Key Points
Haploidentical HCT has similar survival as MUD-HCT in myelofibrosis.
Strategies to prevent graft failure in haploidentical HCT need to be investigated.
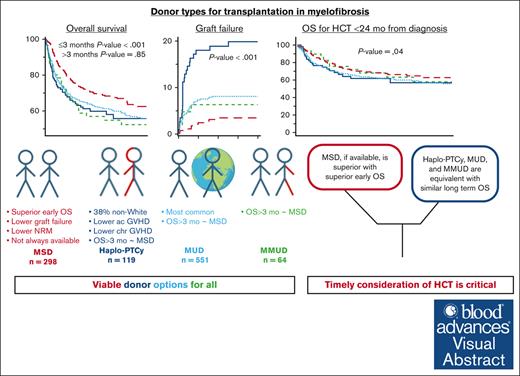
Visual Abstract
We evaluate the impact of donor types on outcomes of hematopoietic cell transplantation (HCT) in myelofibrosis, using the Center for International Blood and Marrow Transplant Research registry data for HCTs done between 2013 and 2019. In all 1597 patients, the use of haploidentical donors increased from 3% in 2013 to 19% in 2019. In study-eligible 1032 patients who received peripheral blood grafts for chronic-phase myelofibrosis, 38% of recipients of haploidentical HCT were non-White/Caucasian. Matched sibling donor (MSD)–HCTs were associated with superior overall survival (OS) in the first 3 months (haploidentical hazard ratio [HR], 5.80 [95% confidence interval (CI), 2.52-13.35]; matched unrelated (MUD) HR, 4.50 [95% CI, 2.24-9.03]; mismatched unrelated HR, 5.13 [95% CI, 1.44-18.31]; P < .001). This difference in OS aligns with lower graft failure with MSD (haploidentical HR, 6.11 [95% CI, 2.98-12.54]; matched unrelated HR, 2.33 [95% CI, 1.20-4.51]; mismatched unrelated HR, 1.82 [95% CI, 0.58-5.72]). There was no significant difference in OS among haploidentical, MUD, and mismatched unrelated donor HCTs in the first 3 months. Donor type was not associated with differences in OS beyond 3 months after HCT, relapse, disease-free survival, or OS among patients who underwent HCT within 24 months of diagnosis. Patients who experienced graft failure had more advanced disease and commonly used nonmyeloablative conditioning. Although MSD-HCTs were superior, there is no significant difference in HCT outcomes from haploidentical and MUDs. These results establish haploidentical HCT with posttransplantation cyclophosphamide as a viable option in myelofibrosis, especially for ethnic minorities underrepresented in the donor registries.
Introduction
Myelofibrosis is a clonal hematological malignancy that remains incurable without an allogeneic hematopoietic cell transplantation (HCT) despite therapeutic advances.1-5 The natural history of chronic-phase myelofibrosis involves variable rates of progression and transformation to blast phase.6 Although HCT is the only known disease-modifying therapy to date, there remains a critical need to improve timely access to and outcomes with HCT.
Various patient-, disease-, and HCT-related factors contribute to the prognosis after HCT in myelofibrosis.7-13 Donor type was shown to significantly affect outcomes of HCT in results from a Center for International Blood and Marrow Transplant Research (CIBMTR) registry study that demonstrated superior overall survival (OS) with matched sibling donors (MSDs) compared with matched unrelated donors (MUDs), which was in turn superior to mismatched unrelated donors (MMUDs) in myelofibrosis.14 This report included transplants before 2010, and no patients had received HLA-haploidentical donor (Haplo) grafts. In myelofibrosis, in which the average age of diagnosis is >65 years, MSDs are often limited by advanced age.15 Furthermore, with the emergence of posttransplantation cyclophosphamide (PTCy) for graft-versus-host disease (GVHD) prophylaxis, Haplos have gained prominence with successful HCT outcomes for diverse hematological malignancies.16,17 Haplos have the advantage of ease of graft acquisition and quicker availability for HCT planning and have offered a donor option for ethnically diverse minority populations, which are not proportionally represented in the donor registries.18,19
The feasibility of Haplos and PTCy for HCT in myelofibrosis has been described thus far in small series.11,20,21 However, the comparative outcomes of different donor types, including Haplo, on HCT outcomes in myelofibrosis have not been elucidated in the contemporary era. Understanding the impact of donor type is crucial not only to improve clinical outcomes with HCT but also to establish a donor pool with viable options and eventually improve access to HCT. This information will appropriately position HCT in the management paradigm of myelofibrosis. Hence, our study was designed to systematically evaluate the association of donor choice on outcomes of HCT in myelofibrosis in a contemporaneous cohort including Haplos.
Materials and methods
Data source
Data were derived from the CIBMTR registry, a research collaboration between the National Marrow Donor Program/Be The Match and the Medical College of Wisconsin. Approximately 375 medical centers worldwide submit clinical data to CIBMTR about HCT and other cellular therapies. CIBMTR collects transplant data on 2 levels, using a Transplant Essential Data (TED) form and a Comprehensive Report Form (CRF). CIBMTR collects TED data on all patients. Using a regularly reviewed, weighted algorithm, CIBMTR selects a subtype of patients for more detailed CRF data collection. For this study, TED and CRF-level data were used to depict donor trends over the years in HCT for myelofibrosis, whereas CRF-level data were available for all patients included in the patient-level analyses.
Patient selection
All consecutive adult patients (aged ≥18 years) who underwent a first HCT for primary or post–essential thrombocythemia or post–polycythemia vera myelofibrosis between January 2013 and December 2019 were included in evaluating donor trends. Because only a small number of patients received bone marrow or cord blood grafts, we only included HCT with peripheral blood grafts for chronic-phase myelofibrosis (<10% blasts in blood and marrow6) for outcome analysis to decrease the heterogeneity of the analyzed population. We excluded patients who received Haplo-HCT without PTCy–based GVHD prophylaxis and those who received ex vivo T-cell depletion or CD34+ selection for GVHD prophylaxis, in accordance with widely accepted HCT platforms (supplemental Figure 1).
End points and main definitions
The primary end point was OS. Death from any cause was counted as an event, and patients who are alive were censored at the last follow-up. Engraftment, nonrelapse mortality (NRM), acute and chronic GVHD, relapse, and disease-free survival (DFS) were secondary outcomes.22 Pertinent patient-, disease-, and HCT-related factors were evaluated for association with differences in clinical outcomes. We conducted additional exploratory analyses to characterize patients experiencing graft failure and evaluate outcomes of patients who underwent HCT within 24 months of diagnosis to include a uniform population in which the time to HCT was not influenced by matched donor availability. Twenty-four months was selected as the time frame because that represented the lowest median time from diagnosis to HCT among the 4 cohorts.
Haplos were mismatched at ≥2 HLA loci, MMUDs were mismatched at ≥1 HLA loci, whereas MUDs were matched at allele level at HLA-A, -B, -C, and -DRB1. Conditioning regimens were classified as myeloablative, nonmyeloablative, or reduced-intensity conditioning, per previously published criteria.23 Neutrophil engraftment was defined as the recovery of neutrophils to >0.5 × 109/L for 3 consecutive days.22 Graft failure was defined as the failure of neutrophil engraftment or decline in absolute neutrophil count to <0.5 × 109/L after engraftment or the need for second transplantation or donor leukocyte infusion for aforementioned reasons.24,25 We recognize that definitions of graft failure vary across studies, and an ongoing effort is under the works to standardize the definitions for graft failure in myelofibrosis. Relapse was captured per institutional reporting. CIBMTR’s practice for data collection on relapse involves evaluation at 6 months after HCT for resolution of cytopenia, splenomegaly, and marrow findings of myelofibrosis, and persistence of these factors at 6 months is labeled as relapse.
Statistical considerations
Kaplan-Meier curve estimates were used to generate probabilities of OS and DFS. Cumulative incidence estimates were calculated for graft failure, relapse, NRM, and GVHD as competing risk outcomes. The Cox proportional hazard model was used to evaluate the impact of donor type on OS and DFS. For competing risk outcomes including relapse, NRM, and graft failure, the cause-specific hazards model was used. The proportional hazard assumptions were checked by testing whether the coefficients of the logarithm of time multiplied by a covariate were equal to 0 for each variable. If violated, it was included as time-dependent variables, which results in a “piecewise” Cox proportional hazards model allowing for different hazards before and after a time cut point. The optimal time cut point was chosen as the one with the highest likelihood among candidate time cut point values. Stepwise variable selection method was used at the significance level .05 for entering or removing effects into the final model. Various patient-, disease-, and HCT-related factors were included in the analysis (listed in supplemental Table 1). Interaction between donor type and significant factors was checked. Center effects were tested using the Score test.26 If there were significant center effects, the marginal Cox model was used to adjust for center effects. All analyses were performed using SAS 9.4 (SAS institute, Cary, NC).
Results
Trends of donor types in myelofibrosis
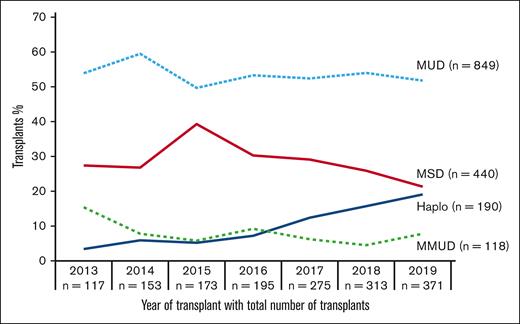
We queried the CIBMTR data sets including all 1597 HCTs (TED and CRF data and marrow and peripheral blood grafts) for myelofibrosis. Figure 1 illustrates a notable increase in the overall number of HCTs performed for myelofibrosis from 117 in 2013 to 371 in 2019. Of the 4 donor sources, we noted a substantial increase in the use of Haplos from 4 (3% of total HCTs) in 2013 to 71 (19% of total HCTs) in 2019 (supplemental Table 2).
Patient, disease, and HCT characteristics
In subsequent analyses, after the exclusion of patients with TED forms only, cord/marrow grafts, nonstandard donor options, ex vivo T-cell–depleted/ CD34+ selection, and blasts ≥10%, per study criteria, we included 1032 patients (supplemental Figure 1). Baseline characteristics of these 1032 patients are outlined in Table 1. There are notable differences at baseline between the 4 cohorts, as expected in an observational study. Patients undergoing MMUD-HCTs were younger (median age, 59 years). Patients of non-White ethnicities were more likely to undergo Haplo-HCT (38% vs 18% MSD-HCT, 10% MUD-HCT, and 22% MMUD-HCT; P ≤ .01). Median time from diagnosis to HCT was longer for Haplo-HCT (33 months) than with MSD (25 months), although not statistically significant (P = .53). Patients undergoing Haplo-HCT had higher-risk disease by Dynamic International Prognostic Scoring System (DIPSS; P ≤ .01). Haplo-HCT was more commonly done using reduced-intensity (37%) or nonmyeloablative conditioning (36%; P ≤ .01). As expected, MSDs were older, whereas Haplos were distributed over the age ranges, and the most of MUDs and MMUDs were aged <30 years (P ≤ .01).
Primary end point: OS by donor type
Median follow-up on the study was 46.5 months (range, 3.7-99.7). Univariate estimates of OS at 3 years were 68.8% (95% confidence interval [CI], 63.3-74.1) with MSD, 59% (95% CI, 49.7-67.9) with Haplo, 61.3% (95% CI, 57.1-65.4) with MUD, and 55.2% (95% CI, 42.7-67.4) with MMUD (P = .03). Detailed univariate analysis is tabulated in supplemental Table 3. Based on these results and using the predetermined variables as outlined in “Methods,” we conducted the multivariable analysis. The proportional hazard assumptions were not valid for donor types in the OS model, which indicates the hazard ratio (HR) between donor groups is not constant over time. To address violated proportional hazard assumptions, the time-dependent donor type was included using the time cut point of 3 months. In the first 3 months after HCT, donor type was independently associated with OS on multivariable analysis (reference MSD) with HR 5.79 (95% CI, 2.54-13.20) for Haplo, HR 4.48 (95% CI, 2.25-8.92) for MUD, and HR 5.24 (95% CI, 1.49-18.42) for MMUD (P < .001; Table 2). Beyond 3 months, OS was not significantly different among all donor cohorts on multivariable analysis (MSD reference; Haplo HR, 1.09 [95% CI, 0.73-1.63]; MUD HR, 1.05 [95% CI, 0.78-1.41]; MMUD HR, 1.21 [95% CI, 0.78-1.88]; P = .85). There was no difference in OS (both ≤3 months and >3 months) among the 3 non-MSD donor options of Haplo, MUD, and MMUD (Figure 2A).
Outcomes of HCT in myelofibrosis by donor type. (A) Adjusted OS. (B) Adjusted graft failure. (C) Adjusted NRM.
Outcomes of HCT in myelofibrosis by donor type. (A) Adjusted OS. (B) Adjusted graft failure. (C) Adjusted NRM.
Age at HCT >60 years, Karnofsky performance score <90, higher HCT-Comorbidity index (≥3), and CD34+ cell dose <8 × 106/kg were significantly associated with inferior OS (Table 2).
Key secondary end points by donor type
Graft failure
On multivariable analysis, the use of MSDs was associated with significantly lower incidence of graft failure than Haplos (HR, 6.11; 95% CI, 2.98-12.54; P < .001) and MUDs (HR, 2.33; 95% CI, 1.20-4.51; P = .01), although not significantly different compared with MMUDs (HR, 1.82; 95% CI, 0.58-5.72; P = .30; Table 3). Cumulative incidence of graft failure at day +100 was 1% (95% CI, 0.20-0.25) for MSDs, 15.3% (95% CI, 9.3-22.3) for Haplos, 5.1% (95% CI, 3.6-7.3) for MUDs, and 4.7% (95% CI, 0.9-11.2; P < .001) for MMUDs. The adjusted rate of graft failure are shown in Figure 2B. Other patient-, disease-, or HCT-related variables did not have a statistically significant association, although the limited number of events may limit reliable accounting for all covariates (Table 3).
GVHD
On multivariable analysis, MMUDs were independently associated with higher acute GVHD grade 3 to 4 (MSD HR reference; Haplo HR, 0.64 [95% CI, 0.38-1.09]; MUD HR, 0.78 [95% CI, 0.57-1.06]; MMUD HR, 1.40 [95% CI, 0.83-2.35]; P = .04), as shown in supplemental Table 4A and supplemental Figure 2. There was no significant difference in chronic GVHD (supplemental Table 4B; supplemental Figure 3).
NRM
On multivariable analysis, MSD was associated with significantly lower NRM with an HR of 1.71 (95% CI, 1.09-2.68) for Haplos, HR 1.58 (95% CI, 1.14-2.18) for MUDs, and HR 1.71 (95% CI, 0.98-2.97) for MMUDs (P = .03; Figure 2C). Notably, there was no significant difference among Haplo, MUD, or MMUD (supplemental Table 4C). Age >60 years and Karnofsky score <90 were also independently associated with higher NRM (supplementary Table 4C). The most common cause of NRM was GVHD in MSD-HCTs (20% of deaths), organ failure or infection in Haplo-HCTs (25% of deaths each), and infection in MUD-HCTs (21% of deaths) as well as MMUD-HCTs (32% of deaths).
Relapse and DFS
There was no statistically significant difference in relapse or DFS by donor type (supplemental Table 4D-E). Adjusted probabilities are shown in supplemental Figures 4 and 5. Splenomegaly at HCT was independently associated with increased relapse (HR, 1.36; 95% CI, 1.11-1.66; P = .003) and inferior DFS (HR, 1.37; 95% CI, 1.17-1.62; P < .001). Age >60 years was associated with inferior DFS (HR, 1.38; 95% CI, 1.17-1.64; P < .001). Disease relapse was the most common cause of death in MSD-HCT (31%), Haplo-HCT (28%), and MUD-HCT (24%). In MMUD-HCT, 17% of deaths were attributed to relapse.
Exploratory analyses
Patients with graft failure
The lower incidence of graft failure had a major contribution to the reduced NRM and improved early OS observed with MSDs. Hence, we conducted a comprehensive review of patients who experienced graft failure, and a descriptive breakdown of pertinent variables is detailed in supplemental Table 5. Eighty-four patients (8%) experienced graft failure. Although not compared in a statistical model, patients who experienced graft failure had a longer time to HCT (median, 31.5 vs 26.7 months), more advanced disease at HCT, that is, lower hemoglobin (<10 g/dL in 79% vs 71%), lower platelets (<50 000/μL in 27% vs 21%), and higher DIPSS scores (intermediate-2 or high in 54% vs 40%), splenomegaly at HCT (56% vs 47%), and more commonly received nonmyeloablative conditioning (14% vs 6%). After graft failure, mortality was high (59/84 [70.2%]), attributed to graft failure itself (12/59 [20%]), resulting infection (15/59 [25%]), hemorrhage (4/59 [6.7%]), organ failure/toxicity (13/59 [21.7%]), or disease relapse (5/59 [8.3%]).
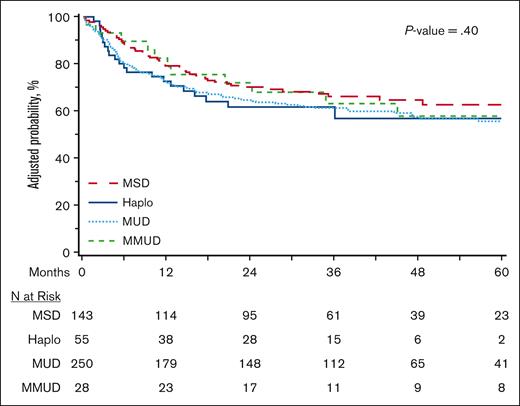
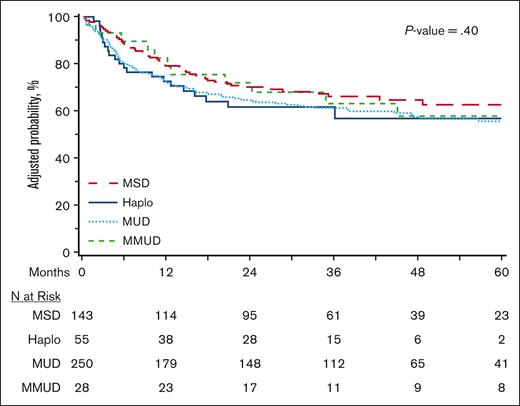
Outcomes for HCT within 24 months from diagnosis
The current donor selection paradigm influences the timing of HCT because recipients of MSDs are more likely to receive transplants more promptly, whereas alternative donor sources are more likely to be offered later in the disease course. We therefore evaluated OS in the subset of patients who underwent HCT within 24 months of diagnosis, as an exploratory outcome, to allow for the inclusion of a more homogenous population in which HCT was not delayed due to lack of matched donor availability (n = 481). As depicted in Figure 3 and supplemental Table 6, multivariable analysis did not demonstrate a significant difference in OS between the 4 donor types (MSD reference; Haplo HR, 1.40 [95% CI, 0.83-2.36]; MUD HR, 1.32 [95% CI, 0.93-1.86]; MMUD HR, 1.12 [95% CI, 0.58-2.16]; P = .40). Univariate estimates of graft failure were significantly lower with MSD in this cohort (at day +100, MSD 1.4%, Haplo 10.9%, MUD 5.9%, and MMUD 3.4%), whereas NRM and relapse were not significantly different (supplemental Table 7).
Discussion
In this study, we leveraged the registry of prospective observational data to compare HCT outcomes of available donor options in a large cohort of patients with myelofibrosis. The current practice favors HLA-matched donors over -mismatched donors based on prior studies in historical context.13,14,27 Our study, which included Haplo-HCT from a more contemporary cohort reflecting the current practice shift with the growing adoption of Haplo-HCT since 2013, demonstrates a significant trend in reducing the survival gap when using mismatched donors and establishes Haplo-HCT with PTCy as an acceptable approach in patients with myelofibrosis in need of an allograft. These findings advocate for an approach of donor selection emphasizing on timely HCT without necessitating exclusive prioritization of fully matched donors. This has implications on clinical practice because it expands the donor pool and promotes equitable access to HCT for patients with myelofibrosis from diverse ethnic backgrounds (38% non-White in this study). Similar comparisons between donor types have been performed in acute leukemias and lymphomas and have consistently demonstrated no significant differences in survival between different donor types, underscoring the improved access to HCT due to the availability of Haplos with PTCy, without compromising outcomes.16,28-32 Furthermore, because NRM continues to improve with better supportive care and improved GVHD management,33-35 we anticipate that this diminishing disparity between donor types will continue to disappear in the future.
The superiority of OS with MSD in the early post-HCT period appears predominantly due to superior engraftment and consequently lower NRM noted in the early post-HCT period. This analysis identifies graft failure as a critical unmet need in myelofibrosis HCTs, especially with Haplos, and that designing strategies to lower graft failure in Haplo-HCT is important to improve outcomes.25 Notably, the graft failure rates noted in this study with Haplos are higher than those reported in other multi-institutional and single-center studies (6%-9%), likely due to differences in definitions for graft failure.10,11,20 Considering that the reduced sample size for the cohort with graft failure limits definitive conclusions despite the multivariable analysis, we explored the characteristics of patients who experienced graft failure to highlight differences that can guide clinical practice. One striking difference was the more prevalent use of nonmyeloablative conditioning regimens (than reduced-intensity and myeloablative conditionings) in patients who experienced graft failure, which increase the risk of graft rejection due to higher persistence of the host immune system.36-38 This is especially pertinent considering that approximately one-third of patients who underwent Haplo-HCT received nonmyeloablative conditioning, compared with only a negligible fraction in the other donor groups. Hence, in myelofibrosis HCT, in which the disease characteristics such as splenomegaly and marrow fibrosis can impede engraftment, it is recommended to use at least reduced-intensity conditioning, instead of nonmyeloablative conditioning, to reduce the risk of graft failure. This aligns with the single-center data that reported no graft failures with the use of increased dose of total body irradiation to 400 cGy from 200 cGy, with which 10% rate of graft failure was noted.10 In the absence of statistically significant association of conditioning regimen, it also appears that myeloablation may not be required in all patients in myelofibrosis. Strategies for improved disease control, such as adequate spleen size reduction, in addition to optimal conditioning regimens should be evaluated in future studies to identify the optimal bridge to HCT.
Another consideration for improving outcomes is timely consideration for HCT. Prior studies and recommendations have consistently favored early consideration for HCT in patients with myelofibrosis especially those with DIPSS intermediate-1 or higher disease.9,27,39-41 Exclusive prioritization of matched donors can delay HCT and likely worsen disease status at the time of HCT, possibly increasing the risk of inferior outcomes. This finding is underscored in our exploratory analysis, which reveals no significant difference in OS among patients who underwent HCT within 24 months of diagnosis. Although this analysis carries the limitation of sample size attrition, the narrowing of effect size suggests equivalent outcomes, regardless of donor type, in patients who undergo HCT early in the disease course.
This analysis is limited by the absence of somatic mutation data that have been correlated with HCT outcomes in the published literature10,42-44 but were not available in CIBMTR data for most patients. Another methodological limitation is that donor age cofounds with the main effect of analysis, that is, patient age correlates with donor age in the Haplo cohort, and hence, cannot be included in the multivariable model. This is especially relevant with emerging data demonstrating that younger donor age is significantly associated with improved outcomes with HCT for diverse hematological malignancies.30,45,46 We warrant caution interpreting results for MMUD-HCT because only 17% MMUD-HCTs were done using PTCy, which may not be reflective of contemporary practice. Prospective randomized studies to evaluate the impact of different donor types are logistically impractical given the rarity of the disease; and in the absence of such studies, prospectively collected observational data from large registries such as the CIBMTR serve as important source to guide clinical practices.
Conclusion
The survival gap between MSD and alternative donor sources has reduced over time. Haplos with PTCy have made HCT more accessible especially to patients of non-White ethnicities. Although MSD donors have more favorable early survival due to lower NRM and graft failure, survival of donor types other than MSD are similar. Timely consideration of HCT is important, and the availability of fully matched donor should not delay HCT. Further prospective studies and research are urgently needed to reduce the risk of graft failure in myelofibrosis.
Acknowledgments
Center for International Blood and Marrow Transplant Research is supported primarily by the Public Health Service U24CA076518 from the National Cancer Institute, the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases; 75R60222C00011 from the Health Resources and Services Administration; and N00014-23-1-2057 and N00014-24-1-2057 from the Office of Naval Research. Support is also provided by the Medical College of Wisconsin, National Marrow Donor Program, Gateway for Cancer Research, and Pediatric Transplantation and Cellular Therapy Consortium and from the following commercial entities: AbbVie; Actinium Pharmaceuticals; Adaptive Biotechnologies Corporation; ADC Therapeutics; ADIENNE SA; Alexion; AlloVir, Inc.; Amgen; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics; BeiGene; BioLineRx; Blue Spark Technologies; bluebird bio; Blueprint Medicines; Bristol Myers Squibb; CareDx; CSL Behring; CytoSen Therapeutics; Deutsche Knochenmarkspenderdatei; Elevance Health; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Gamida Cell, Ltd; Gift of Life Biologics; Gift of Life Marrow Registry; GlaxoSmithKline; HistoGenetics; Incyte Corporation; Iovance; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc; Karius; Kashi Clinical Laboratories; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Labcorp; Legend Biotech; Mallinckrodt Pharmaceuticals; Med Learning Group; medac GmbH; Merck & Co; Mesoblast; Millennium, the Takeda Oncology; Miller Pharmacal Group, Inc; Miltenyi Biotec; MorphoSys; MSA-EDITLife; Neovii Pharmaceuticals AG; Novartis Pharmaceuticals Corporation; Omeros Corporation; Optum Health; Orca Biosystems, Inc; OriGen BioMedical; Ossium Health, Inc; Pfizer, Inc; Pharmacyclics, LLC, an AbbVie company; Pharmaceutical Product Development, LP; REGiMMUNE; Registry Partners; Rigel Pharmaceuticals; Sanofi; Sarah Cannon; Seagen Inc; Sobi, Inc; STEMCELL Technologies; Stemline Therapeutics; STEMSOFT; Takeda Pharmaceuticals; Talaris Therapeutics; Vertex Pharmaceuticals; Vor Biopharma Inc; and Xenikos BV.
Authorship
Contribution: T.J. designed the study, analyzed data, interpreted the data analysis, wrote the first draft of the manuscript, revised the manuscript, and approved the final draft; N.E.-M. and J.D. collected the data from registry, analyzed data, and approved the final draft; M.Q.S. and R.K. designed the study, participated in data interpretation and writing the manuscript, and approved the final draft; S.K. and M.C. analyzed data and participated in writing the manuscript; W.S. and V.G. designed the study, analyzed data, participated in data interpretation and writing the manuscript, and approved the final draft; and the remaining authors participated in writing and editing the manuscript.
Conflict-of-interest disclosure: T.J. reports receiving institutional research support from CTI BioPharma, Kartos Therapeutics, Incyte, and Bristol Myers Squibb (BMS); and advisory board participation with CareDx, BMS, Incyte, AbbVie, CTI, Kite, Cogent Biosciences, Blueprint Medicine, Telios Pharma, Protagonist Therapeutics, TScan Therapeutics, Karyopharm, and MorphoSys. R.K. reports advisory board participation for Vertex Pharmaceuticals. M.A.C. reports becoming an AstraZeneca employee while on Hospital del Mar; and advisory board or consulting fees from Novartis, Pfizer, BMS, Takeda, and Sanofi. H.E. reports advisory board for Shoreline Biosciences; and research funding from BMS. T.B. reports advisory board fees from Pfizer, Takeda, and MorphoSys. K.B. reports receiving research funding to her institution from Stemline Therapeutics. V.R.B. reports participating in safety monitoring committee for Protagonist, serving as an associate editor for the journal Current Problems in Cancer and as a contributor for BMJ Best Practice; consulting fees from Taiho, Sanofi, Imugene, Genentech, Incyte, Servier Pharmaceuticals LLC, and AbbVie; research funding (institutional) from MEI Pharma, Actinium Pharmaceutical, Sanofi US Services, AbbVie, Pfizer, Incyte, Jazz, and National Marrow Donor Program; and drug support (institutional) from Chimerix for a trial. Z.D. reports research support from Incyte, Corp, REGiMMUNE, Corp, and Taiho Oncology, Inc; and consulting fees from Sanofi, Incyte, Corp, MorphoSys AG, Inhibrx, PharmaBiome AG, and Ono Pharmaceutical. N.F. has been an advisory board member for Incyte; received speaker fees from Incyte; is on the data safety monitoring committee for Chronic Graft-versus-Host Disease Consortium; and is the medical monitor for Blood and Marrow Transplant Clinical Trial Network. A.P.G. reports advisory board/research funding from Orca Bio and Jasper Therapeutics. U.G. reports consulting and speaker bureau fees from Incyte. M.R.G. reports consulting fees from AbbVie, Amgen, Astellas, Blueprint Medicines, BMS, Cardinal Health, Sobi/CTI BioPharma, Daiichi Sankyo, Gamida Cell, Genentech, Gilead Sciences, GlaxoSmithKline (GSK)/Sierra Oncology, Incyte, Invitae, Jazz, Novartis, Ono Pharmaceutical, Pfizer, Pharmacosmos, Premier, Servier/Agios, and Stemline Therapeutics; research support from Incyte and Janssen; and stock ownership in Medtronic. N.H. reports advisory board participation with Novartis, Gilead, Janssen, AbbVie, Pfizer, Incyte, Roche, Takeda, Jazz Pharmaceuticals, Link Pharmaceuticals, and Mallinckrodt. B.K.H. reports advisory committees/consultancy fees from Nkarta, Sanofi, Incyte, and Equilium; DSMB fees from Angiocrine Bioscience; and adjudication committee fees from CSL Behring. O.J. reports advisory board participation for Ascentage Pharma. M.A.K.-D. reports grant/research support from BMS, Novartis, and Pharmacyclics; and lecture/speaking engagement fees from Kite Pharma. C.U. reports honoraria for being in speaker bureau for Takeda and Blueprint. J.R.W. reports consultant fees from Ansun, Celgene, Cidara Therapeutics, F2G, Orca Bio, and Takeda. T.N. reports clinical trial support by Novartis to the institution; and clinical trial support (drug only supply) by Karyopharm to the institution. M.A.P. reports honoraria from Adicet Bio, Allogene, Allovir, Caribou Biosciences, Celgene, BMS, Equilibrium, ExeVir, ImmPACT Bio, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, Orca Bio, Sanofi, Syncopation, VectivBio AG, and Vor Biopharma; serves on DSMBs for Cidara Therapeutics and Sellas Life Sciences; and the scientific advisory board of NexImmune; has ownership interests in NexImmune, Omeros, and Orca Bio; received institutional research support for clinical trials from Allogene, Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis; serves in a volunteer capacity as a member of the board of directors of the American Society for Transplantation and Cellular Therapy and on the Center for International Blood and Marrow Transplant Research Cellular Immunotherapy Data Resource Executive Committee; and previously served on the board of directors of Be The Match (National Marrow Donor Program). V.G. reports consulting fees from AbbVie, BMS/Celgene, GSK, Novartis, Incyte, CTI BioPharma, and Pfizer; and participation in the data safety monitoring board or advisory board for AbbVie, Incyte, GSK, BMS/Celgene, Pfizer, and Roche. The remaining authors declare no competing financial interests.
Correspondence: Tania Jain, Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, 1650 Orleans St, Rm 3M88, Baltimore, MD 21202; email: tjain2@jhmi.edu.
References
Author notes
W.S. and V.G. contributed equally to this study.
Center for International Blood and Marrow Transplant Research (CIBMTR) supports accessibility of research in accordance with the National Institutes of Health Data Sharing Policy and the National Cancer Institute Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases deidentified data sets that comply with all relevant global regulations regarding privacy and confidentiality.
The full-text version of this article contains a data supplement.