TO THE EDITOR:
In chronic lymphocytic leukemia (CLL), treatments containing Bruton tyrosine kinase or B-cell lymphoma 2 inhibitors have surpassed chemoimmunotherapy (CIT) and represent the standard of care in both frontline and relapse settings.1-10 Updated European Society for Medical Oncology and National Comprehensive Cancer Network guidelines no longer recommend the use of CIT.11,12 However, there is still interest in the long-term results of CIT regimens, for example to determine late-onset toxicity or to support hypothesis building when designing new treatments. A recent study reported long-term outcome of patients treated frontline with fludarabine, cyclophosphamide, and the type I CD20-antibody rituximab (FCR).13 Almost 50% of patients with mutated immunoglobulin heavy chain variable gene (mIGHV) had durable remissions and long-lasting progression-free survival (PFS) of >15 years, but secondary myeloid neoplasia occurring in >6% of the patients remained a serious therapy-related risk. CIT with obinutuzumab/chlorambucil was developed for patients deemed unfit for FCR due to age or comorbidities. Compared with FCR, this regimen contains milder chemotherapy but a glycoengineered type II instead of a type I CD20-antibody. The modes of binding to CD20 antigen differ between the 2 antibodies.14 Primary and updated results of the CLL11 study comparing the efficacy and safety of obinutuzumab (G) or rituximab (R) plus chlorambucil (G-Clb or R-Clb) vs Clb alone (stage 1) and G-Clb vs R-Clb (stage 2) in patients with treatment-naïve CLL demonstrated superior PFS with G-based compared with R-based CIT.15,16 However, it was unknown whether superiority in PFS could translate into overall survival (OS) benefits or would last in the long term. Here, we report the results of the final survival analysis of CLL11 together with very long-term data from a subset of CLL11 participants followed up in a national registry after study completion.
CLL11 (ClinicalTrials.gov identifier: NCT01010061) is an international, multicentric, open-label, randomized phase 3 study. Details on the trial design, eligibility criteria, diagnostic and therapeutic interventions (including drug dosing), and patient baseline characteristics have been reported previously.15,16 These are summarized in the supplemental Material and illustrated in supplemental Figure 1 and supplemental Table 1. Overall, 781 patients were treated with Clb alone (n = 118), G-Clb (n = 333), or R-Clb (n = 330). After median observation time of 62.5 months for G-Clb vs Clb, 57.7 months for R-Clb vs Clb and 59.4 months for G-Clb vs R-Clb, no new safety signals were identified. Rates of late-onset adverse events including late neutropenias (13%) and secondary malignancies (11%) were comparable between antibody arms (supplemental Table 2). In the G-Clb arm, there was 1 case of myelodysplasia but no cases of acute leukemia. B-cell depletion was more persistent after treatment with G-Clb than with R-Clb (median time to recovery: 550 vs 105 days, supplemental Figure 2), whereas there was no difference in immunoglobulin depletion.
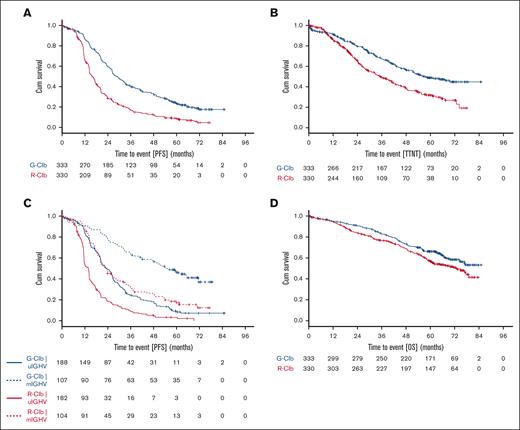
Compared with Clb alone (stage 1), G-Clb treatment significantly improved PFS and OS whereas R-Clb improved PFS, but not OS (supplemental Figure 3). Compared with R-Clb (stage 2), G-Clb treatment demonstrated statistically significant and clinically meaningful improvements in PFS (median 28.9 vs 15.7 months, hazard ratio [HR], 0.49; 95% confidence interval [CI], 0.41-0.58; P < .0001; Figure 1A) and time-to-next treatment (median 56.4 vs 34.9 months; HR, 0.58; 95% CI, 0.46-0.73; P < .0001; Figure 1B). Five-year PFS rates (95% CI) were 23% (19-28) and 9% (6-12), respectively. The PFS benefit was consistent across subgroups, including patients with unmutated IGHV (uIGHV), but excluding patients with del(17p) (supplemental Figure 4). Median PFS was longer in patients with mIGHV than in patients with uIGHV (median in months: 54.8 for G-Clb/mIGHV vs 23.4 for G-Clb/uIGHV, 23.5 for R-Clb/mIGHV vs 13.8 for R-Clb/uIGHV; Figure 1C).
CLL11 final survival analysis. Kaplan-Meier curves showing probability of PFS (A), time-to-next treatment (TTNT) (B), PFS by genetic risk (C), and OS (D) for the comparison of G-Clb vs R-Clb in the CLL11 study.
CLL11 final survival analysis. Kaplan-Meier curves showing probability of PFS (A), time-to-next treatment (TTNT) (B), PFS by genetic risk (C), and OS (D) for the comparison of G-Clb vs R-Clb in the CLL11 study.
OS was significantly longer with G-Clb than with R-Clb (median not reached vs 73.1 months, HR, 0.76; 95% CI, 0.60-0.97; P = .0245; Figure 1D). Five-year OS rates (95% CI) were 66% (61-72) and 57% (51-62), respectively. Subgroup analyses were in support of an OS benefit of G-Clb over R-Clb (supplemental Figure 4). In comparison to the G-Clb arm, patients in the R-Clb arm received more subsequent treatments. The total number of patients receiving inhibitors as salvage treatment was low (supplemental Table 3). Causes of death are summarized in supplemental Table 4.
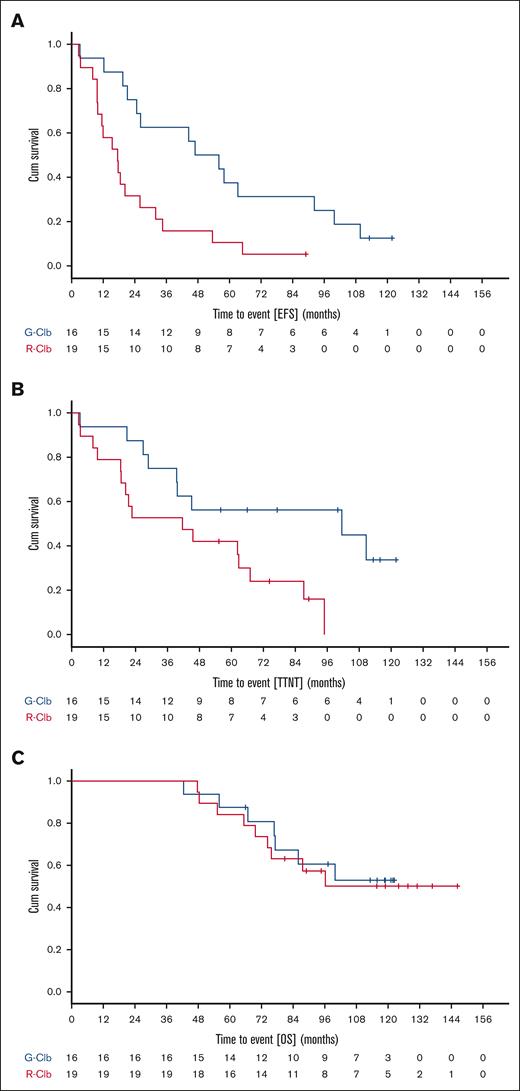
Of the patients who had received G-Clb or R-Clb as part of the CLL11 study, a subset of n = 35 was further followed up in a national registry of the German CLL Study Group (ClinicalTrials.gov identifier: NCT02863692) after the end of the study in August 2017. Methods and inclusion criteria for the registry patients are detailed in the supplemental Material. Baseline characteristics of these 35 patients largely matched those of the whole CLL11 population (supplemental Table 5). Median (range) observation times were 118.7 (42.4-122.3) months for G-Clb and 124.0 (47.7-146.3) months for R-Clb. There were 5 secondary neoplasms (all solid) in 4 of 16 G-Clb patients and 9 secondary neoplasms (including 1 case of acute myeloid leukemia) in 5 of 19 R-Clb patients (supplemental Table 6). More R-Clb than G-Clb–treated patients required antileukemic retreatment (supplemental Table 7). Causes of death in the patients are summarized in supplemental Table 8. When comparing efficacy of G-Clb vs R-Clb in the 35 registry patients, 8-year OS rates were 61% and 57% (medians not reached; HR, 0.86; 95% CI, 0.32-2.32; P = .768), 8-year time-to-next treatment rates were 56% and 0% (median 101.5 vs 41.6 months; HR, 0.36; 95% CI, 0.14-0.88; P = .025), and 8-year event-free survival rates were 25% and 5% (median 46.9 vs 17.5 months; HR, 0.35; 95% CI, 0.16-0.76; P = .008) (Figure 2). Remarkably, 2 of 16 G-Clb patients (13%) remained event-free beyond 8 years. One of these had mIGHV and both lacked del(17p).
CLL11 long-term survival data. Kaplan-Meier curves showing probability of EFS (A), TTNT (B), and OS (C) for the comparison of G-Clb and R-Clb in a subset of CLL11 study participants with extended follow-up beyond end of study. Cum, cumulative; EFS, event-free survival.
CLL11 long-term survival data. Kaplan-Meier curves showing probability of EFS (A), TTNT (B), and OS (C) for the comparison of G-Clb and R-Clb in a subset of CLL11 study participants with extended follow-up beyond end of study. Cum, cumulative; EFS, event-free survival.
These data suggest that CIT of CLL with obinutuzumab/chlorambucil could achieve long-lasting remissions over many years in patients with favorable genetic risk and that secondary myeloid neoplasia is uncommon. However, long-term remissions appear to be rarer than after FCR. No “plateau” was seen in PFS and event-free survival curves, as was not the case in newer studies that included G-Clb as a control arm and had a median follow-up between 34.1 and 76.2 months.9,17-19 These studies showed inferior PFS of G-Clb even in low-risk disease (mIGHV), thereby providing a rationale for restricting its use to exceptional settings and no longer including G-Clb as a control arm in clinical trials.20 Our results also suggest that superiority of G-Clb over R-Clb in disease control is sustained over long time, although the generalizability of this finding is limited by the moderate number of analyzable patients with very long-term data. Although durable control of CLL is a desirable therapeutic goal, an ultimate purpose of treatment is to prolong OS. In our study, the most striking benefit observed for CLL11 patients receiving G-Clb instead of R-Clb was prolongation of OS. This could be due to the different abilities of the 2 antibody regimens to eradicate minimal residual disease in CLL. In CLL11 and in a more recent study (CLL13), obinutuzumab achieved higher rates of undetectable minimal residual disease compared with rituximab when combined with chemotherapy or with venetoclax, respectively.4,21 Adding obinutuzumab to Bruton tyrosine kinase or B-cell lymphoma 2 inhibitors improved disease control. When combined with venetoclax, obinutuzumab outperformed rituximab for PFS.22 A PFS benefit was also observed by adding obinutuzumab to acalabrutinib.18
In summary, the results of our study provide evidence that obinutuzumab is an effective CD20-antibody with potential to provide long-term benefits including survival. Findings of our study are in support of current strategies to explore this antibody as part of modern combination regimens.
Acknowledgments: The German CLL Study Group thank all CLL11 study investigators and site staff, as well all patients and their families. CLL11 was sponsored by F. Hoffmann-La Roche Ltd. The authors acknowledge Michele Porro Lura for reviewing the manuscript on behalf of F. Hoffmann-La Roche Ltd.
Contribution: V.G., K.F., and M.H. contributed to the study design and interpretation; S.R. and A.G. contributed to the data analysis and interpretation; M.J.S.D., M.J.E., L.M., and L.S. contributed to the data acquisition and interpretation; N.K., A.F., and S.S. contributed to the study design, data acquisition, data analysis and interpretation; and all authors helped write the manuscript and approved the final version for publication.
Conflict-of-interest disclosure: V.G. received honoraria and travel support and was advisory board member for AbbVie, AstraZeneca, Berlin Chemie, Gilead, Heel, Janssen, Norgine, Novartis, and Roche. K.F. received honoraria from and was advisory board member for AbbVie, AstraZeneca, and Roche. M.J.S.D. received research funding from Roche. L.S. received honoraria and travel support and was advisory board member for AbbVie, AstraZeneca, and Janssen. N.K. received research funding, honoraria and travel support from AstraZeneca, Celgene, Gilead, and Janssen. A.F. received research funding and travel support from AbbVie and AstraZeneca. S.S. received research funding, honoraria, and travel support from and was advisory board member for AbbVie, Amgen, AstraZeneca, Celgene, Gilead, Glaxo Smith Kline, Janssen, Novartis, and Roche. M.H. received research funding, honoraria, consulting fees, and travel support from and was advisory board member for AbbVie, AstraZeneca, Bristol Myers Squibb, Gilead, Janssen, and Roche. The remaining authors declare no competing financial interests.
Correspondence: Valentin Goede, Department of Oncogeriatrics, St. Marien Hospital, Kunibertskloster 11-13, 50668 Cologne, Germany; email: valentin.goede@cellitinnen.de.
References
Author notes
V.G. and K.F. are joint first authors.
Original patient outcome data are available on request from the corresponding author, Valentin Goede (valentin.goede@cellitinnen.de).
The full-text version of this article contains a data supplement.