Parsaclisib, a PI3Kδ inhibitor, reduced spleen volume and improved symptom scores when added to ruxolitinib for patients with myelofibrosis.
The safety and tolerability of the combination was acceptable, and daily parsaclisib dosing may provide the greatest benefit.
Visual Abstract
Ruxolitinib reduces spleen volume, improves symptoms, and increases survival in patients with intermediate- or high-risk myelofibrosis. However, suboptimal response may occur, potentially because of signaling via the phosphoinositide 3-kinase (PI3K)/protein kinase B pathway. This phase 2 study evaluated dosing, efficacy, and safety of add-on PI3Kδ inhibitor parsaclisib for patients with primary or secondary myelofibrosis with suboptimal response to ruxolitinib. Eligible patients remained on a stable ruxolitinib dose and received add-on parsaclisib 10 or 20 mg, once daily for 8 weeks, and once weekly thereafter (daily-to-weekly dosing; n = 32); or parsaclisib 5 or 20 mg, once daily for 8 weeks, then 5 mg once daily thereafter (all-daily dosing; n = 42). Proportion of patients achieving a ≥10% decrease in spleen volume at 12 weeks was 28% for daily-to-weekly dosing and 59.5% for all-daily dosing. Proportions of patients achieving ≥50% decrease at week 12 in Myelofibrosis Symptom Assessment Form and Myeloproliferative Neoplasms Symptom Assessment Form symptom scores were 14% and 18% for daily-to-weekly dosing, and 28% and 32% for all-daily dosing, respectively. Most common nonhematologic treatment-emergent adverse events were nausea (23%), diarrhea (22%), abdominal pain and fatigue (each 19%), and cough and dyspnea (each 18%). New-onset grade 3 and 4 thrombocytopenia were observed in 19% of patients, each dosed daily-to-weekly, and in 26% and 7% of patients dosed all-daily, respectively, managed with dose interruptions. Hemoglobin levels remained steady. The addition of parsaclisib to stable-dose ruxolitinib can reduce splenomegaly and improve symptoms, with manageable toxicity in patients with myelofibrosis with suboptimal response to ruxolitinib. This trial was registered at www.clinicaltrials.gov as #NCT02718300.
Introduction
Myelofibrosis is a Philadelphia chromosome–negative myeloproliferative neoplasm (MPN) characterized by blood cell count abnormalities, extramedullary hematopoiesis, elevated expression of proinflammatory cytokines, and bone marrow fibrosis. Clinical manifestations include progressive splenomegaly and debilitating symptoms such as fatigue, night sweats, pruritus, fever, and unintentional weight loss.1,2 Dysregulation of Janus kinase (JAK) 1 and 2 signaling has been reported to play a key role in the pathogenesis of MPNs.3,4
To date, 4 JAK inhibitors (ruxolitinib, fedratinib, momelotinib, and pacritinib) have been approved for treatment of patients with myelofibrosis.5-8 Ruxolitinib, a potent and selective inhibitor of JAK1 and JAK2,9 was approved by the US Food and Drug Administration for the treatment of patients with intermediate- or high-risk myelofibrosis based on the results of the COMFORT trials.5,10,11 Ruxolitinib improved quality of life, reduced symptom burden, and prolonged overall survival.10,12 Despite the efficacy of ruxolitinib in reducing spleen volume (SV) and improving symptoms, unmet needs exist for patients with suboptimal outcomes or relapse after initial response to ruxolitinib.13 Evidence suggests that signaling through the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathway, downstream of JAK-STAT (signal transducer and activator of transcription), may also be dysregulated in myelofibrosis.14-16
Persistent activation of the PI3K/AKT pathway in patients chronically treated with ruxolitinib suggests it may be an important driver of disease progression in patients with suboptimal response to JAK-inhibitor therapy.17-20 The combination of PI3K and JAK1/2 inhibitors demonstrated synergistic inhibition of MPN cell growth in vitro, including in clonogenic assays of hematopoietic progenitors of patients with primary myelofibrosis and knock-in JAK2V617F (most common oncogenic driver in MPN) mouse models,17-21 suggesting an encouraging therapeutic combination for patients with myelofibrosis.
Parsaclisib is an orally bioavailable, potent, and highly selective next-generation inhibitor of PI3Kδ that attenuates downstream signaling of the PI3K/AKT pathway.22 PI3K inhibitors have demonstrated meaningful clinical benefit in other therapy areas such as B-cell lymphomas; however, some adverse events (AEs) seem to be a class effect (eg, transaminitis, diarrhea, colitis, pneumonitis, neutropenia, and rash).23 Parsaclisib was designed with a different molecular structure to limit both on- and off-target toxicities associated with earlier-generation PI3K inhibitors.23 In preclinical studies, combination of parsaclisib and ruxolitinib abrogated abnormal cell proliferation and reduced circulating tumor cells and spleen weight compared with single agents in an ex vivo MPN model with patient-derived CD34+ cells and an in vivo murine model using a JAK2V617F-expressing cell line (data on file, Incyte Corporation). This suggests that the addition of parsaclisib may be a suitable treatment option for patients with suboptimal response or loss of response to ruxolitinib, who otherwise have limited therapy options.24-27 The objective of this study was to assess the safety and efficacy of the addition of parsaclisib to ruxolitinib for patients with myelofibrosis and suboptimal response to ruxolitinib monotherapy.
Methods
Study design and patients
This phase 2, open-label study explored both dose levels and dose regimens and was comprised of 4 parts (supplemental Figure 1): part 1 (safety run-in) determined the safe and tolerable doses of parsaclisib as add-on to ruxolitinib; and parts 2 to 4 (randomized) evaluated the efficacy and safety of different dosing regimens of parsaclisib as add-on to ruxolitinib. The study was carried out in accordance with the principles of the International Conference on Harmonisation guidelines for Good Clinical Practice, the Declaration of Helsinki, and all applicable laws. The protocol was approved by institutional review boards, and all patients provided written informed consent before enrollment. This trial was registered at www.clinicaltrials.gov as #NCT02718300.
Eligible patients were aged ≥18 years with a diagnosis of primary or secondary myelofibrosis treated with ruxolitinib for ≥6 months, and on a stable dose (5-25 mg twice daily) for ≥8 weeks before study start, with suboptimal response when receiving ruxolitinib (see supplemental Methods for definition of suboptimal response). By avoiding a washout of ruxolitinib and requiring maintenance of a fixed ruxolitinib dose, the design assures that responses observed may be attributed to the addition of the second therapy (parsaclisib). Patients were required to have an Eastern Cooperative Oncology Group performance status score of ≤2. Exclusion criteria included use of any other drugs for myelofibrosis such as danazol and hydroxyurea (except ruxolitinib) or splenic irradiation within 6 months, prior therapy with other PI3K inhibitors, inadequate bone marrow reserve, inadequate liver or renal function, uncontrolled cardiac disease, active infections requiring therapy, HIV infection, history of grade 3 or 4 immune-related AEs, or any-grade ocular immune-related AEs. Patients with anemia and transfusion dependence were not excluded.
Study procedures and end points
Ruxolitinib dosing: ruxolitinib was administered orally, twice daily at the stable dose at the time of screening for each patient per the approved product label (see supplemental Methods).
Parsaclisib dosing: parsaclisib was administered orally with water in a fasting state and dosed according to the study protocol.
Part 1: safety run-in. Part 1 (supplemental Figure 1) was an open-label safety run-in to assess the safety and tolerability of parsaclisib add-on to ruxolitinib and to select the dose of parsaclisib for further study. The initial parsaclisib dosing regimen used 8 weeks of once-daily dosing, followed by once-weekly dosing at the same dose strength. This dosing regimen was based on ongoing studies with parsaclisib in lymphoma, in which late-onset gastrointestinal toxicities (including severe diarrhea and colitis, with a median onset of 5.7 months) with once-daily dosing were observed.28 The maximum tolerated dose was planned to be the highest dose tested for which <2 dose-limiting toxicities (DLTs) occurred in a cohort of 6 patients in which evaluable patients had to have received at least 22 out of 28 days of the prescribed dose of parsaclisib plus ruxolitinib for that cohort. After the 28-day evaluation period, patients remained on treatment for as long as adequately tolerated. The primary end point for part 1 was the determination of the doses of parsaclisib that were safe and tolerable in combination with ruxolitinib. No DLTs were observed; the highest dose tested was 20 mg daily.
Parts 2, 3, and 4: randomized phase. Part 2 (supplemental Figure 1) was planned to be a block-randomized, open-label study with 2 treatment groups, receiving either 10 mg or 20 mg parsaclisib daily for the first 8 weeks, then the same dose once weekly through the end of study (daily-to-weekly regimen), while continuing their stable ruxolitinib dose. Part 3 was planned to be an open-label study comparing daily parsaclisib vs weekly long-term doses. Two randomized treatment groups were planned: all patients were to initially receive parsaclisib 20 mg daily plus ruxolitinib; then, after 8 weeks, 1 group would switch to once-weekly dosing of parsaclisib 20 mg plus ruxolitinib (daily-to-weekly regimen) and the other would continue with once-daily dosing but at a reduced dose of parsaclisib 5 mg with ruxolitinib (all-daily dosing regimen). Initial results based on investigator feedback suggested that the all-daily regimens provided more durable response; therefore, enrollment in parts 2 and 3 was suspended upon initiation of part 4, which was designed to compare 2 all-daily dosing regimens. Patients were randomized to receive parsaclisib 5 mg daily for the duration of their participation in the study, or to begin with an induction phase of 20 mg daily, with transition to 5 mg daily after 8 weeks. All patients were dosed indefinitely or until discontinuation criteria were met. Ad hoc consideration of sample sizes available at the time the study was closed suggested that combining the 2 all-daily dosing groups vs daily-to-weekly dosing groups would provide a more rigorous basis to compare efficacy and safety parameters.
The primary end points of parts 2, 3, and 4 were absolute change and percentage change in SV from baseline to week 12. Key secondary end points were absolute change and percentage change in SV from baseline to week 24, changes in Myelofibrosis Symptom Assessment Form (MFSAF) and Myeloproliferative Neoplasms Symptom Assessment Form (MPN-SAF) total symptom score from baseline to week 12 or 24, number of patients with response, Patient Global Impression of Change (PGIC) score, and safety and tolerability. Exploratory end points included change and percentage change in palpable spleen length from baseline to each study visit, and change in bone marrow fibrosis grade from baseline to week 24.
Assessments
Safety was evaluated based on AEs, laboratory assessments, physical examinations, electrocardiograms, and vital signs reported at study visits. AEs of special interest described as occurring with other inhibitors of the PI3K pathway were also assessed (see supplemental Methods). AE severity was graded according to the Common Terminology Criteria for Adverse Events version 4.03.
SV was measured using magnetic resonance imaging or computed tomography imaging at baseline and every 12 weeks through week 108, by an independent central reader. Spleen length (costal margin to point of greatest splenic protrusion) was assessed by manual palpation at every study visit. The proportion of patients with spleen volume reductions (SVR) of 10%, 25%, and 35% were determined to assess the magnitude of response in this treatment population.
Symptoms were assessed using a daily symptom diary (modified MFSAF version 3.029; recorded by the patient every evening on a handheld electronic device from baseline to week 24), along with other patient-reported measures (MPN-SAF30 questionnaire completed by the patient at each study visit, and PGIC31 administered at each visit starting at week 4; both as paper forms). Response rates were assessed according to the 2013 International Working Group consensus criteria for primary myelofibrosis, post–polycythemia vera myelofibrosis, and post-essential thrombocythemia myelofibrosis.32 Effects of treatment on fibrosis grade, plasma protein levels, and mutation status were also assessed (see supplemental Methods).
Statistical methods
The intent-to-treat population included all randomized patients. The safety population included all randomized patients who received ≥1 dose of study drug. For analysis, patients treated in the study were grouped into those receiving parsaclisib 10 mg or 20 mg daily for 8 weeks followed by once weekly at the same dose thereafter (daily-to-weekly dosing group), and those who received parsaclisib 5 or 20 mg daily for 8 weeks followed by 5 mg daily thereafter (all-daily dosing group). Efficacy analyses included all patients who were enrolled and who received ≥1 dose of study drug, and are summarized using descriptive statistics.
Results
Patient characteristics and disposition
At database lock for final analysis (20 July 2022), 74 patients had been enrolled in parts 1 to 4 of the study: 32 patients received daily-to-weekly parsaclisib dosing (parsaclisib 10 mg daily for 8 weeks followed by 10 mg once weekly [n = 14]; parsaclisib 20 mg daily for 8 weeks followed by parsaclisib 20 mg once weekly [n = 18]), and 42 patients received all-daily parsaclisib dosing (parsaclisib 20 mg daily for 8 weeks followed by parsaclisib 5 mg daily [n = 21]; or parsaclisib 5 mg daily for the entire study [n = 21]; Figure 1).
Patient disposition and treatment group allocation. ∗When once daily (QD) regimens were added to the protocol, QD dosing options were made available to patients receiving once weekly (QW) dosing. †Deaths occurring during study treatment were due to pneumonia (2 patients) and intracranial hemorrhage; none attributed to study treatment by the investigator.
Patient disposition and treatment group allocation. ∗When once daily (QD) regimens were added to the protocol, QD dosing options were made available to patients receiving once weekly (QW) dosing. †Deaths occurring during study treatment were due to pneumonia (2 patients) and intracranial hemorrhage; none attributed to study treatment by the investigator.
Demographic characteristics were well matched with no notable differences between the daily-to-weekly group and the all-daily group (Table 1). Median duration of myelofibrosis was 33.0 months (range, 4.9-268.9 months). The median SV and spleen length at enrollment were 1972.5 cm3 (range, 327.1-5323.7 cm3) and 13 cm (range, 5-30 cm), respectively, and the median MFSAF and MPN-SAF scores were 13.6 (range, 0-47.0) and 29.0 (range, 0-83.0), respectively. The median duration of ruxolitinib treatment before enrollment on this trial was 17.2 months (range, 3.7-105.5 months; minimum range of <6 months is because of incomplete ruxolitinib start date data for 1 patient in the daily-to-weekly dosing group).
Patient baseline characteristics
| Characteristic . | Daily-to-weekly dosing (n = 32) . | All-daily dosing (n = 42) . | Total (N = 74) . |
|---|---|---|---|
| Age, median (range), y | 67 (41-89) | 69 (51-84) | 68 (41-89) |
| Male, n (%) | 15 (47) | 20 (48) | 35 (47) |
| Race, n (%) | |||
| White | 25 (78) | 35 (83) | 60 (81) |
| Other∗ | 7 (22) | 7 (17) | 14 (19) |
| Time since first myelofibrosis diagnosis, mo, median (range) | 30.5 (6.7-268.9) | 37.5 (4.9-251.5) | 33.0 (4.9-268.9) |
| Ruxolitinib use, median (range) | |||
| Daily dose, mg | 28.9 (13.8-50.0) | 29.3 (8.7-44.8) | 29.3 (8.7-50.0) |
| Duration, mo | 18.1 (3.7-93.9) | 16.4 (5.1-105.5) | 17.2 (3.7-105.5) |
| Palpable spleen, n | 31 | 42 | 73 |
| Median length, cm (range) | 14 (8-30) | 11 (5-30) | 13 (5-30) |
| SV, n | 29 | 37 | 66 |
| Median, cm3 (range) | 2414.5 (327.1-5323.7) | 1877.5 (434.2-3904.1) | 1972.5 (327.1-5323.7) |
| Total symptom score by MFSAF, n | 28 | 32 | 60 |
| Median (range) | 10.8 (0-47.0) | 16.3 (0.6-38.4) | 13.6 (0-47.0) |
| Total symptom score by MPN-SAF, n | 28 | 37 | 65 |
| Median (range) | 25.5 (0-83.0) | 30.0 (3.0-65.0) | 29.0 (0-83.0) |
| Hemoglobin, g/L, median (range) | 102 (70-159) | 98 (57-155) | 100 (57-159) |
| DIPSS risk level at baseline, n (%) | |||
| High | 5 (16) | 10 (24) | 15 (20) |
| Intermediate-2 | 10 (31) | 19 (45) | 29 (39) |
| Intermediate-1 | 13 (41) | 12 (29) | 25 (34) |
| Low | 4 (12.5) | 1 (2) | 5 (7) |
| Myelofibrosis type, n (%) | |||
| PMF | 17 (53) | 23 (55) | 40 (54) |
| PPV-MF | 12 (38) | 12 (29) | 24 (32) |
| PET-MF | 3 (9) | 7 (17) | 10 (14) |
| Characteristic . | Daily-to-weekly dosing (n = 32) . | All-daily dosing (n = 42) . | Total (N = 74) . |
|---|---|---|---|
| Age, median (range), y | 67 (41-89) | 69 (51-84) | 68 (41-89) |
| Male, n (%) | 15 (47) | 20 (48) | 35 (47) |
| Race, n (%) | |||
| White | 25 (78) | 35 (83) | 60 (81) |
| Other∗ | 7 (22) | 7 (17) | 14 (19) |
| Time since first myelofibrosis diagnosis, mo, median (range) | 30.5 (6.7-268.9) | 37.5 (4.9-251.5) | 33.0 (4.9-268.9) |
| Ruxolitinib use, median (range) | |||
| Daily dose, mg | 28.9 (13.8-50.0) | 29.3 (8.7-44.8) | 29.3 (8.7-50.0) |
| Duration, mo | 18.1 (3.7-93.9) | 16.4 (5.1-105.5) | 17.2 (3.7-105.5) |
| Palpable spleen, n | 31 | 42 | 73 |
| Median length, cm (range) | 14 (8-30) | 11 (5-30) | 13 (5-30) |
| SV, n | 29 | 37 | 66 |
| Median, cm3 (range) | 2414.5 (327.1-5323.7) | 1877.5 (434.2-3904.1) | 1972.5 (327.1-5323.7) |
| Total symptom score by MFSAF, n | 28 | 32 | 60 |
| Median (range) | 10.8 (0-47.0) | 16.3 (0.6-38.4) | 13.6 (0-47.0) |
| Total symptom score by MPN-SAF, n | 28 | 37 | 65 |
| Median (range) | 25.5 (0-83.0) | 30.0 (3.0-65.0) | 29.0 (0-83.0) |
| Hemoglobin, g/L, median (range) | 102 (70-159) | 98 (57-155) | 100 (57-159) |
| DIPSS risk level at baseline, n (%) | |||
| High | 5 (16) | 10 (24) | 15 (20) |
| Intermediate-2 | 10 (31) | 19 (45) | 29 (39) |
| Intermediate-1 | 13 (41) | 12 (29) | 25 (34) |
| Low | 4 (12.5) | 1 (2) | 5 (7) |
| Myelofibrosis type, n (%) | |||
| PMF | 17 (53) | 23 (55) | 40 (54) |
| PPV-MF | 12 (38) | 12 (29) | 24 (32) |
| PET-MF | 3 (9) | 7 (17) | 10 (14) |
DIPSS, Dynamic International Prognostic Scoring System; PET-MF, post-essential thrombocythemia myelofibrosis; PMF, primary myelofibrosis; PPV-MF, post–polycythemia vera myelofibrosis; SV, spleen volume.
Includes Black/African American, Asian, and Other.
The most common reasons for treatment discontinuation among the 74 patients enrolled in the study included rollover to an open-label parsaclisib study (n = 16; 22%), AEs (n = 13; 18%), and progressive disease/lack of efficacy and physician decision (each n = 10; 13.5%); notably, 5 patients (7%) discontinued study treatment in order to proceed to transplantation (Figure 1). The median duration of treatment in all patients was 11.1 months (range, 1.0-49.1 months); for patients receiving daily-to-weekly dosing, this was 11.3 months (range, 1.7-49.1 months), and for all-daily dosing, this was 10.9 months (range, 1.0-36.2 months). Overall, 50 of 74 (68%) were on therapy for ≥6 months, 31 of 74 (42%) for ≥12 months, and 10 of 74 (14%) for ≥24 months (Figure 2). Note that the time on therapy in the present study exceeds the observation timeframe for late-onset gastrointestinal toxicities observed with parsaclisib for patients with lymphoma (see “Study procedures and end points”).
Duration of parsaclisib treatment by parsaclisib dosing regimen. Blue bars represent daily-to-weekly parsaclisib dosing, and red bars represent all-daily parsaclisib dosing.
Duration of parsaclisib treatment by parsaclisib dosing regimen. Blue bars represent daily-to-weekly parsaclisib dosing, and red bars represent all-daily parsaclisib dosing.
Changes in SV and palpable spleen length
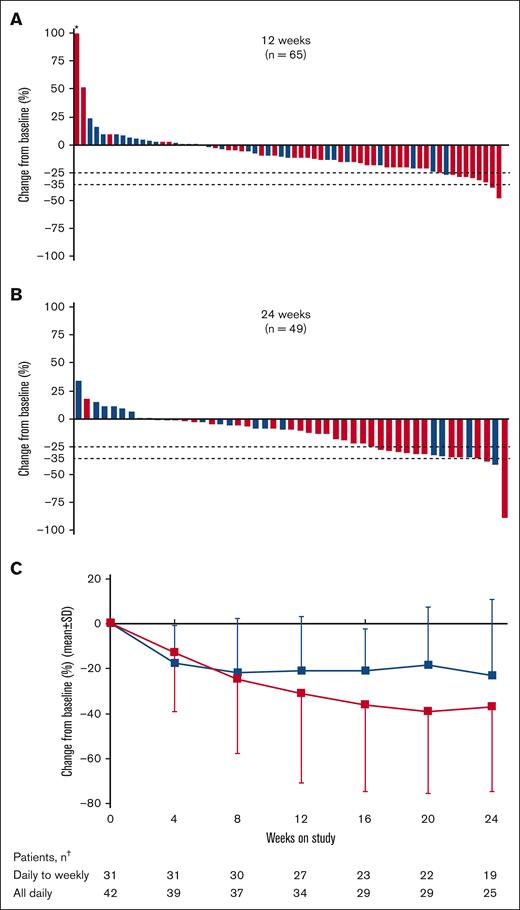
Overall, 65 patients (88%) were evaluable for the primary end point based on SV measurements at baseline and week 12. The median change in SV was −163.6 cm3 (range, −735.6 to 10 173 cm3), with a median percentage change of −11% (range, −47% to 444%; Figure 3A). After 24 weeks, the median SV change in 49 evaluable patients was −192.0 cm3 (range, −2040 to 761.4 cm3), and median percentage change was −10% (range, −89% to 34%; Figure 3B). At both 12 and 24 weeks, the median percentage change was greater among patients who received all-daily dosing than patients who received daily-to-weekly dosing (week 12: −15% vs −2%; week 24: −19% vs −2.5%, respectively).
Change in SV and spleen length from baseline in patients with MF. Percentage change from baseline in SV at (A) 12 weeks and (B) 24 weeks; and (C) change in spleen length by study visit up to 24 weeks in patients treated with add-on parsaclisib plus ruxolitinib. Blue bars and line represent daily-to-weekly parsaclisib dosing, and red bars and line represent all-daily parsaclisib dosing. Evaluable patients were those receiving ≥1 dose of study drug and had SV baseline assessment with a nonzero value. Dotted lines represent 25% and 35% decrease in SV from baseline. ∗Patient had best percentage change from baseline >100%. †The number of patients in each dosing group who had palpable spleen length evaluated at the follow-up time point. SD, standard deviation.
Change in SV and spleen length from baseline in patients with MF. Percentage change from baseline in SV at (A) 12 weeks and (B) 24 weeks; and (C) change in spleen length by study visit up to 24 weeks in patients treated with add-on parsaclisib plus ruxolitinib. Blue bars and line represent daily-to-weekly parsaclisib dosing, and red bars and line represent all-daily parsaclisib dosing. Evaluable patients were those receiving ≥1 dose of study drug and had SV baseline assessment with a nonzero value. Dotted lines represent 25% and 35% decrease in SV from baseline. ∗Patient had best percentage change from baseline >100%. †The number of patients in each dosing group who had palpable spleen length evaluated at the follow-up time point. SD, standard deviation.
All 74 patients enrolled and treated with add-on parsaclisib plus ruxolitinib had a baseline SV scan performed, but owing to early discontinuation, 6 patients did not have the data entered; however, these patients were included in the SVR responder analysis and were assessed as treatment failures. At 12 weeks, 34 patients (46%) had an SVR of ≥10%; this was higher among patients who received all-daily dosing than those who received daily-to-weekly dosing (25 of 42 [59.5%] vs 9 of 32 [28%]; Table 2). At 24 weeks, 25 patients (34%) had achieved SVR of ≥10% (21 of 42 [50.0%] in all-daily dosing, and 4 of 32 [12.5%] in daily-to-weekly dosing), 16 (22%) had achieved an SVR of ≥25% (12 of 42 [29.0%] in all-daily dosing, and 4 of 32 [12.5%] in daily-to-weekly dosing), and 4 (5%) had achieved an SVR of ≥35% (3 of 42 [7%] in all-daily dosing, and 1 of 32 [3%] in daily-to-weekly dosing; Table 2). Response rates based on the presence of the V617F mutation were essentially the same (see supplemental Results).
SV, MFSAF, and MPN-SAF response rates at 12 and 24 weeks, in patients treated with add-on parsaclisib plus ruxolitinib
| Response category . | Daily-to-weekly dosing (n = 32) . | All-daily dosing (n = 42) . | Total (N = 74) . |
|---|---|---|---|
| SV | |||
| Week 12, n | 32 | 42 | 74 |
| Response category, n (%) | |||
| <0% reduction | 13 (41) | 6 (14) | 19 (26) |
| 0 to <10% reduction | 6 (19) | 6 (14) | 12 (16) |
| ≥10 to <25% reduction | 8 (25) | 16 (38) | 24 (32) |
| ≥25 to <35% reduction | 1 (3) | 7 (17) | 8 (11) |
| ≥35% reduction | 0 | 2 (5) | 2 (3) |
| Week 24, n | 32 | 42 | 74 |
| Response category, n (%) | |||
| <0% reduction | 8 (25) | 1 (2) | 9 (12) |
| 0 to <10% reduction | 8 (25) | 7 (17) | 15 (20) |
| ≥10 to <25% reduction | 0 | 9 (21) | 9 (12) |
| ≥25 to <35% reduction | 3 (9) | 9 (21) | 12 (16) |
| ≥35% reduction | 1 (3) | 3 (7) | 4 (5) |
| MFSAF | |||
| Week 12, n | 28 | 32 | 60 |
| Response category, n (%) | |||
| <0% reduction | 9 (32) | 4 (13) | 13 (22) |
| 0 to <25% reduction | 5 (18) | 7 (22) | 12 (20) |
| ≥25 to <50% reduction | 3 (11) | 4 (13) | 7 (12) |
| ≥50% reduction | 4 (14) | 9 (28) | 13 (22) |
| Week 24, n | 28 | 32 | 60 |
| Response category, n (%) | |||
| <0% reduction | 7 (25) | 4 (13) | 11 (18) |
| 0 to <25% reduction | 3 (11) | 3 (9) | 6 (10) |
| ≥25 to <50% reduction | 3 (11) | 6 (19) | 9 (15) |
| ≥50% reduction | 3 (11) | 5 (16) | 8 (13) |
| MPN-SAF | |||
| Week 12, n | 27 | 37 | 64 |
| Response category, n (%) | |||
| <0% reduction | 5 (19) | 4 (11) | 9 (14) |
| 0 to <25% reduction | 6 (22) | 4 (11) | 10 (16) |
| ≥25 to <50% reduction | 4 (15) | 10 (27) | 14 (22) |
| ≥50% reduction | 5 (19) | 12 (32) | 17 (27) |
| Week 24, n | 27 | 37 | 64 |
| Response category, n (%) | |||
| <0% reduction | 2 (7) | 2 (5) | 4 (6) |
| 0 to <25% reduction | 2 (7) | 4 (11) | 6 (9) |
| ≥25 to <50% reduction | 6 (22) | 2 (5) | 8 (13) |
| ≥50% reduction | 5 (19) | 18 (49) | 23 (36) |
| Response category . | Daily-to-weekly dosing (n = 32) . | All-daily dosing (n = 42) . | Total (N = 74) . |
|---|---|---|---|
| SV | |||
| Week 12, n | 32 | 42 | 74 |
| Response category, n (%) | |||
| <0% reduction | 13 (41) | 6 (14) | 19 (26) |
| 0 to <10% reduction | 6 (19) | 6 (14) | 12 (16) |
| ≥10 to <25% reduction | 8 (25) | 16 (38) | 24 (32) |
| ≥25 to <35% reduction | 1 (3) | 7 (17) | 8 (11) |
| ≥35% reduction | 0 | 2 (5) | 2 (3) |
| Week 24, n | 32 | 42 | 74 |
| Response category, n (%) | |||
| <0% reduction | 8 (25) | 1 (2) | 9 (12) |
| 0 to <10% reduction | 8 (25) | 7 (17) | 15 (20) |
| ≥10 to <25% reduction | 0 | 9 (21) | 9 (12) |
| ≥25 to <35% reduction | 3 (9) | 9 (21) | 12 (16) |
| ≥35% reduction | 1 (3) | 3 (7) | 4 (5) |
| MFSAF | |||
| Week 12, n | 28 | 32 | 60 |
| Response category, n (%) | |||
| <0% reduction | 9 (32) | 4 (13) | 13 (22) |
| 0 to <25% reduction | 5 (18) | 7 (22) | 12 (20) |
| ≥25 to <50% reduction | 3 (11) | 4 (13) | 7 (12) |
| ≥50% reduction | 4 (14) | 9 (28) | 13 (22) |
| Week 24, n | 28 | 32 | 60 |
| Response category, n (%) | |||
| <0% reduction | 7 (25) | 4 (13) | 11 (18) |
| 0 to <25% reduction | 3 (11) | 3 (9) | 6 (10) |
| ≥25 to <50% reduction | 3 (11) | 6 (19) | 9 (15) |
| ≥50% reduction | 3 (11) | 5 (16) | 8 (13) |
| MPN-SAF | |||
| Week 12, n | 27 | 37 | 64 |
| Response category, n (%) | |||
| <0% reduction | 5 (19) | 4 (11) | 9 (14) |
| 0 to <25% reduction | 6 (22) | 4 (11) | 10 (16) |
| ≥25 to <50% reduction | 4 (15) | 10 (27) | 14 (22) |
| ≥50% reduction | 5 (19) | 12 (32) | 17 (27) |
| Week 24, n | 27 | 37 | 64 |
| Response category, n (%) | |||
| <0% reduction | 2 (7) | 2 (5) | 4 (6) |
| 0 to <25% reduction | 2 (7) | 4 (11) | 6 (9) |
| ≥25 to <50% reduction | 6 (22) | 2 (5) | 8 (13) |
| ≥50% reduction | 5 (19) | 18 (49) | 23 (36) |
Evaluable patients were those receiving ≥1 dose of study drug, had SV, MFSAF, or MPN-SAF baseline assessment with a nonzero value, and met ≥1 of the following criteria: (1) had week 12 or 24 SV, MFSAF, or MPN-SAF assessments; and (2) had been on the study for a minimum of 89 or 173 days of follow-up; or (3) had discontinued from treatment on, or before, week 12 or 24. Noncompleters were assessed as nonresponders.
Change from baseline in palpable spleen length below the left costal margin was observed early in both daily-to-weekly and all-daily dosing groups; reductions continued with additional time on study, particularly for patients receiving all-daily dosing regimens (Figure 3C). At 12 weeks, the mean (standard deviation) percentage change was −31% (40%) for all-daily dosing and −21% (24%) for daily-to-weekly dosing; at 24 weeks, the change was −37% (38.0%) and −23% (33.5%), respectively.
Changes in myelofibrosis symptoms
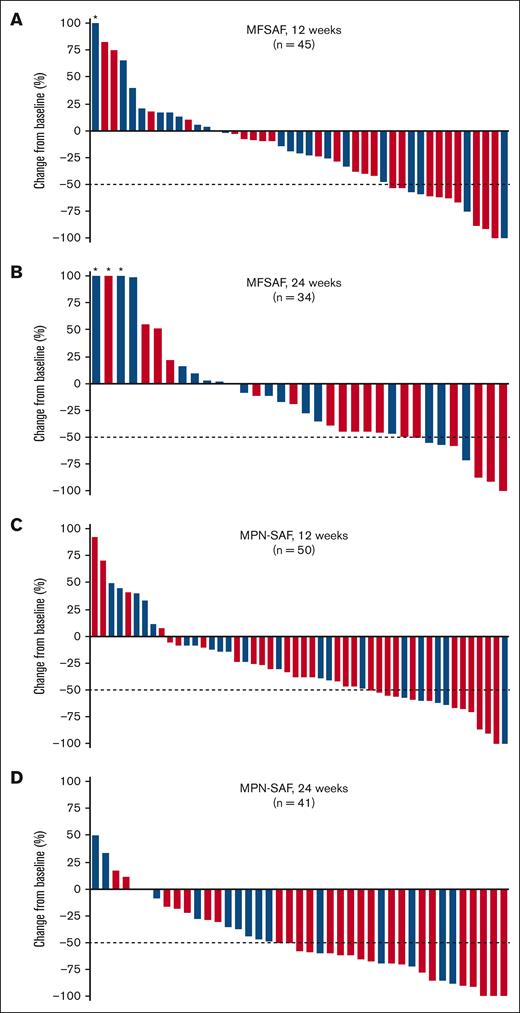
Symptom data were collected using a daily symptoms diary with the MFSAF, and monthly using 7-day recall with the MPN-SAF; the MFSAF data collected daily mitigate some of the day-to-day variation in symptoms, whereas the MPN-SAF serves as potentially corroborating data. Among all patients treated with add-on parsaclisib plus ruxolitinib, the median percentage change in MFSAF from baseline to week 12 was −20.5% (range, −100% to 500%; Figure 4A). The median percentage change at week 12 was greater for patients dosed all-daily than for patients dosed daily-to-weekly (−33% [range, −100% to 84%] vs −14% [range, −100% to 500%], respectively; Figure 4A); a similar trend was also evident at week 24 (−44% [range, −100% to 152%] vs −10% [range, −71% to 222.5%]; Figure 4B). At 12 weeks and 24 weeks, 32 of 60 (53%) and 23 of 60 (38%) patients, respectively, had experienced any reduction in MFSAF. At weeks 12 and 24, more patients who received all-daily dosing than daily-to-weekly dosing achieved a ≥50% reduction in MFSAF (Table 2). Individual symptom responses from the MPN data set for patients receiving all-daily doses were further analyzed. Symptoms related to spleen size (early satiety and abdominal discomfort) decreased by the first assessment (week 4) and remained ∼80% decreased through week 36 (supplemental Figure 2). Cytokine related symptoms such as itching and night sweats also decreased rapidly and durably. Bone pain and concentration problems were not suppressed as much as other symptoms, whereas fatigue and inactivity reached a maximum degree of inhibition and drifted back to baseline levels.
Change in MFSAF and MPN-SAF symptom scores from baseline in patients with MF. Percentage change in MFSAF from baseline at (A) 12 weeks and (B) 24 weeks, and in MPN-SAF from baseline at (C) 12 weeks and (D) 24 weeks in patients treated with add-on parsaclisib plus ruxolitinib. Blue bars represent daily-to-weekly parsaclisib dosing, and red bars represent all-daily parsaclisib dosing. Evaluable patients were those receiving ≥1 dose of study drug and had MFSAF or MPN-SAF baseline assessment with a nonzero value. Dotted line represents 50% decrease in MFSAF or MPN-SAF from baseline. ∗Patients had best percentage change from baseline of >100%.
Change in MFSAF and MPN-SAF symptom scores from baseline in patients with MF. Percentage change in MFSAF from baseline at (A) 12 weeks and (B) 24 weeks, and in MPN-SAF from baseline at (C) 12 weeks and (D) 24 weeks in patients treated with add-on parsaclisib plus ruxolitinib. Blue bars represent daily-to-weekly parsaclisib dosing, and red bars represent all-daily parsaclisib dosing. Evaluable patients were those receiving ≥1 dose of study drug and had MFSAF or MPN-SAF baseline assessment with a nonzero value. Dotted line represents 50% decrease in MFSAF or MPN-SAF from baseline. ∗Patients had best percentage change from baseline of >100%.
In the overall population, median percentage change in MPN-SAF from baseline to week 12 was −37.5% (range, −100% to 93%; Figure 4C) and from baseline to week 24 was −58% (range, −100% to 50%; Figure 4D). The median percentage changes at both weeks 12 and 24 were greater for patients dosed all-daily than for patients dosed daily-to-weekly (−40% [range, −100% to 93%] vs −19% [range, −100% to 50%], respectively, at week 12; and −61% [range, −100% to 17.5%] vs −43% [range, −88% to 50%], respectively, at week 24). At 12 and 24 weeks, 41 of 64 (64%) and 37 of 64 (58%) patients, respectively, experienced any reduction in MPN-SAF. At weeks 12 and 24, more patients who received all-daily dosing than daily-to-weekly dosing experienced ≥50% reduction in MPN-SAF, using a responder analysis in which noncompleters were treated as treatments failures (Table 2).
Median PGIC scores at weeks 12 and 24 were 3.0 (range, 1.0-6.0) and 2.0 (range, 1.0-5.0), respectively, corresponding to “minimal improvement” and “much improvement,” respectively. Overall, 47% of patients had an improvement in PGIC score at 12 weeks and 53% at 24 weeks, whereas 8% and 1% worsened at these time points, respectively (supplemental Table 1). The percentages of patients with an improvement were slightly greater in the all-daily dosing group than in the daily-to-weekly dosing group at both 12 and 24 weeks (supplemental Table 1).
Changes in fibrosis, plasma cytokine proteins, and genomic mutational status
In the daily-to-weekly dosing group, 16 patients had both baseline and on-study values for fibrosis score assessment; 5 patients (31%) showed improvement in their fibrosis scores, 1 patient (6%) had a worsening score, and 10 patients (63%) remained unchanged. For the all-daily dosing group, 25 patients had baseline and on-study data for fibrosis-score analysis; 6 patients (24%) showed improvements in their fibrosis scores, 3 patients (12%) had worsening scores, and 16 patients (64%) remained unchanged (supplemental Table 2). Changes in plasma cytokine proteins are summarized in supplemental Results and supplemental Figure 3.
Genomic analysis at baseline indicated a similar mutational landscape between the 4 dosing regimens, and, as expected, JAK2V617F was the most represented MPN driver mutation for all dosing regiments, followed by CALR exon 9 mutation. The high molecular risk mutation status did not influence the likelihood of achieving a spleen or symptom response during treatment. However, except for 1 patient, the combination of parsaclisib with ruxolitinib did not result in meaningful effect on MPN driver mutation allele burden.
Safety and tolerability
In total, 10 patients were treated in the safety run-in (part 1). No DLTs were observed in the 3 patients enrolled in cohort 1 (parsaclisib 10 mg daily for 8 weeks followed by 10 mg once weekly); cohort 2 enrolled 3 patients initially, followed by 4 additional patients (parsaclisib 20 mg daily for 8 weeks followed by 20 mg once weekly), with no DLTs observed.
Seventy-one patients (96%) experienced ≥1 treatment-emergent AE, 40 of whom experienced events that were grade ≥3. The most common nonhematologic AEs of any grade, irrespective of attribution, were nausea (n = 17; 23%), diarrhea (n = 16; 22%), abdominal pain and fatigue (each n = 14; 19%), and cough and dyspnea (n = 13; 18%) (Table 3). Forty-five (61%) and 35 (47%) patients experienced AEs that were judged by the investigator to be related to parsaclisib or ruxolitinib treatment, respectively (supplemental Tables 3 and 4). The most common nonhematologic AEs (≥5% in all patients) of any grade that were judged by the investigator to be related to parsaclisib were diarrhea, nausea, alanine aminotransferase increased (each n = 5; 7%), and aspartate aminotransferase increased and dizziness (each n = 4; 5%). The most common nonhematologic AEs (≥5% in all patients) of any grade judged by the investigator to be related to ruxolitinib treatment was diarrhea (n = 4; 5%). Serious AEs occurred in 26 patients (35%); pneumonia (n = 6), fall (n = 3), and pyrexia (n = 2) were the only serious AEs that occurred in ≥2 patients (supplemental Table 5).
Most common nonhematologic AEs occurring in 10% or more of patients treated with add-on parsaclisib plus ruxolitinib
| Event, n (%) . | Daily-to-weekly dosing (n = 32) . | All-daily dosing (n = 42) . | Total (N = 74) . | |||
|---|---|---|---|---|---|---|
| All grades . | Grade 3 or 4 . | All grades . | Grade 3 or 4 . | All grades . | Grade 3 or 4 . | |
| Nausea | 10 (31) | 1 (3) | 7 (17) | 0 | 17 (23) | 1 (1) |
| Diarrhea | 9 (28) | 1 (3) | 7 (17) | 0 | 16 (22) | 1 (1) |
| Abdominal pain | 7 (22) | 1 (3) | 7 (17) | 0 | 14 (19) | 1 (1) |
| Fatigue | 9 (28) | 1 (3) | 5 (12) | 1 (2) | 14 (19) | 2 (3) |
| Cough | 7 (22) | 0 | 6 (14) | 0 | 13 (18) | 0 |
| Dyspnea | 5 (16) | 0 | 8 (19) | 2 (5) | 13 (18) | 2 (3) |
| Dizziness | 4 (13) | 0 | 8 (19) | 0 | 12 (16) | 0 |
| Fall | 6 (19) | 2 (6) | 6 (14) | 0 | 12 (16) | 2 (3) |
| Constipation | 6 (19) | 0 | 7 (17) | 0 | 11 (15) | 0 |
| Hyperuricemia | 4 (13) | 0 | 6 (14) | 0 | 10 (14) | 0 |
| Pruritus | 5 (16) | 1 (3) | 5 (12) | 0 | 10 (14) | 1 (1) |
| Arthralgia | 3 (9) | 0 | 6 (14) | 1 (2) | 9 (12) | 1 (1) |
| Back pain | 5 (16) | 1 (3) | 4 (10) | 0 | 9 (12) | 1 (1) |
| Epistaxis | 6 (19) | 0 | 3 (7) | 0 | 9 (12) | 0 |
| Blood creatinine increased | 4 (13) | 1 (3) | 4 (10) | 0 | 8 (11) | 1 (1) |
| Contusion | 8 (25) | 0 | 0 | 0 | 8 (11) | 0 |
| Insomnia | 1 (3) | 0 | 7 (17) | 0 | 8 (11) | 0 |
| Pain in extremity | 4 (13) | 0 | 4 (10) | 0 | 8 (11) | 0 |
| Pneumonia | 3 (9) | 3 (9) | 5 (12) | 2 (5) | 8 (11) | 5 (7) |
| Pyrexia | 5 (16) | 1 (3) | 3 (7) | 0 | 8 (11) | 1 (1) |
| Rash | 5 (16) | 0 | 3 (7) | 0 | 8 (11) | 0 |
| Upper respiratory tract infection | 3 (9) | 0 | 5 (12) | 0 | 8 (11) | 0 |
| Alanine aminotransferase increased | 2 (6) | 2 (6) | 5 (12) | 0 | 7 (10) | 2 (3) |
| Headache | 6 (19) | 0 | 1 (2) | 0 | 7 (10) | 0 |
| Nasal congestion | 4 (13) | 0 | 3 (7) | 0 | 7 (10) | 0 |
| Event, n (%) . | Daily-to-weekly dosing (n = 32) . | All-daily dosing (n = 42) . | Total (N = 74) . | |||
|---|---|---|---|---|---|---|
| All grades . | Grade 3 or 4 . | All grades . | Grade 3 or 4 . | All grades . | Grade 3 or 4 . | |
| Nausea | 10 (31) | 1 (3) | 7 (17) | 0 | 17 (23) | 1 (1) |
| Diarrhea | 9 (28) | 1 (3) | 7 (17) | 0 | 16 (22) | 1 (1) |
| Abdominal pain | 7 (22) | 1 (3) | 7 (17) | 0 | 14 (19) | 1 (1) |
| Fatigue | 9 (28) | 1 (3) | 5 (12) | 1 (2) | 14 (19) | 2 (3) |
| Cough | 7 (22) | 0 | 6 (14) | 0 | 13 (18) | 0 |
| Dyspnea | 5 (16) | 0 | 8 (19) | 2 (5) | 13 (18) | 2 (3) |
| Dizziness | 4 (13) | 0 | 8 (19) | 0 | 12 (16) | 0 |
| Fall | 6 (19) | 2 (6) | 6 (14) | 0 | 12 (16) | 2 (3) |
| Constipation | 6 (19) | 0 | 7 (17) | 0 | 11 (15) | 0 |
| Hyperuricemia | 4 (13) | 0 | 6 (14) | 0 | 10 (14) | 0 |
| Pruritus | 5 (16) | 1 (3) | 5 (12) | 0 | 10 (14) | 1 (1) |
| Arthralgia | 3 (9) | 0 | 6 (14) | 1 (2) | 9 (12) | 1 (1) |
| Back pain | 5 (16) | 1 (3) | 4 (10) | 0 | 9 (12) | 1 (1) |
| Epistaxis | 6 (19) | 0 | 3 (7) | 0 | 9 (12) | 0 |
| Blood creatinine increased | 4 (13) | 1 (3) | 4 (10) | 0 | 8 (11) | 1 (1) |
| Contusion | 8 (25) | 0 | 0 | 0 | 8 (11) | 0 |
| Insomnia | 1 (3) | 0 | 7 (17) | 0 | 8 (11) | 0 |
| Pain in extremity | 4 (13) | 0 | 4 (10) | 0 | 8 (11) | 0 |
| Pneumonia | 3 (9) | 3 (9) | 5 (12) | 2 (5) | 8 (11) | 5 (7) |
| Pyrexia | 5 (16) | 1 (3) | 3 (7) | 0 | 8 (11) | 1 (1) |
| Rash | 5 (16) | 0 | 3 (7) | 0 | 8 (11) | 0 |
| Upper respiratory tract infection | 3 (9) | 0 | 5 (12) | 0 | 8 (11) | 0 |
| Alanine aminotransferase increased | 2 (6) | 2 (6) | 5 (12) | 0 | 7 (10) | 2 (3) |
| Headache | 6 (19) | 0 | 1 (2) | 0 | 7 (10) | 0 |
| Nasal congestion | 4 (13) | 0 | 3 (7) | 0 | 7 (10) | 0 |
Based on laboratory assessment, new-onset grade 3 thrombocytopenia was reported in 6 of 32 (19%) patients receiving daily-to-weekly parsaclisib dosing (3 of 6 patients entered the study at grade 2) and 11 of 42 (26%) patients receiving all-daily parsaclisib dosing (7 of 11 patients entered the study at grade 2); new-onset grade 4 thrombocytopenia was reported in 6 of 32 (19%) patients receiving daily-to-weekly parsaclisib dosing (4 of 6 entered the study at grade 2 or 3) and 3 of 42 (7.1%) patients receiving all-daily parsaclisib dosing (1 of 3 patients entered the study at grade 2). Overall, both platelet and hemoglobin levels remained steady during the conduct of the study (supplemental Figure 4).
AEs of special interest were infrequent. Among patients treated daily-to-weekly (n = 32), 4 (12.5%) had grade ≥2 diarrhea, 2 (6%) had grade ≥3 alanine aminotransferase elevation, 2 (6%) had grade ≥3 aspartate aminotransferase elevation, and 1 (3%) each had grade ≥2 rash, herpes simplex virus infection, and varicella zoster virus infection. Among patients treated with all-daily dosing (n = 42), the only AEs of special interest were herpes simplex virus infection in 2 patients (5%) and varicella zoster virus infection in 2 patients (5%).
AEs leading to parsaclisib discontinuation occurred in 5 of 32 patients (16%) receiving daily-to-weekly dosing and 4 of 42 patients (9.5%) receiving all-daily dosing (supplemental Table 5); thrombocytopenia was the only AE that led to discontinuation in >1 patient among all treated patients (n = 2; 3% overall; both in all-daily dosing). Overall, 6 of 74 patients (8%) who were enrolled and received treatment had fatal AEs, 4 with daily-to-weekly parsaclisib dosing (pneumonia [n = 2], and blood bilirubin increased and metastatic breast cancer [each n = 1]) and 2 with all-daily dosing (pneumonia and intracranial hemorrhage [each n = 1]; supplemental Table 6); none were considered related to either drug.
Discussion
This phase 2 study investigated the safety, tolerability, and efficacy of parsaclisib when added to a stable dose of ruxolitinib, for patients with myelofibrosis with a suboptimal response to ruxolitinib monotherapy. The addition of parsaclisib reduced SV and improved symptom burden; these benefits were observed early and were durable through at least 24 weeks. The combination had a manageable safety profile, and grade 3 or 4 AEs were uncommon in all dose groups. The observed safety, SVR, and plasma cytokine protein results favored all-daily parsaclisib dosing over daily-to-weekly dosing; patients who received all-daily parsaclisib dosing in combination with ruxolitinib had lower rates of serious, grade 3 or 4, or fatal AEs, and AEs leading to discontinuation compared with daily-to-weekly parsaclisib dosing.
We hypothesize that all-daily dosing with a lower parsaclisib dose would give consistent PI3K inhibition resulting in better symptom control for patients with myelofibrosis, while being associated with lower peak plasma parsaclisib concentrations, resulting in fewer AEs, than once-weekly dosing with a higher parsaclisib dose. Pharmacokinetic analyses from studies in patients with B-cell lymphoma showed that plasma concentrations with 5 mg daily parsaclisib were consistently above the reported 90% maximal inhibitory concentration and demonstrated ≥90% inhibition of pAKT over 6 hours at steady state.28 In contrast, plasma concentrations with parsaclisib 20 mg once weekly dropped below the 50% maximal inhibitor concentration at ∼60 hours and reached 0 at ∼80 hours after dosing.28
Splenomegaly in myelofibrosis is highly associated with morbidity and reduced quality of life33,34; therefore, reducing SV and associated symptoms is a key treatment goal. The primary end points in our study were the change and percentage change in SV from baseline to week 12; patients receiving add-on parsaclisib with ruxolitinib experienced reduction in SV from baseline by a median of −163.6 cm3 (−11%) after 12 weeks of study treatment, regardless of parsaclisib dose or regimen. An SVR of ≥35% has been the accepted cutoff for response in patients who are naïve to JAK inhibitors.10,35 However, recent clinical studies of novel agents for patients who have received prior ruxolitinib treatment reveal that a relatively low percentage of these patients achieve an SVR of ≥35%,13,36-39 and some studies include lower cutoffs (eg, ≥25%, ≥20%, and ≥10%) as end points.37,39 SVR as low as 10% can lead to symptom and overall survival improvements, as reported in the pooled analysis from the COMFORT I and II studies of ruxolitinib treatment for patients with myelofibrosis.40 Interim analyses suggested that an SVR of ≥35% might not be achievable in patients with treatment-resistant disease enrolled in our trial; thus, categories of SVR response between 0% and 35% were also analyzed, with ≥10% representing proof of concept for pharmacologic activity in this phase 2 study. At 12 weeks, 60% of patients with suboptimal response to stable-dose ruxolitinib receiving all-daily add-on parsaclisib experienced an SVR of ≥10%, with 21% experiencing SVR of ≥25%, and 5% an SVR of ≥35%. This represents a modest response to parsaclisib add-on treatment in terms of SVR, which may be related to the heavily pretreated population in this phase 2 study.
Substantial proportions of patients experienced ≥50% reduction in MPN-SAF symptom score whether receiving daily-to-weekly or all-daily add-on parsaclisib with ruxolitinib: 32% and 49% after 12 and 24 weeks of all-daily parsaclisib treatment, respectively. Additionally, with all-daily add-on parsaclisib, 28% and 16% of patients experienced ≥50% reduction in MFSAF symptom score at 12 and 24 weeks, respectively. Recent phase 3 studies of novel JAK inhibitors have included symptom scores as the primary or coprimary end points, highlighting the importance of symptom control in patients with myelofibrosis.13,37 Our findings are comparable with results from phase 3 studies for MPN-SAF and MFSAF.13,37 Further studies are needed to fully evaluate individual symptom effects because the number of patients with data decreased beyond 24 weeks in our study, but data presented support a preliminary conclusion that symptom improvement was related to effects from both SV and cytokines. We also assessed the patient-reported outcome PGIC and found that most patients experienced at least a minimal improvement by 24 weeks, with very few patients reporting worsening scores. Similar changes in PGIC have been reported in clinical trials with ruxolitinib and novel JAK inhibitors.13,41,42
We observed modest improvements in fibrosis scores in 24% to 31% of patients, suggesting that the addition of parsaclisib to ruxolitinib may have had disease-modifying activity in some patients. Change in bone marrow fibrosis score is emerging as a potential valuable surrogate end point for disease modification in myelofibrosis.38,39 Worse fibrosis scores have been correlated with shorter overall survival,43 and greater improvements in fibrosis score were associated with longer overall survival in a recent phase 2 study.39
Particular attention was paid to potential PI3Kδ class effects, as well as hematologic and immunologic AEs among patients treated with add-on parsaclisib plus ruxolitinib. New-onset grade 4 thrombocytopenia was reported at a slightly higher rate after daily-to-weekly parsaclisib dosing than all-daily parsaclisib dosing. Overall, the cumulative myelosuppression was low grade and manageable for this add-on combination. A total of 6 patients had fatal AEs in our study, none were attributed by the investigator to be related to study drug; of the 6 patients, 3 had pneumonia that led to deaths (2 receiving daily-to-weekly dosing and 1 receiving all-daily dosing). Other AEs of special interest occurred infrequently in all groups but, as with other safety findings, were potentially more frequent with daily-to-weekly dosing.
In summary, our phase 2 study suggests that the addition of parsaclisib to stable ruxolitinib therapy can reduce splenomegaly and meaningfully improve clinical symptoms without significantly affecting hemoglobin levels, and with manageable thrombocytopenia, in patients with myelofibrosis with suboptimal response to ruxolitinib monotherapy. Safety and tolerability of the combination was acceptable for all dosing regimens tested, and daily dosing may provide the greatest benefit.
Acknowledgments
The authors thank the patients, investigators, and site personnel who participated in this study.
This study was funded by Incyte Corporation (Wilmington, DE) and was partially supported by a Cancer Center support grant/core grant to Memorial Sloan Kettering Cancer Center (P30 CA008748). Medical writing assistance was provided by Kakuri Omari of Envision Pharma Group, Inc (Fairfield, CT) and funded by Incyte Corporation.
Authorship
Contribution: N.D., A.A., S.E.-V., and F.Z. contributed to the conception and design of the study; A.Y., U.B., R.K.R., H.A., E.S.W., A.T.G., G.H., M.K., E.W., C.O., S.G., S.T.O., G.S., J.M., J.P., H.H., S.H., and N.D. acquired the data; A.A., S.E.-V., and F.Z. analyzed the data; and all authors had access to study data and contributed to data interpretation, manuscript writing, editing, and critical review of the manuscript, and approved the final draft of the manuscript.
Conflict-of-interest disclosure: A.Y. was part of an advisory board for AbbVie, Acceleron Pharma Inc, Apellis Pharmaceuticals, Celgene, CTI BioPharma, Gilead Sciences, Incyte Corporation, Notable Labs, Inc, Novartis, PharmaEssentia Corporation, Pfizer, and Servier. U.B. has membership on an entity’s board of directors or advisory committees for AbbVie/Genentech, Daiichi Sankyo, Genentech, Novartis, Pfizer, and Takeda. R.K.R. has acted as a consultant for AbbVie, Blueprint Medicines, Celgene/Bristol Myers Squibb, Constellation Pharmaceuticals, CTI BioPharma, Disc Medicines, Galecto, Incyte Corporation, Jazz Pharmaceuticals, Kartos Therapeutics, PharmaEssentia Corporation, Promedior, Sierra Oncology, and Stemline Therapeutics, and has received research funding from Constellation Pharmaceuticals, Incyte Corporation, and Stemline Therapeutics. H.A. was involved in a speakers’ bureau for Incyte Corporation. E.S.W. acted as a consultant and/or was part of an advisory board for AbbVie, Astellas, Daiichi Sankyo, Dava Oncology (Arog), Gilead Sciences, Genentech, Jazz Pharmaceuticals, Kite Pharmaceuticals, Kura Oncology, MacroGenics, Pfizer, PTC Therapeutics, and Stemline Therapeutics; was part of independent data review committees for AbbVie, Genentech, and Rafael Pharmaceuticals; and was a speaker for Pfizer and Stemline Therapeutics. A.T.G. served on advisory boards for AbbVie, Celgene/Bristol Myers Squibb, Constellation Pharmaceuticals, PharmaEssentia Corporation, and Sierra Oncology. G.H. received research support from Bayer, Constellation Pharmaceuticals, Incyte Corporation, and Merck, and was a member of scientific advisory boards for AbbVie, Celgene/Bristol Myers Squibb, Constellation Pharmaceuticals, and Novartis. M.K. acted as a consultant or was a member of an advisory board for AbbVie, CTI BioPharma, MorphoSys, and Protagonist. C.O. received research funding from Astex Pharmaceuticals and Genentech, and was a member of an advisory board for Astex Pharmaceuticals, Bristol Myers Squibb, Pfizer, and Shionogi. S.T.O. acted as a consultant and/or was part of an advisory board for AbbVie, Blueprint Medicines, Celgene/Bristol Myers Squibb, Constellation Pharmaceuticals, CTI BioPharma, Disc Medicine, Geron, Incyte Corporation, Kartos Therapeutics, Novartis, PharmaEssentia Corporation, and Sierra Oncology. G.S. received research funding and speaker fees from Incyte Corporation and Novartis. J.M. was involved in a speakers’ bureau for Amgen, Bristol Myers Squibb, Incyte, Jazz Pharmaceuticals, Stemline, and Takeda, and acted as a consultant for AbbVie, CTI BioPharma, and Novartis. J.P. received research funding from CTI Biopharma, Incyte Corporation, PharmaEssentia, Protagonist, and Sierra Oncology; acted as a consultant for CTI BioPharma, Protagonist and Sierra Oncology; and was a member of an advisory board for MorphoSys. H.H. received research funding from ADC Therapeutics, Adicet Bio, Allogene, Artiva Biotherapeutics, Autolus, BeiGene, Bristol Myers Squibb, Caribou Biosciences, Genentech, Incyte Corporation, Kite Pharma, and Novartis; acted as a consultant for ADC Therapeutics, AstraZeneca, Bristol Myers Squibb, Crispr Therapeutics, Epizyme, Janssen, Karyopharm Therapeutics, Kite Pharma, Novartis, Rigel Pharmaceuticals, and TG Therapeutics; received honoraria from Bristol Myers Squibb and Kite Pharma; was involved in a speakers’ bureau for Kite Pharma; and reports membership on a board or advisory committee for Exuma Biotech. N.D. acted as a consultant and/or was part of an advisory board for AbbVie, Agios, Astellas, Bristol Myers Squibb, Celgene, Daiichi Sankyo, Dava Oncology (Arog), Genentech, Gilead Sciences, Kite Pharmaceuticals, Novartis, Pfizer, Servier, Shattuck Labs, Sobi, Star Therapeutics, Syndax, Trillium Therapeutics, and Trovagene; and received research funding from AbbVie, Amgen, Astellas, Bristol Myers Squibb, Daiichi Sankyo, Fate Therapeutics, Gilead Sciences, Glycomimetics, Hanmi Pharmaceutical, ImmunoGen, Incyte Corporation, Karyopharm, Newave, Novimmune, Pfizer, Servier, Shattuck Labs, Sobi, Trovagene, and Trillium Therapeutics. A.A., S.E.-V., and F.Z. are employees and stockholders of Incyte Corporation. The remaining authors declare no competing financial interests.
The current affiliation for U.B. is Division of Hematology, The Ohio State University Comprehensive Cancer Center, Columbus, OH.
Correspondence: Abdulraheem Yacoub, Department of Internal Medicine, University of Kansas Cancer Center, 2650 Shawnee Mission Pkwy, Westwood, KS 66205; email: ayacoub@kumc.edu; and Naval Daver, Leukemia Department, University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; email: ndaver@mdanderson.org.
References
Author notes
Presented in abstract form at the 64th annual meeting of the American Society of Hematology, New Orleans, LA, 10 to 13 December 2022.
Incyte Corporation (Wilmington, DE) is committed to data sharing that advances science and medicine while protecting patient privacy. Qualified external scientific researchers may request anonymized data sets owned by Incyte for the purpose of conducting legitimate scientific research. Researchers may request anonymized data sets from any interventional study (except phase 1 studies) for which the product and indication have been approved on, or after, 1 January 2020 in at least 1 major market (eg, United States, European Union, and Japan). Data will be available for request after the primary publication, or 2 years after the study has ended. Information on Incyte’s clinical trial data sharing policy and instructions for submitting clinical trial data requests are available at: https://www.incyte.com/Portals/0/Assets/Compliance%20and%20Transparency/clinical-trial-data-sharing.pdf?ver=2020-05-21-132838-960.
The online version of this article contains a data supplement.