, in which enolase, pyruvate kinase M1, aldolase, GAPDH, and sirtuin 2 all associate to drive glycolytic function.47 Our data argue for a similar organization in GVHD T cells, in a process dependent upon AMPK. We suspect that AMPK phosphorylates a key target (eg, Ser176 on aldolase), whose phosphorylation then organizes glycolytic enzymes into a supramolecular complex, facilitating an increased glycolytic response. Further work is required to fully confirm this hypothesis, whose tenets are supported by reductions in aldolase and GAPDH activity in T cells lacking AMPK.
To our knowledge, this is the first direct evidence of AMPK controlling aldolase activity and suggests a mutual feedback loop in which aldolase promotes AMPK activity when glycolysis is low,48 with activated AMPK redirecting aldolase back toward glycolysis. There are also additional ways in which AMPK might influence glycolytic adaptation. For example, AMPK could promote glycolysis directly, through phosphorylation of 6-phosphofructo-2-kinase,49,50 or indirectly increase surface expression of glucose transporters via phosphorylation of class II histone deacetylases such as histone deacetylase-5 (HDAC5).51 Intriguingly, HDAC5 was identified as a phosphorylation target of AMPK in day 7 T cells (supplemental Table 1), although its exact role in GVHD T cells remains to be clarified.
AMPK is also known to promote IFN-γ production in T cells in vitro and in vivo.1 Here, we uncovered a notable defect in IFN-γ production upon restimulation of day 7 allogeneic AMPK KO T cells, without changes in other proinflammatory cytokines. Disruptions in glycolysis have been previously linked to diminished IFN-γ production, with insufficient acetate production limiting IFN-γ transcription2 and IFN-γ translation being encumbered by the moonlighting role of GAPDH, which sequesters IFN-γ transcripts when glycolysis activity wanes.3 Our data support the hypothesis of disrupted IFN-γ translation in AMPK KO T cells. T-cell–derived IFN-γ plays a complex role after transplant, having been ascribed both a beneficial effect in preventing early lung pathology and a later, more destructive role by promoting a Th1 effector phenotype .4-7 Our data imply that AMPK KO T cells produce sufficient IFN-γ to avoid early lung injury but not enough to drive maximal GVHD severity. Reassuringly, in spite of decreased IFN-γ production, T cells lacking AMPK continue to elicit potent GVL effects,8 including in human T cells targeting leukemia cells in vitro, a phenotype consistent with the idea that IFN-γ production may be dispensable for controlling in vivo leukemia growth.9,10
Human T cells lacking AMPK and cultured in vitro displayed metabolic vulnerabilities identical to those seen in day 7 murine T cells but manifested only impaired glycolysis on day 21 in vivo. This metabolic correction was not due to outgrowth of AMPK positive T cells, because indel rates continued to exceed 85%, so alternative explanations must account for these differences. Murine T cells lose AMPK expression during thymic development, whereas AMPK is acutely deleted in human cells. Sustained loss of AMPK has been linked to mitochondrial dysregulation,15 suggesting that the conditional murine deletion may exaggerate the impact of AMPK loss on mitochondrial function. In addition, different environmental and experimental conditions exist between allogeneic and xenogeneic models (reviewed in Koyama and Hill16), potentially explaining the observed differences. Regardless, in both murine and human T cells, loss of AMPK disrupts oxidative metabolism at early time points and glycolytic metabolism at all time points, coincident with reduced disease severity.
The dramatic improvements in acute GVHD after transplantation of CRISPR-treated cells is believed to be the first demonstration that acute loss of AMPK in human T cells can have profound effects on GVHD progression. The murine data would suggest that an early loss in IFN-γ production, concurrent with reduced glycolysis and a limitation in CD4 T-cell numbers, reduces acute GVHD severity. This idea is echoed in the xenogeneic data, including impaired posttransplant glycolysis and reduced CD4/CD8 ratios. It is intriguing to speculate that AMPK deficiency optimally balances GVHD versus GVL considerations, where reduced GVHD is driven by fewer donor CD4 T cells, while antileukemic potential is maintained secondary to the sustained presence of CD8-mediated cytotoxicity. Regardless, the murine and human data together highlight the mechanistic importance of T-cell AMPK in GVHD pathogenesis.
Although the landscape of therapies to successfully prevent GVHD has improved in recent years,11-14 further novel strategies are required to maintain antitumor responses while still minimizing GVHD pathogenesis. The current results document a prominent role for AMPK in controlling glycolysis in both murine and human GVHD T cells, offer a mechanistic explanation for the observed changes in glycolysis, link metabolic changes to decreased IFN-γ production, highlight a prominent role for AMPK in controlling posttransplant donor CD4 T-cell numbers, and together provide a strong rationale for further development of AMPK inhibition as a clinically translatable strategy for GVHD prevention and treatment.
Acknowledgments
Cellular bioenergetics was performed on the Seahorse Analyzer in collaboration with the Rangos Metabolic Core within the Department of Pediatrics at the University of Pittsburgh, assisted by Core Director Clinton Van’t Land.
This work was supported by grants to C.A.B. from the National Institutes of Health (NIH)–National Heart, Lung, and Blood Institute (K08 HL123631, R01 HL144556), the American Society of Hematology (ASH) Scholar award, and the Be the Match Foundation (Amy Strelzer-Manasevit award). These studies were also made possible by an ASH Research Training Award for Fellows, a training grant for Pediatric subspecialit fellows from the NIH (T32HD071834, PI:Dermody), and a Hyundai Hope on Wheels Young Investigator Award (A.R.) and a St. Baldrick’s Foundation fellowship and Young Investigator Awards from the Hyundai Motor Company and Alex’s Lemonade Stand Foundation (E.L.B.). The University of Pittsburgh holds a Physician-Scientist Institutional Award from the Burroughs Wellcome Fund that supported the work of both A.R. and E.L.B. E.L.B. was additionally supported on a University of Pittsburgh Cancer Immunology Training Program T32 grant (5T32CA082084) and a Eunice Kennedy Shriver National Institute of Child Health and Human Development K12 grant (HD052892). F.K. was supported on Interdisciplinary Training in Transplantation Biology training grant (5T32AI074490) and work by R.C. and W.H. was supported by NIH grant (R01 AI175111).
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Authorship
Contribution: A.R. designed and performed experiments, analyzed data, and drafted and reviewed the manuscript; E.L.B. designed and performed experiments, analyzed data, and wrote, reviewed, and edited the manuscript; L.-K.S. performed experiments, analyzed data, and drafted the manuscript; D.M. designed and performed experiments; F.K. and M.Q. performed experiments and reviewed the manuscript; C.W., M.J.R., and R.C. performed experiments; W.H. designed experiments, analyzed data, generated figures, and drafted the manuscript; and C.A.B. drafted the manuscript with assistance from A.R., E.L.B., L.-K.S., and W.H., designed and performed experiments, analyzed data, generated figures, and revised the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Craig A. Byersdorfer, Division of Pediatric Blood and Marrow, Transplantation & Cellular Therapy, 420 Delaware Street SE, Mayo Mail code 366, Minneapolis, MN 55455; email: byer0012@umn.edu.
References
Author notes
A.R. and E.L.B. contributed equally to this study.
The raw mass spectrometric data have been deposited to the PRIDE database (www.ebi.ac.uk/pride/) as AMPK metabolics in T cells during GVHD (accession number PXD052355).
The full-text version of this article contains a data supplement.