Primary testicular lymphoma is characterized by high risk of contralateral testis and central nervous system relapse in historical series.
Combined intravenous and intrathecal prophylaxis is feasible and effective in preventing central nervous system relapses, improving outcome.
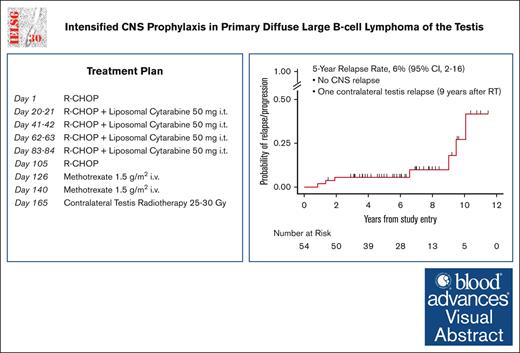
Visual Abstract
Primary testicular diffuse large B-cell lymphoma (PTL) is characterized by high risk of contralateral testis and central nervous system (CNS) relapse. Chemoimmunotherapy with intrathecal (IT) CNS prophylaxis and contralateral testis irradiation eliminates contralateral recurrences and reduces CNS relapses. The IELSG30 phase 2 study investigated feasibility and activity of an intensified IT and IV CNS prophylaxis. Patients with stage I/II PTL who had not received treatment received 2 cycles of IV high-dose methotrexate (MTX) (1.5 g/m2) after 6 cycles of the R-CHOP regimen (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone, every 21 days). IT liposomal cytarabine was administered on day 0 of cycles 2 to 5 of 21-day R-CHOP regimen. Contralateral testis radiotherapy (25-30 Gy) was recommended. Fifty-four patients (median age: 66 years) with stage I (n = 32) or II (n = 22) disease were treated with R-CHOP, 53 received at least 3 doses of IT cytarabine, 48 received at least 1 dose of IV MTX, and 50 received prophylactic radiotherapy. No unexpected toxicity occurred. At a median follow-up of 6 years, there was no CNS relapse; 7 patients progressed, and 8 died, with 5-year progression-free and overall survival rates of 91% (95% confidence interval [CI], 79-96) and 92% (95% CI, 81-97), respectively. Extranodal recurrence was documented in 6 patients (in 2 without nodal involvement). In 4 cases, the relapse occurred >6 years after treatment. Causes of death were lymphoma (n = 4), second primary malignancy (n = 1), cerebral vasculopathy (n = 1), unknown (n = 2). Intensive prophylaxis was feasible and effective in preventing CNS relapses. Late relapses, mainly at extranodal sites, represented the most relevant pattern of failure. This trial was registered at www.clinicaltrials.gov as #NCT00945724.
Introduction
Diffuse large B-cell lymphoma (DLBCL) with primary testis localization is a rare entity, accounting for <2% of all non-Hodgkin lymphoma, however, it represents the most frequent testicular neoplasm in older patients.1 Primary testicular DLBCL (PTL) belongs to the activated B cell–like lymphoma subtype of DLBCL,2,3 characterized by the constitutive activation of nuclear factor κB (NF-κB) signaling, which may result from somatically acquired mutations in MYD88.4,5 It is recognized as a unique condition characterized in historical series by high long-term risk of contralateral testis and central nervous system (CNS) relapse, despite most patients having a low CNS International Prognostic Index score.1,6-8 Peculiar molecular features have been related to its preferential manifestation in these immune-privileged sites.9,10
Retrospective series provided some evidence of the benefit of prophylactic radiotherapy (RT) of the contralateral testis in reducing the risk of relapse at this site.3 CNS relapses involving both the meningeal and parenchymal compartment represent a major challenge in clinical management of this entity.1,6,7 The association of rituximab to the CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy regimen improved survival3 but did not reduce the risk of CNS relapse.3,11 In a large retrospective survey investigating the impact of single-route CNS prophylaxis, in the subset of cases with testis involvement (n = 69), intrathecal (IT) prophylaxis was administered to 44 patients and IV high-dose methotrexate (HD-MTX) to 25. The CNS relapse occurred in 11.6% of cases.8 The IELSG10 phase 2 trial coordinated by the International Extranodal Lymphoma Study Group (IELSG), demonstrated a significant outcome improvement of stage I/II PTL with the combination of R-CHOP, prophylactic IT chemotherapy, and contralateral testis irradiation.12 However, despite a significant reduction of their frequency, CNS relapses in the IELSG10 study continued to occur, with a detrimental impact on outcome.
Here, we report the results of the subsequent IELSG30 phase 2 trial, designed to assess whether an intensified CNS prophylaxis program including IV HD-MTX together with IT liposomal cytarabine can further improve PTL outcomes.
Methods
Study design
The IELSG30 trial was a multicenter phase 2 prospective study aimed at evaluating the progression-free survival (PFS) of 6 cycles of the standard 21-day R-CHOP (CHOP21) regimen; 4 doses of IT liposomal cytarabine; 2 cycles of IV HD-MTX; and prophylactic RT to the contralateral testis. The study was designed by the IELSG and conducted in collaboration with the Fondazione Italiana Linfomi (FIL).
The study protocol was approved by the ethical review committees of all participating institutions and was carried out in accordance with the principles of the Declaration of Helsinki. All patients provided written informed consent.
Patients
The trial enrolled patients with previously untreated PTL aged ≥18 years and Ann Arbor stage IE and IIE at presentation, including bilateral testis involvement, which was considered stage IE or IIE according to the extent of regional node involvement. Mandatory orchiectomy had to be performed within 2 months before study entry. Other eligibility conditions were Eastern Cooperative Oncology Group performance status score of ≤2 and normal kidney, liver, and heart function. Patients with CNS involvement were excluded from the study. Measurable or evaluable disease was required, but patients who have had all disease removed by surgery were eligible. The study protocol with the complete inclusion and exclusion criteria is available upon request.
Treatment plan
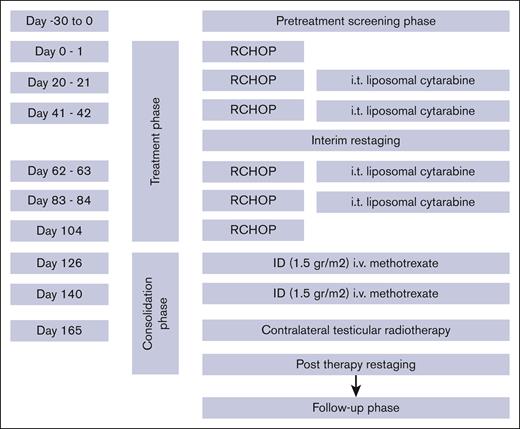
All patients had diagnostic orchiectomy. The treatment consisted of R-CHOP21, which included rituximab (375 mg/m2 per day IV) on day 0 or day 1, cyclophosphamide (750 mg/m2 per day IV) on day 1, doxorubicin (50 mg/m2 day IV) on day 1, vincristine (1.4 mg/m2 per day IV, maximum 2 mg) on day 1, and prednisone (40 mg/m2 per day, orally) on day 1 to 5. The cycles were repeated every 21 days. All patients were restaged after 3 cycles of R-CHOP21. Patients with stage I and II disease received a total of 6 cycles of R-CHOP21. All patients were planned to receive CNS prophylaxis with 4 doses of IT liposomal cytarabine (50 mg) given on day 0 of cycles 2, 3, 4, and 5 of R-CHOP21 and 2 cycles of IV HD-MTX (1.5 g/m2) given at the end of the chemoimmunotherapy program. Prophylactic irradiation to the contralateral testis (recommended dose of 25-30 Gy) was planned in all patients (Figure 1).
Baseline and response evaluation
Baseline assessment included full laboratory workup; hepatitis B virus, hepatitis C virus, and HIV serology; lumbar puncture with cytologic and cytometric examination of the cerebrospinal fluid; bone marrow biopsy; testicular ultrasound; chest, abdomen, and pelvis computed tomography (CT) scans. CT of the head and neck were planned at the discretion of the treating physician. 18F-fluorodeoxyglucose positron emission tomography/CT (PET/CT) was recommended but not mandatory.
Intermediate response was assessed after the third course of R-CHOP21 with CT scan and testicular ultrasound. PET/CT was advisable, if previously positive, according to local policy, but it was not mandatory in this phase.
Patients were restaged after the immunochemotherapy program with CT scan and testicular ultrasound. End-of treatment PET/CT scan (to be performed 3 months after RT) was mandatory. Final response was assessed according to the 2007 Cheson criteria.13
Sample size estimation and statistical methods
At the time the IELSG30 study was designed, the previous IELSG10 trial was still ongoing and no sound information on long-term PFS was available for sample size estimation. Hence, the study began with a planned inclusion of 35 patients in 4 years, to be followed by a calculation of a final sample size that could identify a clinically relevant PFS improvement of 15% (primary end point). Once mature results of the IELSG10 were available showing a PFS of 67% at 7 years,12 the final sample size of 54 patients was subsequently defined to allow the detection, in a single arm study, of a 15% PFS increase (from 67% to 82%), with 80% power and α at .05 (1-sided).
Time-related end points were defined according to the revised National Cancer Institute criteria; PFS was calculated from the date of diagnosis (date of orchiectomy) to relapse/progression or death as a result of any cause or to the date of last follow-up visit for event-free patients; overall survival (OS) was calculated from the date of diagnosis to death as a result of any cause or to the date of last follow-up visit.13 The median follow-up was computed by the reverse Kaplan-Meier method. Survival probabilities were calculated using the life-table method, survival curves were estimated by the Kaplan-Meier method. Binomial exact 95% confidence intervals (CIs) were calculated for percentages. P ≤ .05 (2-sided test) were considered to indicate statistical significance. Statistical analysis was conducted using the STATA/SE 11.2 statistical software (StataCorp, College Station, TX).
Results
Patient characteristics
From October 2009 to July 2017, 54 consecutive patients were enrolled (Table 1). The median age was 66 years (range, 37-79 years), with 31% patients aged >70 years; 32 patients had stage I and 22 had stage II disease. All but 3 patients, qualified with stage I disease in 1 case and stage II in 2 additional cases, had a baseline PET/CT. DLBCL histology was confirmed by expert pathologist review in all patients.
One patient with stage II disease had bilateral testicular involvement at diagnosis. Lactate dehydrogenase levels above normal values were observed in 7 patients.
Feasibility
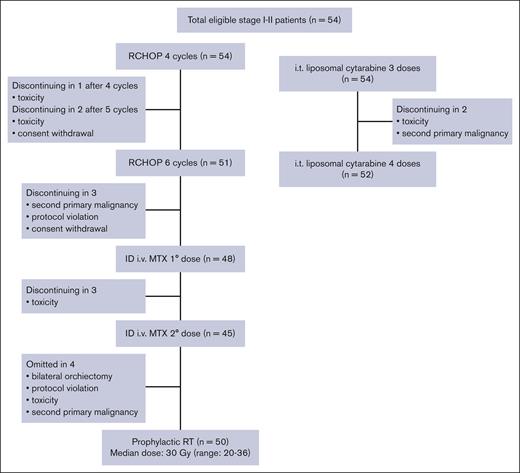
Of the 54 patients, 51 (94%) completed R-CHOP21 as planned; 2 patients interrupted treatment because of toxicity after 4 and 5 cycles, respectively; and 1 patient withdrew his consent to treatment after 5 cycles (Figure 2). Three patients did not receive any IV dose of MTX because of the diagnosis of second primary malignancy, protocol violation, and consent withdrawal to treatment in 1 patient each. A total of 45 patients received both doses of IV MTX; 3 patients interrupted the treatment after the first dose because of toxicity.
IT CNS prophylaxis was completed as planned in 52 patients (96%); in 2 patients the treatment was interrupted after 3 doses because of the diagnosis of second primary malignancy and toxicity.
Because of transient liposomal cytarabine shortage occurred during study, IT cytarabine hydrochloride was delivered in 5 patients: 1 had a single dose, 2 had 2 doses, and 2 had 3 doses.
In 4 patients RT was omitted: in 1 because of bilateral orchiectomy at diagnosis, in 1 case to treatment interruption because of prior toxicity, in 1 case to the diagnosis of second primary malignancy, and in 1 patient to protocol violation. Fifty patients received RT to the contralateral testis, with median delivered dose of 30 Gy (range, 20-36 Gy). One patient with stage I and 5 patients with stage II disease had retroperitoneal plus contralateral testis RT (Figure 2).
Toxicity and safety
The R-CHOP21 treatment was well tolerated with no unexpected adverse events. Two patients discontinued treatment after 4 and 5 cycles, respectively, because of toxicity (in both cases febrile neutropenia complicated by pneumonia). The adverse events induced by IT prophylaxis were mild and occurred at low frequency (only 1 patient discontinued treatment after 3 doses because of systemic toxicity related to R-CHOP21). IV HD-MTX was feasible, with only 3 patients discontinuing treatment after the first dose because of adverse events (intracranial hemorrhage, acute kidney failure, and grade 3 mucositis, in 1 patient each; Table 2).
Response and outcome
In all but 4 patients an interim evaluation of disease response was performed after 3 cycles of R-CHOP21 therapy (29 patients with stage I disease and 21 patients with stage II disease). An interim PET/CT was performed in a total of 17 patients (5 patients with stage I and 12 patients with stage II disease). Among these 50 patients evaluated for response, 29 patients had resected stage I disease, and persistent complete remission, whereas a partial remission was reported in 6 of 21 evaluable patients with stage II disease, confirmed by a PET/CT scan in 4 of them.
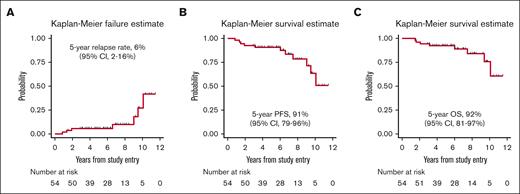
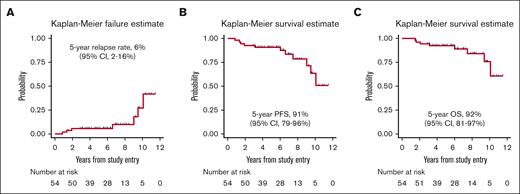
At the end of the treatment, 53 patients (98%) were in CR (confirmed by a PET/CT scan in all but t3 cases), and only 1 experienced progressive disease at nodal and extranodal sites and died 9 months later. At a median follow-up of 6.1 years, 7 patients experienced relapse (1 with stage I disease, and 6 with stage II disease at diagnosis, P = .009). One patient had nodal relapse, and 2 patients relapsed only at extranodal sites (skin and adrenal gland). Both nodal and extranodal relapses were reported in 4 cases, the latter including gastrointestinal tract and pleurae in the same patient, as well as the kidney, the spinal canal (extra-axial), and the contralateral testis (9 years after RT) 1 patient each. Notably, 4 of 7 relapses occurred after 6 to 10 years from diagnosis. No CNS relapse was reported. The 5-year cumulative incidence of progression was 6% (95% CI, 2-16; Figure 3A). Four of these patients died of lymphoma after 0.2 to 9 months after disease relapse, and 3 were alive after 2.5 to 20 months.
Disease outcome. (A) Kaplan-Meier estimates of cumulative incidence of relapse; (B) PFS; and (C) OS.
Disease outcome. (A) Kaplan-Meier estimates of cumulative incidence of relapse; (B) PFS; and (C) OS.
With a median follow-up of 6.1 years, the 5-year PFS was 91% (95% CI, 79-96; Figure 3B) and the 5-year OS rate was 92% (95% CI, 81-97; Figure 3C). Eight patients died: 4 of progressive disease, 1 of second primary malignancy, 1 of cerebral vasculopathy, and 2 due to unknown cause. Stage II disease had a significantly inferior PFS (5-year PFS: 81.8% [95% CI, 58.5-92.8] vs 96.4% [95% CI, 77.2-99.5], P = .0281). Moreover, a trend toward an inferior OS was observed in stage II disease (5-year OS: 85.9% [95% CI, 62.4-95.2] vs 96.4% [95% CI, 77.2 to 99.5], P = .0520).
Discussion
The IELSG30 prospective trial demonstrates that the administration of IV HD-MTX in combination with IT liposomal cytarabine and the R-CHOP21 program is feasible and effective in a patient population with very high risk of CNS relapse and advanced age at diagnosis in many cases. Ninety-six percent of the enrolled patients received all the planned IT treatment, and 83% of them completed the program of systemic prophylaxis; >90% are alive and/or progression-free at 5 years. The major limitation of the study is the single-arm design. However, in a rare disease such as PTL, implementing a randomized phase 3 trial was deemed unrealistic.
These very promising outcomes should be properly discussed in the context of the prior IELSG studies.
More than a decade ago, the IELSG10 trial demonstrated that systemic treatment of localized PTL with R-CHOP, combined with intrathecal prophylaxis and contralateral testicular radiotherapy and nodal radiotherapy in stage II, led to a dramatic improvement in the very dismal prognosis (5-year OS of 54% for patients with stage I/II disease) described in the earlier, large IELSG5 survey.1 The 5-year PFS and OS rates in the IELSG10 trial were 74% and 85%, respectively. The 5-year cumulative incidence of CNS relapse was 6%12 whereas in the IELSG5 survey, the CNS recurrence rate was 13% in patients with localized stage.1
Comparing the IELSG10 and IELSG30 studies requires caution because of several potential influencing factors, including the relatively low number of patients in each study, slight differences in patient populations, changes in the study timeframe, and the possibility of increased expertise among investigators, along with improved supportive therapies in recent years. Despite these limitations, it is important to note that the 5-year survival rates in the IELSG30 trial appear more favorable, and in this study with intensified CNS prophylaxis no CNS relapses were observed.
CNS prophylaxis with IT and/or IV administration of blood-brain barrier–crossing agents is widely used in patients with DLBCL considered at high risk of CNS dissemination.14,15 However, no our knowledge, no randomized study has been performed, and the evidence supporting this practice is questionable.16
Retrospective analysis suggested that HD-MTX may represent an effective CNS prophylaxis in DLBCL.17-19 More recent surveys, however, reported discordant results, with many studies showing little or no benefit.8,20-22
PTL represents a subgroup of DLBCLs with a known high risk of CNS recurrence, for which there is definite consensus on CNS prophylaxis, which is considered mandatory. The aforementioned IELSG10 prospective study provided evidence for the use of IT prophylaxis. However, IV (but not IT) prophylaxis improved survival without reducing the risk of CNS relapse in a retrospective series of 192 patients with PTL treated with curative anthracycline-based chemotherapy, with or without rituximab.23
The value of prophylactic IT chemotherapy in DLBCL is indeed controversial, and leptomeningeal relapse is also reported in patients who have received IT chemotherapy.23-25 In the IELSG10 trial the CNS prophylaxis comprised 4 doses of IT MTX, and CNS relapses were observed in 3 of 53 patients (2 meningeal and 1 parenchymal).12
Based on these open issues and the aim to reducing the CNS recurrence, we added, in this trial, IV MTX at the end of R-CHOP. The efficacy of IV MTX depends on drug dose: although standard dose MTX does not cross the blood-brain barrier, doses at 1 to 3 g/m2 result in tumoricidal levels in the brain parenchyma, and doses of >3 g/m2 yield tumoricidal levels in the cerebrospinal fluid. Higher doses of MTX increase the risk of significant renal toxicity and mucositis, which can affect clinical outcomes.26,27 In our predominantly elderly patient population, as is typical for PTLs, we opted for a total of 2 cycles of MTX at a dose of 1.5 g/m2. We considered this approach safe and well tolerated, while also maintaining a good balance between risk and benefit.
At this dose the concentration of MTX in the leptomeningeal compartment is relatively low,28 thus, the contribution of the concomitant IT prophylaxis to the outcome may be relevant. Moreover, in comparison with the standard IT MTX used in the prior IELSG10 study, the use of the liposomal formulation may have ensured higher and more prolonged cytarabine concentrations in the cerebrospinal fluid.
Indeed, controlled clinical trials have shown that liposomal cytarabine have a better clinical profile than the classical treatments of neoplastic meningitis29 with, in lymphomatous meningitis, superior response rates, improved quality of life, and a prolongation of the time to neurological progression.30 More recent literature also provided some evidence of its safety and activity in different prophylaxis settings.31-33
Although liposomal cytarabine may optimize the IT chemotherapy, the compound was permanently withdrawn from the market because of persistent manufacturing process problems. This withdrawal has made it challenging to further elucidate its role in controlling disease spread into the CNS, as suggested by our results. Nevertheless, the IELSG30 study also indicates potential benefit of IV HD-MTX in a patient population at very high risk of CNS recurrence. Notably, the administration IV HD-MTX was planned at the end of chemoimmunotherapy to prevent an adverse impact of MTX toxicity on the regular and complete delivery of the R-CHOP program. Our results are in line, in terms of safety and tolerability, with recent data from an international survey demonstrating that the administration of HD-MTX during R-CHOP program significantly increases the risk of R-CHOP delay and that the administration of HD-MTX at the end of treatment with R-CHOP is not inferior to early administration with regards to CNS relapse risk.34 The single-arm study design does not allow to define the relative role of the IT and IV components of our prophylaxis program in determining the treatment outcome. However, the experience gained in this study suggests that the administration of IV HD-MTX at the dose of ≥1.5 g/m2 per 2 courses, in combination with IT chemotherapy, may reduce the risk of CNS relapse, or that the addition of IV HD-MTX may have contributed to an overall improved outcome with a more effective systemic treatment. Only a randomized trial could draw definitive conclusion in this setting, but this is not feasible in a rare disease as PTL. Finally, this trial confirms our previous observations1,12 and provides further evidence of the efficacy of RT to prevent the risk of contralateral relapse; moreover, the lack of isolated retroperitoneal relapses in patients with stage II disease suggests that irradiation of retroperitoneal lymph nodes may be omitted in stage II disease without impairing the outcome.
Late relapses, mainly involving extranodal sites, still represent a clinical challenge in the management of PTL. Hopefully, a better knowledge of the molecular pathogenesis of the disease will help refine the optimal therapeutical strategy.35-37
Acknowledgments
The authors are indebted to the study participants and their families for their commitment. The authors thank all the clinical investigators and research nurses. The authors appreciate the excellent assistance of the study coordinators at each study center as well as the administrative support in data collection and study conduction from the clinical project manager and central study team at the International Extranodal Lymphoma Study Group (IELSG) coordinating center (Bellinzona, Switzerland) and at the Fondazione Italiana Linfomi coordination centers (Alessandria and Modena, Italy). The authors also express gratitude to Rita Gianascio Gianocca for the excellent secretarial assistance.
The IELSG is supported by the Swiss Cancer Research Foundation and the Swiss Cancer League. The IELSG30 academic trial was sponsored by the IELSG and was funded, in part, by an unrestricted research grant from Mundipharma.
The funders had no role in the study design, data collection, analysis, interpretation, or writing of this study.
Authorship
Contribution: U.V. and E.Z. designed the trial and wrote the study protocol; A. Conconi and A. Chiappella analyzed the data and wrote the manuscript; A. Conconi, A. Chiappella, U.V., and E.Z. accessed and verified the trial data; L.B. and M.C. coordinated regulatory activities, and collection, assembly, and management of the data; the remaining authors registered and treated patients or provided follow-up data; and all authors provided critical review of the manuscript and approved the definitive version and its submission.
Conflict-of-interest disclosure: A. Conconi received speaker fees from Roche, AbbVie, Incyte, Takeda, and AstraZeneca, and was a member of advisory boards of Regeneron. A. Chiappella reports honoraria for lectures/educational activities from AstraZeneca, Bristol Myers Squibb/Celgene, Gilead-Sciences, Incyte, Janssen, Novartis, Roche, and Takeda, and was a member of advisory boards of Bristol Myers Squibb/Celgene, Gilead-Sciences, Ideogen, Janssen, Roche, Secura Bio, and Takeda. A.J.M.F. received speaker fees from Gilead and Roche; was a member of advisory boards of Gilead, Juno, Novartis, PletixaPharm, AstraZeneca, Bristol Myers Squibb, and Roche; currently receives research grants from ADC Therapeutics, Bayer HealthCare Pharmaceuticals, BeiGene, Bristol Myers Squibb, Genmab, Gilead, Hutchison Medipharma, Incyte, Janssen Research & Development, MEI Pharma, Novartis, PletixaPharm, Pharmacyclics, Protherics, Roche, and Takeda; and holds patents on NGR-hTNF in brain tumors and NGR-hTNF/R-CHOP in relapsed or refractory primary central nervous system lymphomas and SNGR-hTNF in brain tumors. A.S. reports research funding from AbbVie, ADC Therapeutics, Amgen, AstraZeneca, Bayer, Cellestia, Incyte, Loxo Oncology, Merck MSD, Novartis, Pfizer, Philogen, and Roche; consultancy honoraria from Roche, Janssen, Gilead, Bristol Myers Squibb/Celgene, and BeiGene; expert testimony for Bayer and Eli Lilly; and travel support from AstraZeneca and Incyte. G.G. is a member of advisory boards of AbbVie, AstraZeneca, BeiGene, Incyte, Janssen, and Roche, and is a member of the speaker’s bureau of Janssen. F.M. was a member of Takeda, Roche, Janssen, Gilead, MSD, Incyte, and Novartis, and reports that some travel expenses were covered by Takeda, Janssen, and Novartis. M.C.P. received a travel grant from BeiGene. U.V. is a member of advisory boards of Genmab, Incyte, AbbVie, and Gilead; lectures for Janssen, AbbVie, Incyte, and Roche; and was a research support for MorphoSys. E.Z. was a member of advisory boards of BeiGene, Bristol Myers Squibb, Curis, Eli/Lilly, Incyte, Janssen, Merck, Miltenyi Biomedicine, and Roche; received research support form AstraZeneca, BeiGene, Bristol Myers Squibb/Celgene, Incyte, Janssen, and Roche; and received travel grants from BeiGene, Janssen, Gilead, and Roche. The remaining authors declare no competing financial interests.
The current affiliation for F.E. is Hematology, Department of Biomedicine and Prevention, University Tor Vergata, Rome, Italy.
Correspondence: Annarita Conconi, Hematology Division, Ospedale degli Infermi di Biella, via dei Ponderanesi, 2, Ponderano, 13875 Biella, Italy; email: annarita.conconi@aslbi.piemonte.it.
References
Author notes
A. Conconi and A. Chiappella contributed equally to this study.
U.V. and E.Z. are joint senior authors.
The clinical trial data set is not publicly available because of legal restrictions. Deidentified data sharing could be possible on a reasonable request addressed to the International Extranodal Lymphoma Study Group (ielsg@ior.usi.ch). The clinical trial (NCT00945724/EudraCT 2009-011789-26) protocol will be made available upon request to the International Extranodal Lymphoma Study Group (ielsg@ior.usi.ch).