Key Points
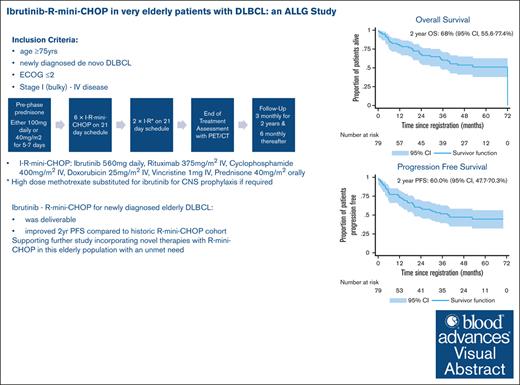
Ibrutinib–R-mini-CHOP was a deliverable combination in patients with DLBCL aged ≥75 years.
Ibrutinib–R-mini-CHOP improves PFS compared with historical rates reported for R-mini-CHOP, but this study did not demonstrate a superior OS.
Visual Abstract
The multicenter, prospective phase 2 Australasian Leukaemia & Lymphoma Group NHL29 trial was conducted to assess the addition of ibrutinib to R-mini-CHOP (dose attenuated R-CHOP; rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) in patients aged ≥75 years with newly diagnosed diffuse large B-cell lymphoma (DLBCL). Treatment consisted of six 21-day cycles of ibrutinib–R-mini-CHOP followed by two 21-day cycles of R-ibrutinib. Coprimary end points were deliverability and 2-year overall survival (OS). The median average relative total dose and average relative dose intensity for the entire regimen were both 97% (interquartile range, 82-100 and 88-100, respectively). With a median follow-up of 35.5 months, the 2-year OS was 68% (95% confidence interval [CI], 55.6-77.4) with a 2-year progression-free survival (PFS) of 60.0% (95% CI, 47.7-70.3). Median OS and PFS were 72 months (95% CI, 35 to not reached) and 40 months (95% CI, 20.4 to not reached), respectively. The overall response rate was 76% (61/79) of patients, with a complete response rate of 71% (56/79). Deaths occurred in 34 of 79 patients (43%), including 17 from progressive disease and 5 treatment related. Overall, 67% patients experienced at least 1 serious adverse event. Most common adverse events were infections and diarrhea (the majority grade 1-2). In both health-related quality of life measures, there was an improvement in functional and symptom scales, median health state classification score, and median visual analogue scale in responders over time. In conclusion, this study showed that the addition of ibrutinib to R-mini-CHOP was both deliverable and efficacious in elderly DLBCL patients.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma. Approximately 40% of DLBCL occurs in patients aged >70 years.1 DLBCL is curable with R-CHOP (rituximab, cyclophosphamide, doxorubicin, Oncovin [vincristine], and prednisone) therapy, but the potential for cure is reduced in older patients with a poorer risk disease biology and less capacity to tolerate intensive chemotherapy because of increased comorbidities and functional decline.2-4 Historically DLBCL clinical trials have defined “elderly patient” as aged 60 to 80 years4,5; however, with the marked increase in the fit aging population globally there is an unmet need for evidence-based treatment paradigms for truly elderly patients with DLBCL.
For these patients, dose attenuated R-CHOP, so-called R-mini-CHOP, is a standard of care reported by Peyrade et al more than a decade ago.6 In the landmark phase 2 study, 150 patients with a median age of 83 years received 6 cycles of R-mini-CHOP achieving a 2-year overall survival (OS) of 59% (95% confidence interval [CI], 49-67), median OS of 29 months (95% CI, 21 to not reached), 2-year PFS of 47% (95% CI, 38-56), and median progression-free survival (PFS) of 21 months (95% CI, 13 to not reached). Of 58 deaths, 12 (21%) were due to toxicity of therapy, mostly infections. Subsequently there has been no improvement over this regimen by substituting ofatumumab or obinutuzumab for R.7,8 More recently a phase 3 study demonstrated the addition of lenalidomide to R-mini-CHOP in patients aged ≥80 years does not improve OS and results in more adverse events (AEs).9
Ibrutinib is a first-in-class oral covalent inhibitor of Bruton tyrosine kinase (BTK). In a phase 3 placebo-controlled study (PHOENIX), ibrutinib in combination with R-CHOP demonstrated efficacy in patients aged <60 years in a nonprespecified subgroup analysis, however toxicity limits the ability to complete therapy in patients aged ≥60 years.10 In patients aged ≥60 years, all key end points, which included event-free survival (hazard ratio [HR], 1.228; 95% CI, 0.887-1.699), PFS (HR, 1.200; 95% CI, 0.866-1.664), and OS (HR, 1.440; 95% CI, 0.963-2.152), were inferior in the ibrutinib plus R-CHOP arm. This was attributed to increased rates of AEs, including serious AEs, leading to early R-CHOP discontinuation in this population, which compromised treatment exposure and likely decreased efficacy.
Herein, we report the safety and efficacy data from the Australasian Leukaemia & Lymphoma Group (ALLG) NH29 (IRiC) study using ibrutinib in combination with R-mini-CHOP in patients aged ≥75 years. We hypothesized the addition of ibrutinib to R-mini-CHOP would be both tolerable and efficacious, improving the 2-year OS of elderly patients with DLBCL compared with the Peyrade6 cohort.
Methods
Patients
This prospective, single-arm phase 2 multicenter cooperative group trial IRiC (Australian New Zealand Clinical Trials Registry: ACTRN12615000551594) was sponsored by the ALLG. Eligible patients were aged ≥75 years with newly diagnosed, histologically proven de novo CD20+ DLBCL. Key inclusion criteria were Ann Arbor stage I bulky to stage IV disease, at least 1 measurable site of disease, Eastern Cooperative Oncology Group (ECOG) performance status score of ≤2 (after prephase prednisone), left ventricular ejection fraction within institutional normal limits, and a minimum life expectancy of 3 months.
Key exclusion criteria were central nervous system (CNS) involvement; creatinine clearance of ≤ 40 mL/min per 1.73 m2; hepatic transaminases of >3 × upper limit of normal, and/or total bilirubin >1.5 × upper limit of normal unless bilirubin rise was due to nonhepatic origin; poor bone-marrow reserve, defined as absolute neutrophil count of <1.0 × 109/L independent of growth factor support and/or platelet count of <100 ×109/L (< 50 × 109/L if bone marrow involvement) independent of transfusion support; history of malignancy during the past 3 years; concurrent anticoagulation with warfarin (or equivalent vitamin K antagonists) and/or dual antiplatelet therapy (eg, aspirin and a P2Y12 antagonist); required treatment with strong CYP3A inhibitors; prior anthracycline exposure of ≥150 mg/m2; history of stroke or intracranial hemorrhage within 6 months of enrolment; known bleeding disorders; clinically significant cardiovascular disease; and known history of HIV, active hepatitis C virus infection, or active hepatitis B virus infection.
Patients provided written informed consent. The study was approved by an independent research ethics committee and conducted in accordance with the International Conference on Harmonization Good Clinical Practice Guidelines, the Declaration of Helsinki (1996), and applicable local regulatory requirements and laws. An ALLG independent safety and data monitoring committee reviewed safety, risk and/or benefit throughout the clinical trial at 6 monthly intervals, as well as in August 2018 after the reporting of increased toxicity and reduced deliverability of the combination of ibrutinib with full-dose R-CHOP in patients aged >60 years in the PHOENIX study.
Treatment and assessments
All patients received prephase prednisone (100 mg or 40 mg/m2 daily) for 5-7 days before commencing IRiC. Treatment consisted of 6 cycles of ibrutinib-R-mini-CHOP at 21-day intervals followed by a further 2 × 21-day cycles of ibrutinib-R. Ibrutinib-R-mini-CHOP consisted of ibrutinib 560 mg orally daily, R 375 mg/m2 IV, cyclophosphamide 400 mg/m2 IV, doxorubicin 25 mg/m2 IV, vincristine 1 mg IV on day 1, and prednisone 40 mg/m2 or 100 mg orally on days 1 through 5.
Supportive medications included mandatory granulocyte colony-stimulating factor each cycle delivered as per local guidelines, and oral fluoroquinolone prophylaxis for cycle 1 only from day 7 until recovery of neutropenia. Tapering of prednisone and replacement therapy was permitted during R-mini-CHOP therapy as required. Serum vitamin D level was checked in all patients at screening and vitamin D replacement therapy was mandated in patients with deficiency with a vitamin D level of <50 nmol/L.
CNS prophylaxis with intrathecal with/without IV methotrexate was permitted for high-risk patients only (fulfilling all 3 of the risk factors for CNS disease: elevated lactate dehydrogenase, ECOG performance status score of >1, and involvement of ≥2 extranodal sites or with lymphomatous involvement of the bone marrow; supplemental Data).
Safety assessments including history, examination, and blood tests were performed before each cycle. Patients underwent a 18F-fluorodeoxyglucose positron emission tomography/computed tomography scan at baseline and after completion of therapy. A computed tomography scan of neck/chest/abdomen/pelvis was performed before therapy, after cycle 4, at end of treatment, 6 monthly for 2 years, then annually until 5 years. Quality-of-life questionnaires (European Organisation For Research And Treatment Of Cancer Core Quality of Life questionnaire (EORTC QLQ-C30), EuroQOL EQ-5D-5L) were performed at baseline, on day 1 of each cycle of therapy, 1 month after the end of treatment, then 6 monthly for 2 years. A comprehensive geriatric assessment was performed at registration with the cumulative illness rating scale for geriatrics (CIRS-G) and the Cancer and Ageing Research Group (CARG) geriatric assessment tool. The CARG was also assessed at cycle 3 day 1, 1 month, and 6 months after treatment.
Available diagnostic tissue samples were analyzed for cell of origin (COO) by both centralized immunohistochemistry (IHC) review using the Hans classification11 and gene expression profiling (GEP) of formalin-fixed paraffin-embedded biopsy tissue using Lymph2CX assay (NanoString Technologies, Seattle, WA).12
End points
The 2 safety and efficacy coprimary end points were (1) deliverability as assessed using the average relative total dose (ARTD) and average relative dose intensity (ARDI); and (2) 2-year OS, respectively.
ARTD is defined as the average delivered dose of the chemotherapy dose as a percentage of the target dose. It is calculated by averaging the relative total doses of the individual drugs.13 The ARDI is defined as the average delivered dose of the chemotherapy regimen per unit time (week) as a percentage of the target dose. This is calculated by averaging the relative total dose intensity of the individual drugs.14
OS was defined as time from registration on study to death from any cause.
Secondary end points were: PFS, disease-free survival (DFS), investigator assessed response rates according to Lugano 2014 response criteria,15 and toxicity, according to National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03). Exploratory end points included patient-reported outcomes using the EORTC (QLQ-C30) and EuroQol questionnaire (EQ-5D-5L); correlating GEP gene mutations, microRNA with clinical outcomes and mechanisms of resistance; and correlation between geriatric assessment tools, outcomes, and tolerability of therapy. PFS was defined as the time from registration on the study to death from any cause, relapse for patients with complete response, progression during or after treatment, or any change of therapy during treatment, censored at the date of last contact. DFS was defined as the time from the date of complete response until the date of progression or death.
Statistical analysis
A sample size of 58 patients provided 80% power with 2-sided α of .05 to detect a 15% improvement in 2-year OS (from 59%6 to 74%), whereas a sample size of 77 patients over 3 years, followed up for a minimum of 2 years, provided 80% power to detect a 15% improvement in 2-year PFS (from 47% to 62%). A sample size of 80 was chosen in order to have sufficient power to detect differences in the secondary PFS end point.
All eligible patients who received at least 1 dose of R-mini-CHOP were included in the intention-to-treat and safety analyses. Quantitative variables were summarized in tables displaying sample size, mean, standard deviation, median, and range. Qualitative variables were described in terms of numbers of each response category, which were converted into percentages of the number of patients or AEs examined, depending on the unit under investigation. Time-to-event outcomes were calculated using Kaplan-Meier methods, including 95% confidence intervals (CIs). Data were censored at the last observation (eg, last response assessment for PFS or DFS, or last known date of survival status for OS). Differences were explored using log-rank tests. Cox regression was used to explore the impact of prognostic factors on time to event outcomes. All analyses were conducted 2 sided, with α set at .05 using Stata MP version 17 for Mac (StataCorp, College Station, TX).
Results
Study population
A total of 80 patients were enrolled at 21 Australian sites between 5 November 2015 and 26 November 2018; 1 patient died from sepsis before commencing treatment and is excluded from the analysis. Patient characteristics are summarized in Table 1. Median age was 81.5 years (range, 75-95) with 65% aged >80 years; 81% had advanced stage disease, 55% had an age-adjusted International Prognostic Index (IPI) score of 2 to 3. Non-germinal center B-cell–like phenotype COO as determined by centralized IHC using the Hans classification11 was identified in 45 of 79 patients (57%). Gene expression was available in 48 of 80 patients (60%), and showed 16 (33%), 27 (56%), and 5 (10%) activated B-cell, germinal center B-cell, and unclassified, respectively.
Deliverability
All 6 cycles of R-mini-CHOP were completed in 63 of 79 patients (80%), and 57 of 79 (71%) completed all 8 cycles of therapy. Reasons for not completing therapy included toxicity (13 patients, including 5 deaths), progressive disease (5 patients), patient choice (3 patients), and withdrawal of consent (1 patient; Figure 1).
Table 2 shows ibrutinib-R-mini-CHOP deliverability. The median ARTD and ARDI for the entire regimen was 97% with an interquartile range of 82-100 and 88-100, respectively. The median RDI was 99.1% (66.9-109.7) for cyclophosphamide and 99.5% (59.5-104.5) for doxorubicin. Ibrutinib was temporarily ceased in 59% (47/79) of patients with a median of 7 days off ibrutinib (interquartile range, 1-21), reasons being AEs (45/47), biopsy (1/47), and pharmacy error (1/47). Ibrutinib was permanently ceased in 25% (20/79) of patients, again mainly because of AEs (16/20). Progressive disease (3/20) and intracranial hemorrhage (1/20) were the other reasons.
CNS prophylaxis with IV methotrexate was administered to 6 patients, with 2 only receiving 1 cycle; all of these patients received the 6 cycles of R-mini-CHOP. There were no CNS relapses in the entire patient cohort.
Efficacy
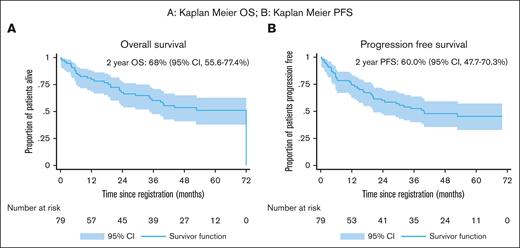
With a median follow-up of 35.5 months (0.2, 71.7), the 2-year OS of 68.0% (95% CI, 55.6-77.4) was not significantly higher than the expected 59.0% (95% CI, 49.0-67.0) from historical controls6 (P = .11). The median OS was 72 months (95% CI, 35 to not reached; Figure 2A).
Overall Survival & Progression Free Survival (A) Kaplan-Meyer OS; (B) Kaplan-Meyer PFS.
Overall Survival & Progression Free Survival (A) Kaplan-Meyer OS; (B) Kaplan-Meyer PFS.
The median 2-year PFS of 60.0% (95% CI, 47.7-70.3) was significantly improved compared with the reference cohort6 of 47.0% (P = .03; Figure 2B).
The 2-year DFS was 64.5% (95% CI, 52.5-74.8) and median DFS was not reached (95% CI, 26.5 to not reached).
The overall response rate at the end of treatment was 77% (61/79) of patients, with a complete response rate of 71% (56/79) of patients.
The median time from progression to death in the 26 patients with progressive disease was 4.2 months (95% CI, 1.8-6.7).
Safety
A total of 34 deaths were reported during treatment and follow-up, 17 due to progressive disease, 5 related to treatment toxicity (6.7%), 9 deaths deemed not related to study treatment, and the cause in 3 was unknown. The 5 fatal AEs were due to respiratory failure (n = 2), pneumonia (n = 1), upper gastrointestinal hemorrhage (n = 1), and cardiac arrest (n = 1).
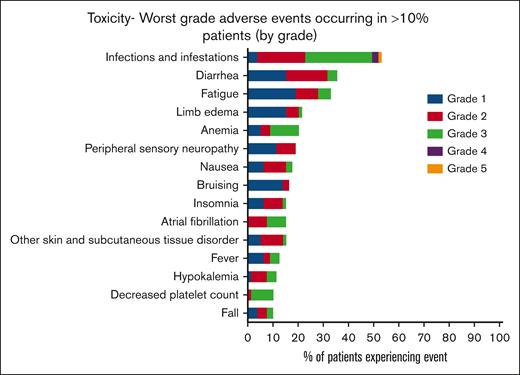
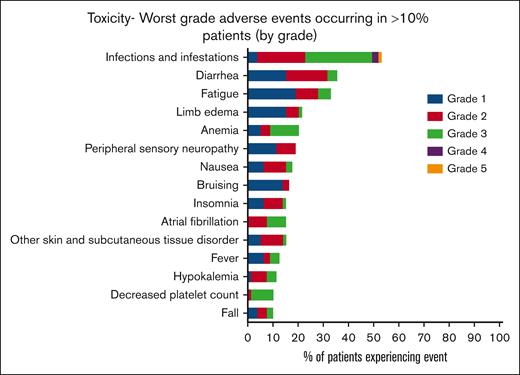
AEs were almost universal in this cohort of patients (78/79 [99%]), with 96% (76/79) experiencing >1 AE. The most common AEs were infections (53.2%), diarrhea, (44.3%) fatigue, (40.5%), and limb edema (29.1%; Figure 3). Hematological AEs included anemia in 17 of 79 patients (21.5%), neutropenia in 6 of 79 (7.6%), febrile neutropenia in 7 of 79 (8.9%), and thrombocytopenia in 9 of 79 (11.4%). Overall, 68% of patients experienced at least 1 grade ≥3 AE, the most common being lung infection (12.7%) and other infection (11.4%; supplemental Table 1). Two-thirds of patients experienced a serious adverse event (SAE) (53/79, 67%), again with infections being the most common. AEs of special interest included atrial fibrillation (AF)/flutter in 14 of 79 patients (17.2%), including 2 of 14 patients with a past or current history of AF/flutter at registration. Other cardiac conduction disorders occurred in 3 of 79 patients (3.8%) with 1 cardiac arrest. Of 79 patients, bleeding occurred in 9 (11.4%), bruising in 15 (19%), hematoma in 7 (8.9%), and arthralgia in 3 (3.8%). Diarrhea occurred in 35 patients (44%); conversely, constipation rates were low (18/79 [23%]). There were only 2 recorded episodes of new or worsening hypertension with hypotension more commonly reported (8/79 [10.1%]).
Clinical prognostic factors
In univariate analyses, baseline Karnofsky performance status, Ann Arbor stage, age-adjusted International Prognostic Index, lactate dehydrogenase, and a history of falls were predictive of both OS and PFS (Table 3). Conversely, baseline albumin level, timed up-and-go walk test, gross cognitive impairment on the CARG, and ECOG performance status score were predictive of neither. When prognostic variables were entered into a multivariate model, none remained significant for either. COO by IHC11 had no significant association with OS, PFS, or disease response (supplemental Figure 2). Similarly COO as determined by GEP in the 47 patients with samples available for testing, had no impact on either OS or PFS; however, there were only low numbers of samples available for testing (supplemental Figure 3).
Quality of life
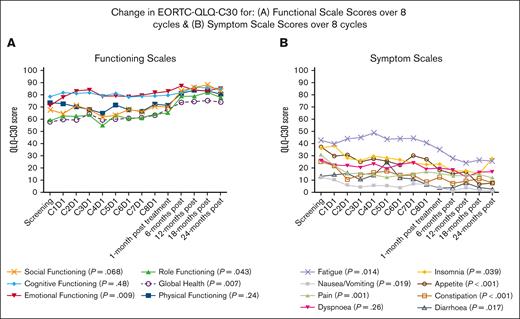
The EORTC QLQ-C30 demonstrated improvement in functional and symptom scales during and after treatment in responders (n = 78 at screening, 57 at day 1 cycle 8, and 29-30 at 24 months after treatment). There was a significant improvement in global health, emotional functioning, and role functioning with no improvement in social, cognitive, or physical functioning (Figure 4, Supplemental Table 2). The symptom scales showed a reduction in fatigue, nausea/vomiting, pain, constipation, diarrhea, appetite loss, and insomnia, with no change in dyspnea (Figure 4, Supplemental Table 3).
On the EQ-5D-5L survey, there was a significant improvement in EQ-5D-5L index and median visual analogue scale thermometer score over time; however, this significance was lost when only comparing the scores of the 26 responding patients at all 3 critical time points (screening, day 1 cycle 8, and 18 months after treatment).
Discussion
This phase 2 study of patients aged ≥75 years with newly diagnosed DLBCL treated with ibrutinib-R-mini-CHOP demonstrated high deliverability of the combination. Although there was a significantly longer 2-year PFS than a prior prospective study of R-mini-CHOP alone6 there was no significant improvement in 2-year OS.
The treatment was deliverable; the median ARTD and ARDI for the entire regimen of ibrutinib-R-mini-CHOP was 97%, and 80% of patients completed all 6 cycles of R-mini-CHOP. This compares favorably with 73.7% of patients aged ≥60 in the PHOENIX study who received 6 cycles of R-CHOP (any ≥1 components) in the ibrutinib arm.10 We were able to maintain the high RDI as demonstrated in the original report of R-mini-CHOP6 despite the addition of ibrutinib.
With a median follow-up of almost 3 years, the 2-year OS was 68% (55.6%-77.4%) with encouraging median OS of 72 months (95% CI, 35 to not reached) in this elderly population. Median 2-year PFS was 60.0% (47.7%-70.3%) with a median PFS of 40 months (95% CI, 20.41 to not reached). The superior PFS compared to with the historic control6 may reflect the slightly lower median age or improved supportive care with prephase prednisone, and routine use of granulocyte colony-stimulating factor in the modern era. However, we are encouraged by the similar deliverability of ibrutinib-R-mini-CHOP as with R-mini-CHOP in the comparable SENIOR study.9 Other studies assessing the addition of other agents such as lenalidomide,9 ofatumumab,7 or obinutuzumab8 have failed to show either tolerability or an advantage over R-mini-CHOP alone and our PFS and OS rates compare favorably with these studies. Similarly, our PFS and OS rates are higher than those reported with R-mini-CHOP in recent real-world data studies.16,17 There were no CNS relapses despite low rates of CNS prophylaxis in our study, which may be because of small sample size or attributed to previously reported data showing that ibrutinib penetrates the blood-brain barrier.18,19
The toxicities were not unexpected in this elderly population with infection the most common. With the exception of infection, anemia, and thrombocytopenia, the majority of AEs were grade 1/2 and reflected the known toxicities of chemotherapy and ibrutinib: diarrhea, fatigue, edema, bruising, and cytopenia. One AE of special interest was the high rate of new AF. At baseline, 15% of patients had a history of AF, rising to 30% during treatment and follow-up, suggesting a potential interaction between ibrutinib and anthracycline use in very elderly patients.
There were comparable SAE infection (27.8% vs 27%) and febrile neutropenia rates (8.9% vs 9%) to a historic R-mini-CHOP cohort,6 despite the addition of ibrutinib. Similarly, our 6.3 % treatment-related mortality (TRM) was comparable with the 8.1% reported with R-mini-CHOP alone.6 This TRM is also comparable with the 5.6% TRM in the R-mini-CHOP arm of the SENIOR study, which had similar safety requirements.9 In the PHOENIX study,10 the challenges of delivery seen with full-dose ibrutinib-RCHOP in patients aged >60 years validated our selection of mini-CHOP in this significantly elderly population.
Geriatric assessment is crucial in any study analyzing treatment in elderly populations; however, in this study, comprehensive geriatric assessment was challenging because of the time-consuming and lengthy questionnaires that were poorly filled out by patients. More sophisticated and simplified geriatric assessment tools such as the simplified frailty score and elderly prognostic index, are now available.20-22 Unfortunately, Instrumental Activities of Daily Living (IADL) and Activities of Daily Living (ADL) scores are not able to be calculated from the CARG questionnaire and so we were unable to evaluate their prognostic impact in this population. This is important because several studies have now shown the prognostic significance of IADLs in elderly patients.20-22 In this study, a self-reported falls history within the last 6 months (42%) was strongly predictive of inferior OS, suggesting that this as an easily obtained maker of frailty worth assessing in future studies. Our quality-of-life improvements are promising especially given the addition of ibrutinib to R-mini-CHOP.
A limitation of this study was its single-arm, nonrandomized design with ambitious target improvements on historical PFS and OS. Notwithstanding this, with a potential PFS advantage compared with the historical control, it is, to our knowledge, the first study to suggest any improvement over R-mini-CHOP alone with the addition of novel agents. Another limitation was the low number of patients with adequate diagnostic tissue to perform GEP. Within the limits of this sample size, there appeared to be a disconnect between IHC and GEP in DLBCL subtyping, with a lower rate of ABC genotype than expected in this elderly population.23 Although acknowledging the putative action of ibrutinib on ABC type disease, the sample size was insufficient to make any meaningful comparisons between ABC and germinal center B-cell–like phenotypes.
In an era of a growing, fitter aging population, with a poorer risk DLBCL biology, there is a pressing need to improve on outcomes of R-mini-CHOP alone. The addition or substitution of novel therapies offer the opportunity to improve efficacy while maintaining an acceptable toxicity profile. With the greater specificity of the second generation or noncovalent BTK inhibitors, potentially with lower rates of gastrointestinal side effects, AF, and bleeding, perhaps pairing a novel BTK inhibitor with R-mini-CHOP may further improve outcomes. There are also other novel agent combinations currently being assessed in elderly DLBCL. These include phase 3 studies of the addition of polatuzumab to R-mini-CHP (ClinicalTrials.gov identifier: NCT04332822), chemotherapy-free regimens (ClinicalTrials.gov identifier: NCT05179733); and phase 3 studies assessing the combination of rituximab and chemotherapy with T-cell–engaging antibodies. We acknowledge further evaluation of IRiC is not proceeding in DLBCL. However, the demonstration of deliverability and a promising PFS result with this combination validates this line of research and supports continued efforts into incorporating novel therapies with R-mini-CHOP in this difficult population with a clear unmet need.
Acknowledgments
This study was supported by research funding from Janssen Cilag Pty Ltd (54179060LYM2002) to the Australasian Leukaemia & Lymphoma Group (ALLG). Statistical analysis for this study was funded by the ALLG.
Authorship
Contribution: E.V. and J.T. designed the research; E.V., J.T., A.J., N.P., E.A.H., H.P.L., T.C., C.Y.C., R.F., D.P., H.S., A.K.E., C.B., N.M., and J.C. recruited patients to the study and analyzed study results; B.E.B. performed the statistical analyses; M.K.G. and K.L. performed the correlative studies; M.W. coordinated the trial data collection; E.V. and J.T. wrote the first version of the manuscript; and all authors read, reviewed, and approved the manuscript.
Conflict-of-interest disclosure: E.V. has received research funding paid to her institution for clinical trials from Janssen, BeiGene, AbbVie, Loxo Oncology, and Roche; and reports a role in an advisory board for BeiGene. J.T. has received research funding paid to her institution for clinical trials from Roche, Bristol Myers Squibb, BeiGene, Cellectar, Pharmacyclics and Janssen. T.C. has received research funding from BeiGene. E.A.H. has received research funding paid to her institution from Bristol Myers Squibb, Merck KgaA, AstraZeneca, Roche, and TG Therapeutics; reports a role in an advisory board for Roche, Antengene, Bristol Myers Squibb, Gilead, AstraZeneca, and Regeneron; and serves on a speakers bureau for Regeneron, Janssen, and AstraZeneca. C.Y.C. consults for, serves on advisory boards of, and receives honoraria from Roche, Janssen, Gilead, AstraZeneca, Eli Lilly, BeiGene, Menarini, Dizal, AbbVie, Genmab, and Bristol Myers Squibb; and reports research funding from Bristol Myers Squibb, Roche, AbbVie, Merck Sharp & Dohme, and Eli Lilly. M.K.G. receives study drug from Janssen for a clinical trial. B.E.B. is an independent statistician who consults to a wide variety of pharmaceutical and device companies. The remaining authors declare no competing financial interests.
A complete list of the members of the Australasian Leukaemia & Lymphoma Group involved in this study appears in “Appendix.”
Correspondence: Emma Verner, Department of Haematology, Concord Repatriation General Hospital, Hospital Rd, Concord, NSW 2040, Australia; email: emma.verner@health.nsw.gov.au.
Members of the Australasian Leukaemia & Lymphoma Group involved in this study but not listed as authors are Douglas Lenton, John Taper, Peter Presgrave, Jason Butler, Peter Mollee, George Kannourakis, and Christopher Steer.
References
Author notes
The study metadata are listed at https://www.allg.org.au/datasets/. Deidentified individual participant data (IPD) are available indefinitely. Proposals to request the trial protocol and deidentified IPD can be made to info@allg.org.au.
The full-text version of this article contains a data supplement.