TO THE EDITOR:
Novel and mutation-specific therapeutic approaches are desperately needed for therapy-related myeloid neoplasm (t-MN), an aggressive leukemia that develops following exposure to cancer-directed therapies. Here, we demonstrate that mutations in the Rat-Sarcoma virus family of oncogenes (NRAS and KRAS) were highly enriched in TP53 wild-type (TP53wt) t-MN and were associated with poor prognosis, equivalent to mutant TP53 (TP53mut).
RAS proteins play a pivotal role in signaling pathways regulated by growth factors. Mutations in RAS (RASmut) family member genes are reported in 10% to 30% of acute myeloid leukemia (AML)1 and 2% to 3% of myelodysplastic syndrome (MDS).2 However, the incidence and prognostic impact of RASmut in t-MN is not well characterized. Therefore, we analyzed the frequency and prognostic relevance of RASmut in 597 t-MN patients managed at the Local Health Network (Adelaide, SA, Australia) and Mayo Clinic (Rochester, MN). The research was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committees (HREC/15/RAH/496 and institutional review board number 19-007595/19-007568).
The median follow-up of the cohort was 36.6 months (interquartile range, 16.0-61.7) with a median overall survival of 13.9 months (95% confidence interval, 12.7-15.6) (supplemental Figure 1A). Cohort details and comparison of RASwt and RASmut cases are provided in the supplemental Methods and supplemental Table 1, respectively.
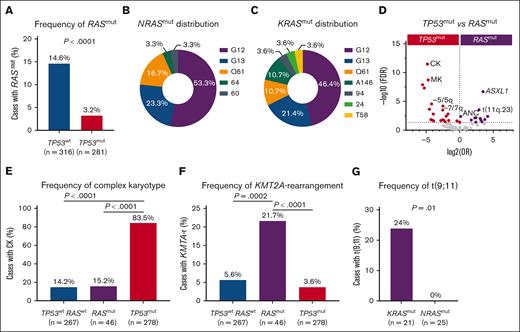
Although the frequency of RASmut was 9% (n = 55), consistent with a reported frequency ranging from 5% to 24%,3,4RASmut were enriched in TP53wt t-MN, compared with TP53mut t-MN (15% vs 3%; P < .0001) (Figure 1A). Most RASmut cases were TP53wt (83.6%; n = 46); however, 9 cases (16.4%) were TP53mut and RASmut comutated. TP53mut represents one of the most aggressive subsets of t-MN.5 Therefore, we compared clinical features (supplemental Table 2) and evaluated outcomes of TP53wt t-MN with or without RASmut and compared these with TP53mut t-MN. Of the 46 TP53wt and RASmut cases, 18 (39.1%), 18 (39.1%), and 10 (21.7%) harbored NRAS, KRAS, or multiple NRAS/KRAS mutations, respectively. The median variant allele frequency (VAF) of RASmut was 17.7% (interquartile range, 6.0-37.8), and 27 (52.9%) of the evaluable cases had VAF 20%. There was no significant difference in VAF between NRAS and KRAS (12.8% vs 21.9%; P = .52) (supplemental Figure 1B).
RASmut were more prevalent in TP53wt and are enriched in KMT2A-r. (A) RASmut were highly prevalent in TP53wt compared with TP53mut t-MNs; (B-C) most prevalent mutational hot spots (G12, G13, and Q61) in NRASmut and KRASmut (G12, G13, and Q61); (D) volcano plot comparing clinical, cytogenetic, and mutational profiles between TP53mut and RASmut. Poor-risk cytogenetics were enriched in TP53mut, whereas KMT2A-r and other somatic mutations are more frequent in RASmut t-MN; (E) complex karyotype (CK) was highly prevalent in TP53mut but not in RASmut t-MN; (F) KMT2A-r was enriched in RASmut cases compared to TP53mut and RASwt t-MN; and (G) t(9;11) was highly prevalent in KRASmut compared to NRASmut.
RASmut were more prevalent in TP53wt and are enriched in KMT2A-r. (A) RASmut were highly prevalent in TP53wt compared with TP53mut t-MNs; (B-C) most prevalent mutational hot spots (G12, G13, and Q61) in NRASmut and KRASmut (G12, G13, and Q61); (D) volcano plot comparing clinical, cytogenetic, and mutational profiles between TP53mut and RASmut. Poor-risk cytogenetics were enriched in TP53mut, whereas KMT2A-r and other somatic mutations are more frequent in RASmut t-MN; (E) complex karyotype (CK) was highly prevalent in TP53mut but not in RASmut t-MN; (F) KMT2A-r was enriched in RASmut cases compared to TP53mut and RASwt t-MN; and (G) t(9;11) was highly prevalent in KRASmut compared to NRASmut.
The most prevalent hot spots were missense mutations in the guanosine triphosphatase domain, specifically G12, G13, and Q61, accounting for 93.3% of NRASmut and 81.5% of KRASmut (Figure 1-C). This is consistent with the profile of de novo AML, in which these hot spot mutations account for 80% to 95% of NRAS and 40% to 95% of KRAS mutations.4 We also observed co-occurrence of RASmut with ASXL1 (P = .0003), STAG2 (P = .002), FLT3 (P = .03), and PTPN11 mutations (P =.04) (supplemental Figure 1C).
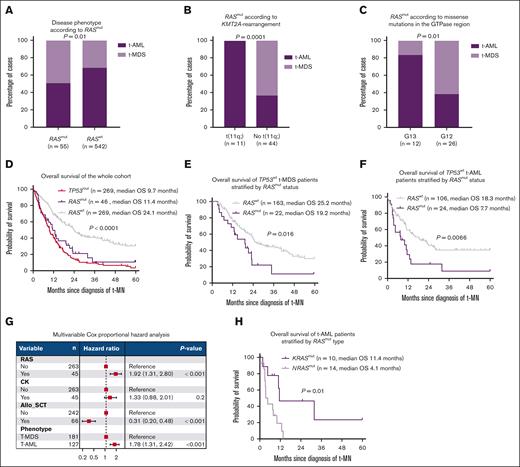
As expected, adverse-risk karyotypes, including complex karyotypes, were highly prevalent in TP53mut cases compared with RASmut (83.5% vs 15.2%; P < .0001) (Figure 1D-E) and RASwt cases (14.2%; P < .0001) (Figure 1E), consistent with the known role of RAS in growth factor signaling rather than genomic stability. In contrast, KMT2A (11q23) rearrangements (KMT2A-r) were enriched in RASmut compared to both RASwt (22% vs 6%; P = .0002) and TP53mut t-MN (22% vs 4%; P < .0001) (Figure 1F; supplemental Figure 1D). Enrichment of RASmut has been reported in KMT2A-r de novo AML and acute lymphoblastic leukemia and is associated with poor outcomes.6 Therefore, we examined the specific RAS family members mutated in KMT2A-r cases. Surprisingly, t(9;11) (p22;q23)/MLLT3:KMT2A was highly prevalent in KRASmut compared with NRASmut t-MN (24% vs 0%; P = .01) (Figure 1G), underscoring differences between RAS family members in co-occurrence.7 Furthermore, RASmut were more likely to present with t-AML than RASwt (49.1% vs 31.7%; P = .01) (Figure 2A), even more so in KMT2A-r t-MN (Figure 2B). In terms of mutation type, substitution at amino acid 13 (G13D/C) was highly enriched in t-AML (83.3%; n = 11; NRAS or KRAS). Conversely, substitution at amino acid 12 (G12D/V/C) was more frequently associated with t-MDS (65.5%; n = 16) (P = .01) (Figure 2C), highlighting phenotypical differences in RAS variant subtypes.
RASmut is enriched in t-AML and is associated with poor prognosis. (A) t-AML was more prevalent in RASmut than RASwt t-MN. (B) t-AML was especially more prevalent in the presence of KMT2A-r. (C) Substitutions at amino acid position 13 (G13D/C) were highly enriched in t-AML. In contrast, mutations at position 12 (G12D/V/C) were more frequently associated with t-MDS. (D) RASmut was associated with survival comparable to TP53-mutated t-MN, (E) t-MDS, and (F) t-AML. (G) Multivariable Cox proportional hazard analysis demonstrated that RASmut was associated with poor survival independent of allo-SCT, t-MN phenotype, and CKs. (H) NRASmut was associated with significantly poorer OS than KRASmut t-AML. Allo-SCT, allogeneic stem cell transplant; OS, overall survival.
RASmut is enriched in t-AML and is associated with poor prognosis. (A) t-AML was more prevalent in RASmut than RASwt t-MN. (B) t-AML was especially more prevalent in the presence of KMT2A-r. (C) Substitutions at amino acid position 13 (G13D/C) were highly enriched in t-AML. In contrast, mutations at position 12 (G12D/V/C) were more frequently associated with t-MDS. (D) RASmut was associated with survival comparable to TP53-mutated t-MN, (E) t-MDS, and (F) t-AML. (G) Multivariable Cox proportional hazard analysis demonstrated that RASmut was associated with poor survival independent of allo-SCT, t-MN phenotype, and CKs. (H) NRASmut was associated with significantly poorer OS than KRASmut t-AML. Allo-SCT, allogeneic stem cell transplant; OS, overall survival.
Finally, and somewhat surprisingly, RASmut was associated with significantly poor survival compared with RASwt (11.4 vs 24.1 months; P = .0001) (Figure 2D; supplemental Figure 1E). The median survival was equally poor when RASmut were subclonal (VAF < 20%) or clonal (≥20%), with single or multiple comutations (supplemental Figure 2A-B). Notably, the poor survival of RASmut compared with the RASwt subset was evident in both t-MDS (19.2 vs 25.3 months; P = .016) and t-AML (7.7 vs 18.3 months; P = .0066) (Figure 2E-F) with no difference depending on blast count (supplemental Figure 2C).
This dismal outcome of RASmut t-MN was comparable to TP53mut t-MN (11.4 vs 9.7 months; P = .18) (Figure 2D). Notably, the median survival of RASmut t-MDS was comparable to RASwt t-AML (19.2 vs 18.3 months) (supplemental Figure 3A). Because KMT2A-r is associated with poor outcomes but enriched in RASmut (Figure 1F), we evaluated survival without KMT2A-r. Even in the absence of KMT2A-r, RASmut was associated with poor survival compared with RASwt cases (12.9 vs 24.1 months; P = .0002) (supplemental Figure 3B) as shown previously in AML.8
Multivariable Cox proportional hazard analysis of TP53wt t-MN subset, after adjusting for covariates including allogeneic transplant, t-AML vs t-MDS, and complex karyotype, further substantiated RASmut as an independent factor associated with poor prognosis (Figure 2G). Survival of KRASmut and NRASmut t-MN (13 vs 10 months; P = .1) and t-MDS (19.4 vs 13 months; P = .67) were similar (supplemental Figure 3C-D). However, NRASmut t-AML was associated with poor survival compared with KRASmut t-AML (4.1 vs 11.4 months; P = .01) (Figure 2H). Similarly, there was a trend toward poor survival in KMT2A-r RASmut t-AML compared to KMT2A-r RASwt t-AML (9.2 vs 28.3 months; P = .27), though the sample size was limited.
Mutation in CBL and PTPN11 was also associated with poor survival (supplemental Figure 4A), with the median overall survival of RAS pathway–mutated t-MN poor (supplemental Figure 4B). Although RASmut is often associated with an aggressive phenotype, its impact varies across MN therapy. For example, in younger patients with AML treated with intensive chemotherapy, RASmut was not associated with adverse outcomes and not included in the adverse-risk AML in the European LeukemiaNet genetic risk classifications7 and showed increased sensitivity to cytarabine with reduced relapse rates.9 However, emerging data suggest poor prognosis of RASmut AML with nonintensive therapies,10,11 particularly hypomethylating agents plus venetoclax.12 In our retrospective cohort, the poor prognosis of RASmut t-MN managed with supportive care (4.3 vs 21.9 months; P = .001) could partly be overcome by treatment with intensive chemotherapy (12.9 vs 23.2 months; P = .09) or hypomethylating agents (14.8 vs 22.5 months; P = .38) (supplemental Figure 5A-C). In our cohort, only 3 TP53wtRASmut cases had venetoclax plus hypomethylating agent as first-line therapy. Validation of our findings is required in an independent large cohort.
This comprehensive study provides compelling evidence that RASmut signifies a key poor prognostic marker not previously described, penetrant for both t-MDS and t-AML and comparable to the effect conferred by TP53mut. Second, this study uncovers a unique association between KMT2A-r, hot spot RASmut, and t-MN phenotype that will be critical for designing future trials. For instance, some current RAS inhibitors are variant specific, such as KRAS G12C.13 Furthermore, a combination of menin (SNDX-5613 [revumenib]) and MEK inhibitors (VTP-50469 [selumetinib]) is showing promising results in patients with KMT2A-r and RAS pathway mutations, respectively.14
Acknowledgments: The authors thank their patients and their families. The authors gratefully acknowledge the support of the South Australia Cancer Research Biobank.
M.S. was supported by Bridget Kiely Clinician Career Development in Transplant Research and Kennedy Bacon Funds for Leukemia and Bone Marrow Transplant Research. D.K.H. is supported by the National Health and Medical Research Council (NHMRC)/Medical Research Future Fund Investigator grant (MRF1195517), Cancer Australia (APP2013617), and the Leukemia Foundation Australia. D.T. is supported by Commonwealth Serum Laboratories Centenary Fellowship, Medical Research Futures Fund, the NHMRC Ideas Grants, and Leukemia & Lymphoma Society with support from the Mike & Sofia Segal Foundation.
Contribution: C.H.K. performed the statistical analysis and wrote the article; D.T. provided critical inputs and edited the article; K.L. edited the article; M.M.K. edited the article and curated figures; C.N.H., H.S., and A.B. assisted with mutational profiling and editing; R.H., A.A.-K., H.A., A.N.S., A. Matin, N.G., A. Mangaonkar, K.B., K.H., P.G., W.J.H., and M.P. contributed patients and edited the article; M.S. contributed patients, provided critical inputs, and edited the article; D.K.H. designed the study, contributed patients, analyzed the data, and wrote the article; and all authors agreed to the final version of the article.
Conflict-of-interest disclosure: M.S. received research funding for the institution from Astellas, AbbVie, Celgene, Kura Oncology, and Marker Therapeutics. D.K.H. is a member of the board of directors or advisory committees of AbbVie and Novartis. N.G. served on the advisory board for Agios and Disc Medicine. P.G. served on an advisory board for AbbVie. A.N.S. received research funding for the institution from Kura Oncology and Chordia Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Devendra K. Hiwase, Royal Adelaide Hospital and South Australian Health and Medical Research Institute, North Terrace, Adelaide, SA 5000, Australia; email: devendra.hiwase@sa.gov.au; and Mithun Shah, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; email: shah.mithun@mayo.edu.
References
Author notes
C.H.K., A.A.-K., and D.T. contributed equally to this work.
M.S. and D.K.H. contributed equally to this work.
Original data are available on request from the corresponding author, Devendra K. Hiwase (devendra.hiwase@sa.gov.au) or Mithun Shah (shah.mithun@mayo.edu).
The full-text version of this article contains a data supplement.