Posttransplant maintenance with inotuzumab is safe and feasible for patients with ALL who are at high risk of relapse.
The high progression-free survival and low relapse rate for these patients at high risk is encouraging.
Visual Abstract
The curative potential of allogeneic hematopoietic transplantation (allo-HCT) in patients with acute lymphoblastic leukemia (ALL) is hampered by relapse. Inotuzumab ozogamicin (INO) is an anti-CD22 monoclonal antibody bound to calicheamicin, which has significant activity against ALL. We hypothesized that low-dose INO would be safe and feasible after allo-HCT. Therefore, we conducted a phase 1 study to determine the dose and safety in this setting. Patients were eligible if they were aged 16 to 75 years, had undergone allo-HCT for CD22+ ALL, were in complete remission (CR) after allo-HCT, had high risk of recurrence, were between day 40 and 100 after allo-HCT with adequate graft function, and did not have a history of sinusoidal obstruction syndrome (SOS). The objectives of this trial were to define INO maximum tolerated dose (MTD), to determine post–allo-HCT INO safety, and to measure 1-year progression-free survival (PFS). The trial design followed a “3+3” model. The treatment consisted of INO given on day 1 of 28-day cycles. Dose levels were 0.3 mg/m2, 0.4 mg/m2, 0.5 mg/m2, and 0.6 mg/m2. Median age was 44 years (range, 17-66 years; n = 18). Disease status at transplantation was first CR (n = 14) or second CR or beyond (n = 4). Preparative regimen was of reduced intensity in 72% of patients who received transplantation. Most common toxicity was thrombocytopenia. There were no instances of SOS; the MTD was 0.6 mg/m2. One-year nonrelapse mortality was 5.6%. With a median follow-up of 18.1 months (range, 8.6-59 months) 1-year post–allo-HCT PFS and overall survival is 89% and 94%, respectively. Low-dose INO has a favorable safety profile and was associated with high rates of 1-year PFS. This trial was registered at www.clinicaltrials.gov as #NCT03104491.
Introduction
Allogeneic hematopoietic transplantation (allo-HCT) is a curative option for adult and pediatric patients with acute lymphoblastic leukemia (ALL). Leukemia relapse, however, remains the most common cause of treatment failure after transplantation, at rates of 20% to 50%.1,2 After relapse, the chemotherapeutic options for disease control are limited and prognosis is poor, even with second allo-HCT.3-5 However, chimeric antigen receptor T-cell therapy after allo-HCT may result in improved outcomes for certain patients.
Several disease, treatment, and patient characteristics have been associated with high recurrence rates. Measurable residual disease (MRD) before transplantation6 is linked to relapse rates as high as 80%.7-10 Similarly, MRD after allo-HCT is considered a harbinger of ALL relapse.11,12 Patients who received transplantation in second or third remission are also at high risk of ALL relapse within a range of 25% to 50%.13-15 Finally, the use of reduced intensity conditioning (RIC) or nonmyeloablative (NMA) conditioning regimens is generally associated with less toxicity but more relapses.16,17 Preparative regimen intensification, conversely, leads to fewer relapses but higher incidence of nonrelapse mortality (NRM) and major toxicities, including sinusoidal obstruction syndrome (SOS) of the liver.
This intensity conundrum could be addressed by effective post–allo-HCT interventions that would eradicate MRD and/or reduce relapse risk without jeopardizing graft function. Intervening after preparative regimen toxicities have abated also carries the potential to minimize the risk of toxicities. As an example, in Philadelphia chromosome–positive (Ph+) ALL, some studies have shown a benefit to continuing tyrosine kinase inhibitors (TKIs) after allo-HCT.18-21 These studies suggest that post–allo-HCT therapy may be beneficial in controlling or eliminating disease after allo-HCT if started before overt relapse, ideally allowing time for the graft-versus-leukemia effect to occur. Currently, there is no standard post–allo-HCT therapy for patients with ALL other than TKIs in Ph+ ALL to reduce the likelihood of relapse. Thus, the development of novel strategies to reduce recurrence is an important goal.
Inotuzumab ozogamicin (INO) is a CD22-targeted monoclonal antibody bound to calicheamicin, which has been shown to have significant activity against ALL.22,23 INO gained regulatory approval for the treatment of relapsed/refractory ALL based on a randomized phase 3 trial that showed an overall complete remission (CR) rate of 81% in the INO arm as compared with 29% in the standard arm.24 INO has been used for patients with relapsed/refractory ALL as a bridge to allo-HCT. In a publication by Marks et al, patients treated with INO before hematopoietic stem cell transplantation had an overall survival (OS) at 2 year after transplantation of 41% (95% confidence interval, 32-51).25 However, SOS was seen in 19% of these patients who were heavily pretreated. Recently, evidence has emerged of the effectiveness of lower doses of INO combined with chemotherapy.26,27
We hypothesized that low-dose post–allo-HCT INO will be well tolerated and safe after allo-HCT. Herein, we present the results of a phase 1 study designed to test this hypothesis.
Methods
Clinical trial design
This phase 1 clinical trial was conducted at 3 sites: University Hospitals of Cleveland, Cleveland Clinic, and Memorial Sloan Kettering Cancer Center (see supplemental Material). The study was approved by the respective institutional review boards, and all patients provided consent. Patients were enrolled between transplantation day 40 and 100.
Eligibility criteria included patients aged 16 to 75 years and a diagnosis of CD22+ ALL or lymphoid blast crisis of chronic myeloid leukemia that was in CR at day 30 after allo-HCT but were considered as being at high risk of relapse. High risk of relapse was defined as having MRD before or after allo-HCT, having received transplantation in second CR or beyond, or having received RIC or NMA conditioning. Further requirements included adequate graft function after transplantation (platelets >50x10^3/uL and absolute neutrophil count >1x10^3/uL), and absence of active grade 3/4 acute graft-versus-host disease (GVHD) or any active liver GVHD, and no history of SOS. All donor types were allowed. Pretreatment tests included a bone marrow biopsy and aspirate to assess disease state and MRD.
The trial design was a 3+3 model28 with 4 INO dose levels: 0.3 mg/m2, 0.4 mg/m2, 0.5 mg/m2, and 0.6 mg/m2. Administration of INO was on day 1 of each 28-day cycle. The first cycle was initiated between day 40 and 100 after allo-HCT. After enrolling 15 patients, the number of allowed cycles of INO was increased from 4 to 12. Concomitant treatment with TKIs was allowed for patients with ALL that was Ph+.
The first 2 cycles were used to determine dose-limiting toxicities (DLT). Predefined DLT included SOS, prolonged grade >3 cytopenia (>28 days), nonhematologic grade >3 adverse events (graded according to the Common Terminology Criteria for Adverse Events) ascribed to INO, and death. Delays of 7 days and 50% dose reductions were allowed for cytopenia or unresolved symptoms. Patients were followed-up for up to 3 years after their last dose of INO or 1 year after the last enrolled patient completed the final dose of INO, whichever came first.
MRD was assessed at the following time points per protocol: 14 days before administration of the first dose of INO; at 3, 6, and 9 months after allo-HCT; and at 30 days after the end of INO maintenance.
End points and assessments
The primary end point was to determine the maximum tolerated dose (MTD) of INO. Secondary end points included the safety profile of INO, the rate of SOS, NRM, progression-free survival (PFS), and OS at 1-year after allo-HCT, and MRD status after INO. Disease assessment consisted of bone marrow biopsy and aspirate at 3, 6, and 9 months after start of therapy; the end of treatment visit; and 1 year after the last dose of INO. MRD was defined as evidence of disease by flow cytometry, polymerase chain reaction, or clonoSEQ testing (Adaptive Biotechnologies, Seattle, WA). The study protocol is included as a data supplement in the supplemental Material.
Statistical analysis
OS was defined as the time from date of first dose to death due to any cause and was censored at the date of last follow-up for those alive.
PFS was defined as the time from allo-HCT dose to the date of disease relapse (ie, ≥5% blasts in blood or bone marrow, or evidence of extramedullary disease after CR/CR with incomplete count recovery), or death due to any cause, whichever occurs first. OS was defined as the time from allo-HCT to death due to any cause. PFS and OS at 1-year were estimated using the Kaplan-Meier method.29 Time-to-event end points (PFS and OS) were summarized using the Kaplan-Meier method and displayed graphically when appropriate. Difference in time-to-event end points was tested using a 2-sided log-rank test at a significance level of .05.
NRM was defined as the time from date of first dose to death due to any cause without prior relapse, with death from relapse as competing risk, and NRM was censored at the date of last follow-up for those alive. For the analysis of NRM, the cumulative incidence rate was computed using a method by Fine and Gray, adjusting for competing risks.30
Results
Nineteen patients were enrolled between 31 July 2017 and 18 October 2021 (Figure 1). One patient was not treated because they did not meet safety criteria before dosing, and is not included in this report (Table 1). All patients completed follow-up with a median time of 18.1 months (range, 8.6-59 months). Thirteen patients qualified on the basis of undergoing RIC/NMA conditioning, but many patients had overlapping qualifying characteristics. For example, in those that underwent RIC/NMA conditioning, 2 were in second CR and 4 had MRD. Further descriptive information is available in supplemental Table 1.
Patient characteristics
Median age was 44 years (range, 17-66 years), with an equal distribution of men and women (Table 1). Fourteen patients (78%) were in first CR, whereas 3 were in second CR and 1 in third CR at allo-HCT. Nine (50%) patients had adverse cytogenetics and 6 (33%) patient had Ph+ ALL.31 Patients had received a median of 2 lines of therapy before transplantation (range,1-5). Nine patients received blinatumomab before allo-HCT, and 3 patients received INO (dose not available) before allo-HCT. In patients with Ph+ ALL, 4 received dasatinib (in combination with hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone), 3 received ponatinib, and 1 received nilotinib; some patients were sequentially treated with 2 TKIs. MRD was detected before (n = 4), after allo-HCT but before INO maintenance (n = 1), or both before and after transplantation but before INO maintenance (n = 1), as measured by flow cytometry, polymerase chain reaction, or next-generation sequencing. The most common allo-HCT donor type was a matched unrelated donor in 67% and peripheral blood was the most common donor source in 67%. The majority of patients (n = 13; 72%) received RIC/NMA conditioning, and a calcineurin inhibitor combined with methotrexate was the most common GVHD prophylaxis (n = 13; 72%). The median age for those receiving myeloablative conditioning (MAC) was 29 years (range, 17-32 years) and the median age for those receiving RIC was 56 years (range, 27-67 years). The median time to first INO dose was 84 days (range, 47-100 days) after allo-HCT, and the median number of INO doses was 3 (range, 1-9). The median platelet count before the first cycle of INO was 133 × 109/L. The following is a list of the number of patients at each dose level and the number of cycles received: 4 patients received 0.3 mg/m2 (1 patient was replaced because this patient only received 1 dose of INO and 2 doses were required to assess the MTD; the patient was followed-up for safety and outcomes), 2 of whom received 4 cycles, 1 received 3 cycles, and 1 patient 1 cycle; 3 patients received 0.4 mg/m2 doses, of whom 2 received 4 cycles and 1 received 3 cycles; 6 patients received a 0.5 mg/m2 dose (expanded to 6 patients because of 1 DLT), 1 of whom received 4 cycles, 3 received 3 cycles, 1 received 2 cycles, and 2 patient received 1 cycle (because of DLT); and 5 patients received doses of 0.6 mg/m2, 1 of whom received 8 cycles, 3 received 4 cycles, and 1 patient received 2 cycles. The most common reasons for INO discontinuation were patient or physician decision to withdraw (n = 8) and cytopenias (n = 6).
Safety and MTD
There was 1 DLT (prolonged thrombocytopenia) at the 0.5 mg/m2–dose level resulting in a cohort expansion to 6 patients. Subsequently, the study reached the final predefined dose of 0.6 mg/m2. No SOS was observed. The most common toxicity was thrombocytopenia (42% of patients; 32% grade 3/4), neutropenia (32% of patients; 16% grade 3/4), and nausea/vomiting (42% of patients, all grade 1/2; Table 2). There was no statistically significant difference in the number and grade of adverse events between dose levels.
PFS and relapse
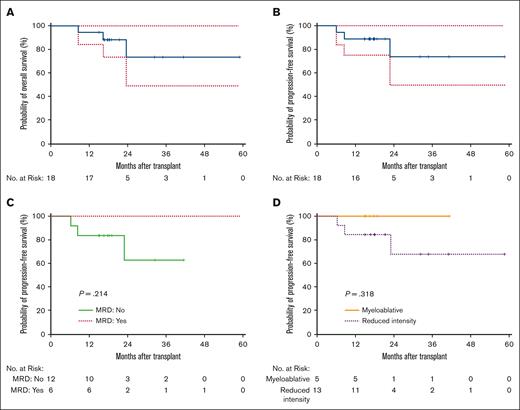
One-year PFS was 89% (Figure 2). For patients with MRD before or after allo-HCT, 1-year PFS was 100%. The 1-year PFS for recipients of RIC (n = 13) and MAC (n = 5) was 83% and 100%, respectively. One patient, in the 0.5 mg/m2 cohort, relapsed 7 months after receiving transplantation. This was the only relapse within 1 year for all patients enrolled in this study. Another patient relapsed from Philadelphia-like ALL, 23 months after allo-HCT.
OS and PFS. (A) OS of the entire cohort. (B) PFS of the entire cohort. (C) PFS of patients who are MRD positive vs those who are MRD negative. (D) PFS of patients who underwent MAC vs RIC or NMA conditioning.
OS and PFS. (A) OS of the entire cohort. (B) PFS of the entire cohort. (C) PFS of patients who are MRD positive vs those who are MRD negative. (D) PFS of patients who underwent MAC vs RIC or NMA conditioning.
MRD after treatment with INO
Six of the patients enrolled had MRD as measured by flow cytometry, polymerase chain reaction, clonoSeq testing, or next-generation sequencing. Of the patients who were MRD positive before transplantation (n = 4), after transplantation (n = 1), and before and after transplantation (n = 1), 4 became negative after treatment with INO. Of the 2 patients who did not become MRD negative after INO maintenance, 1 had Ph+ ALL, remained on a concomitant TKI, and was in remission at the time of last follow-up; the other patient had an unknown MRD status after completion of INO but remained alive and in remission at the time of last follow-up.
OS and NRM
One-year OS was 94% (Figure 1). One-year NRM was 5.6%. For patients with MRD before or after allo-HCT, 1-year OS was 100% whereas for those patients who were MRD negative, the 1-year OS was 90%. For patients who underwent MAC, 1-year OS was 100%, whereas 1-year OS was 92% for recipients of RIC or NMA conditioning transplantation.
Acute and chronic GVHD
Acute GVHD was diagnosed in 13 patients (72.2%), of which 5 were grade 1, 7 grade 2, and 1 grade 4. One patient died of acute GVHD on the 0.6 mg/m2 dose level, accounting for the only NRM within the first year after allo-HCT. New acute or chronic GVHD, occurring after the start of INO, was observed in 8 patients (44.4%). The majority of those diagnoses were mild chronic GVHD (5 of 8 patients). Chronic severe GVHD occurred in 1 patient after INO maintenance was completed.
Discussion
We demonstrate that post–allo-HCT INO is feasible, and its use did not lead to excess toxicity, with no observed SOS or graft failure. The MTD studied in this setting is 0.6 mg/m2. Thrombocytopenia is common and should be carefully monitored, along with other blood counts.
Lower doses of INO have been investigated in relapsed and refractory ALL, in an effort to maintain efficacy and reduce toxicity. Jabbour et al investigated a fractionated induction INO dose of 0.9 mg/m2 and a fractionated consolidation dose of 0.6 mg/m2 in combination with mini hyperfractionated cyclophosphamide, vincristine, and dexamethasone, with or without blinatumomab.27 The complete response rate was 57% and the SOS rate was 3%, which compares favorably with studies of standard dose INO in which the rate of SOS is on the order of 14% to 23%.24,25,32,33 These data suggest that there is a minimal effective dose of INO, which is significantly less toxic, when compared with standard dose INO. Similarly, lower doses of INO have been used to eliminate MRD in patients with Ph+ or Ph− ALL who did not achieve MRD negativity or had MRD-positive relapse after frontline or salvage therapy, including allo-HCT.34
Previous attempts at post–allo-HCT maintenance therapy for patients with ALL have had variable degrees of effectiveness and toxicity, leading to no change in standard practice. In Ph+ ALL, small studies have shown a benefit to continuing TKIs after allo-HCT.18-21 For example, imatinib can be given when neutrophil and platelet engraftment have been demonstrated, with minimal side effects. Currently the National Comprehensive Cancer Network guidelines suggest that practitioners consider post–allo-HCT TKI maintenance for at least 1 year with periodic monitoring for MRD for patients with Ph+ ALL.31,35
Cellular therapy after allo-HCT has focused on donor lymphocyte infusions (DLI). However, DLI has variable effectivity, and acute and chronic GVHD are serious risks.36 A retrospective analysis of DLIs in patients with acute leukemia (n = 318; for patients who are MRD positive after allo-HCT and those with mixed chimerism, or as prophylaxis for patients at high risk) demonstrated an acute or chronic GVHD rate of 40% with 6% of the patients dying from GVHD complications. The leukemia-free survival at 5 years was 58%, with a cumulative incidence of relapse of 29%.37 Additionally, the feasibility of DLIs is limited by a number of factors including donor availability. In a prospective trial of prophylactic low-dose DLI for 108 patients with high-risk leukemia, only 44 were able to receive DLIs.38 In addition, combinations of DLI and chemotherapy have yielded poor results in the treatment of relapsed ALL after allo-HCT.39
Regimen intensity is known to affect the rate of relapse in patients with acute leukemia.40 For patients treated with RIC, retrospective data suggest a 1-year ALL relapse rate of 30% to 50%.41 Additionally, MRD before or after transplantation is associated with even greater relapse.9,10,42 Thus, in this trial, the low relapse rate for patients undergoing either NMA conditioning/RIC or those with MRD before or after allo-HCT is encouraging. The change of 4 patients from being MRD positive to being MRD negative after allo-HCT supports the hypothesis that low-dose post–allo-HCT INO can eliminate MRD. Furthermore, although this cohort was enriched for patients with high HCT-specific comorbidity index, the low 1-year NRM suggests that this maintenance is safe and tolerable.
It is important to note that this phase 1 cohort mostly comprised recipients of RIC/NMA conditioning regimens, with regimens that did not contain busulfan, and it is unclear how the doses and schedules proposed here would apply to patients receiving myeloablative doses of busulfan. We recognize that the population of this study is heterogeneous and that the efficacy, in terms of PFS, may be overinterpreted. Given this heterogeneity, we have included a supplemental Table 1 that details single patient characteristics, follow-up, and outcomes.
There is an important question that this study does not answer: what is the maximum or minimum number of INO cycles that can safely and feasibly be given after HCT? It is not clear a priori what number of cycles of INO would be safe and effective in reducing relapse after HCT and eradicating MRD. To answer this question, we will need to understand the interplay between the pharmacokinetics of INO after HCT, INO cycle number, PFS, and the rate of MRD eradication. We hope to obtain the answer to this question in a phase 2 trial.
In conclusion, the low observed relapse rate and favorable safety profile justify investigating low-dose INO as maintenance therapy after allo-HCT in a phase 2 trial (www.clinicaltrials.gov identifier: #NCT03104491) focused on PFS and MRD eradication.
Acknowledgments
This research was supported, in part, by Pfizer and Case Comprehensive Cancer support grant P30 CA043703, the National Institutes of Health (award number P01 CA23766), and National Institutes of Health/National Cancer Institute Cancer Center support grant P30 CA008748 (C.C., S.G., and M.A.P.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: L.L.M. designed and performed research, analyzed data, and wrote the manuscript; R.S. and C.C. performed research and was a site principal investigator; P.F. and J.W. collected and analyzed data; S.M. analyzed data; L.C., S.K., and R.D.C. collected data; N.M., P.F.C., F.O., B.W.C., M.G., E.M., B.T., A.T.G., M.-A.P., and B.H. performed the research; S.G. performed and designed the research; and M.d.L. designed and performed research, and wrote the manuscript.
Conflict-of-interest disclosure: L.L.M. serves on the speaker’s bureau of Incyte and served on an advisory board for Pfizer. M.d.L. served on an advisory board for Pfizer. M-A.P. reports honoraria from Adicet, Allogene, Allovir, Caribou Biosciences, Celgene, Bristol Myers Squibb, Equillium, ExeVir, ImmPACT Bio, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, OrcaBio, Sanofi, Syncopation, VectivBio AG, and Vor Biopharma. He serves on the data safety monitoring board for Cidara Therapeutics and Sellas Life Sciences and the scientific advisory board for NexImmune. He has ownership interests in NexImmune, Omeros, and OrcaBio. He has received institutional research support for clinical trials from Allogene, Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis. The remaining authors declare no competing financial interests.
Correspondence: Leland L. Metheny, Hematology and Cell Therapy Division, Seidman Cancer Center, University Hospitals, 11100 Euclid Ave, Cleveland, OH 44106; email: Leland.metheny@uhhospitals.org.
References
Author notes
Presented in abstract form at the 64th annual meeting of the American Society of Hematology, New Orleans, LA, 10 to 13 December 2022.
Deidentified individual participant data that underlie the manuscript results will be made available 3 months after publication for a period of 5 years after the publication date. Proposals for access should be sent to the corresponding author, Leland L. Metheny (leland.metheny@uhhospitals.org).
The full-text version of this article contains a data supplement.