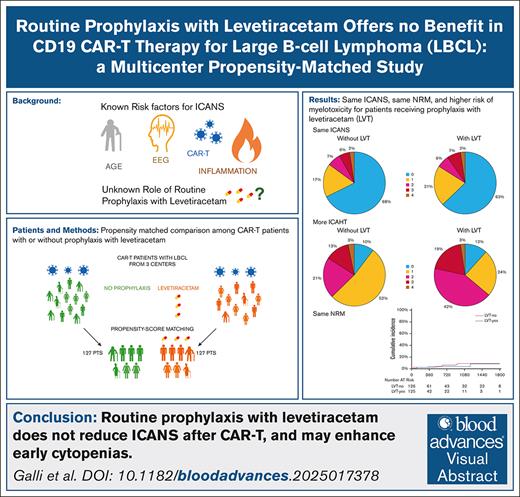
Key Points
Routine prophylaxis with LVT does not reduce the risk of any-grade or severe ICANS after CAR-T therapy for LBCL.
LVT may induce deeper, early ICAHT.
Visual Abstract
This study aims to evaluate the impact of levetiracetam (LVT) prophylaxis on the incidence and severity of immune effector cell–associated neurotoxicity syndrome (ICANS) in patients undergoing anti-CD19 chimeric antigen receptor T-cell (CAR-T) therapy for LBCL (large B-cell lymphoma). A propensity score–matched cohort of 254 patients was analyzed, comparing those receiving LVT prophylaxis (LTV-yes) with those not receiving it (LTV-no), in a 1:1 ratio. The results showed no significant difference in the occurrence of ICANS of any grade between the 2 groups (32.3% in LVT-no vs 37.1% in LVT-yes; P = .29), or in severe ICANS (grades 2-4, 15.1% vs 16.1% [P = .80]; grade 3-4, 7.9% vs 9.7% [P = .71]). The use of LVT was associated with a higher incidence of early immune effector cell–associated hematotoxicity (ICAHT), with grade 2 to 4 ICAHT occurring in 37.3% vs 63.9% (P < .001) of patients in the LVT-no and LVT-yes groups, respectively. Overall survival and progression-free survival did not differ significantly between the 2 groups (P = .337 and .670). Nonrelapse mortality rates were comparable (P = .77). These findings suggest that routine use of LVT as prophylaxis for ICANS in CAR-T therapy is not effective, and further research is needed to refine its role in selected populations or after ICANS treatment.
Introduction
Immune effector cell–associated neurotoxicity syndrome (ICANS) occurs in approximately one-third of patients receiving chimeric antigen receptor (CAR) T-cell (CAR-T) therapy. It typically develops after the onset of cytokine release syndrome (CRS), with a median onset of 5 to 8 days after infusion. Risk factors for ICANS may be related to the patient (eg, age, sex, baseline electroencephalogram [EEG] abnormalities), the disease (eg, primary mediastinal B-cell lymphoma, tumor burden), the CAR-T product (eg, brexucabtagene autoleucel, ciltacabtagene autoleucel), or biological parameters (eg, inflammation, modified endothelial activation and stress index [mEASIX] score).1-3
Several scientific societies and expert consensus panels have addressed the question of whether prophylactic antiepileptic drug administration should be recommended; however, their conclusions have not always been consistent. Levetiracetam (LVT) is an anticonvulsant agent of the pyrrolidine class, which modulates synaptic neurotransmission by regulating vesicle exocytosis and other membrane channels, particularly during pathological activations4; LVT is the preferred antiepileptic agent for prophylaxis due to its favorable safety profile, low incidence of side effects, and minimal pharmacological interactions. Moreover, LVT has limited impact on both cardiac and renal function. Among its reported adverse effects, trilineage cytopenias are relatively common.
Two main prophylactic strategies have been identified: one advocates for universal prophylaxis, administering LVT to all patients undergoing CAR-T therapy, whereas the other supports a risk-adapted approach, in which only patients deemed at high risk receive prophylaxis. Notably, some centers do not administer prophylaxis at all.
A summary of the main recommendations from scientific societies is provided in Table 1. The CARTOX consortium (United States, 2017)5 and the Italian expert panel (2023)11 recommend prophylactic administration of LVT to all patients undergoing CAR-T therapy. In contrast, guidelines from the American Society of Clinical Oncology (2021),6 as well as those from United Kingdom, French, and European (European Hematology Association, European Society for Blood and Marrow Transplantation [EBMT], the Joint Accreditation Committee of International Society for Cell and Gene Therapy and EBMT) working groups (2021-2022), advocate a risk-adapted approach, limiting LVT use to high-risk individuals.7-9 High-risk categories include patients receiving CAR-T products with a higher incidence of ICANS, central nervous system (CNS) involvement, previous seizures, abnormal EEGs, or neurological comorbidities. Finally, the 2023 Chinese consensus recommends LVT only after ICANS onset, not as prophylaxis.10
Regarding clinical trials, antiepileptic prophylaxis is generally neither mentioned nor recommended in most study protocols. However, notable exceptions include the ZUMA-1 trial (cohort 3) and the BELINDA trial. In ZUMA-1 (cohort 3), LVT 750 mg twice daily) was recommended for all patients, whereas in the BELINDA trial, it was advised specifically for those with a previous history of seizures.
In 2019, a survey conducted by the American Society for Blood and Marrow Transplantation investigated real-world practices regarding antiepileptic prophylaxis in the setting of CAR-T therapy. Among >50 participating institutions, 65% routinely prescribed LVT as standard prophylaxis; 15% limited its use to high-risk patients; and 20% did not use primary prophylaxis, instead opting for secondary prophylaxis after the development of ICANS.12
Similarly, a more recent survey conducted by the Cellular Therapy and Immunobiology Working Party of the EBMT collected data from 78 centers across 20 countries, exploring real-world experiences with antiepileptic prophylaxis in the context of CAR-T therapy. Among the responding centers, 68% reported the use of prophylactic LVT, with varying initiation strategies: 14% began treatment before lymphodepletion, 16% at the time of CAR-T infusion, 11% at the onset of CRS, and 32% at the onset of ICANS.13
In summary, although most expert guidelines advocate for prophylactic LVT in high-risk patients, its routine administration to all patients undergoing CAR-T therapy is not universally endorsed. Nonetheless, indiscriminate prophylaxis appears to be the predominant approach in real-world clinical practice, as reflected in recent surveys, with approximately two-thirds of centers adopting this strategy.
To date, however, no high-quality controlled studies have been published to definitively establish the efficacy of primary antiepileptic prophylaxis in preventing ICANS, underscoring the need for further prospective research.
The aim of our study was to conduct a large, international, matched case-control study with a standardized patient selection process, designed to compare the benefits and toxicities of LVT prophylaxis. Our findings are intended to provide robust evidence to inform future recommendations and updates to clinical guidelines.
Patients and methods
All consecutive patients treated with anti-CD19 CAR-Ts at 3 major hub centers (Hôpital Saint-Louis, Paris, France; Fondazione Policlinico Gemelli IRCCS, Rome, Italy; and Policlinico Umberto I/University La Sapienza, Rome, Italy) were considered for inclusion in the study. The inclusion criteria were as follows: age of >18 years; treatment with anti-CD19 CAR-T therapy between 2019 and 2024, with at least 3 months of follow-up; and diagnosis of large B-cell lymphoma (LBCL). Patients were excluded if they were not treated with commercially available CAR-T products or had a history of, or current, CNS involvement due to lymphoma. Data were collected systematically from electronic medical records.
Local practices varied between the centers: at Saint-Louis Hospital, Assistance Publique-Hôpitaux de Paris, no prophylactic LVT was administered (group: LVT-no), whereas all patients at the 2 centers in Rome, Italy, received LVT prophylaxis (500 mg twice daily, starting either at lymphodepletion or infusion, tapered over 6 to 8 weeks after discharge until discontinuation; group: LVT-yes). Demographic, biological, and clinical variables were analyzed for both groups.
Propensity score matching was used to adjust for differences between the 2 groups (LVT-no vs LVT-yes); a 1:1 ratio and the nearest-neighbor method were applied without replacement and with a caliper of 0.5 standard deviation of the propensity score distribution.
The primary outcomes included the occurrence of ICANS of any grade and severe ICANS. Secondary outcomes included the evaluation and severity of CRS, early immune effector cell–associated hematotoxicity (ICAHT), and the use of treatments for inflammatory toxicities (including tocilizumab, dexamethasone, and prednisone). Additional outcomes assessed included intensive care unit (ICU) admission, progression-free survival, and overall survival.
CRS, ICANS, and ICAHT were graded and treated according to current international recommendations.7,14
Statistical analysis was performed using IBM SPSS statistics version 27.0 and R version 4.1.2, with the MatchIt library for propensity score matching.
All participants provided informed consent for the anonymized use of their data. The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee (identity 4879 Prot 0020777/22; amendment, September 2024).
Results
A total of 528 patients treated with anti-CD19 CAR-Ts were screened across the 3 participating centers. A total of 96 patients were excluded due to treatment for conditions other than LBCL or receipt of noncommercial CAR-T products. Additionally, 33 patients were excluded due to a history of, or ongoing, CNS involvement (27 in the LVT-no group, and 6 in the LVT-yes group).
The final eligible cohort consisted of 393 patients (comprising 250 LVT-no and 143 LVT-yes patients). Demographic, disease-related, and treatment-related characteristics were analyzed and compared between the 2 groups (supplemental Table 1).
The propensity score was calculated incorporating the following variables, which were unbalanced at baseline: Eastern Cooperative Oncology Group (ECOG) performance status (PS; 2 vs 0-1), type of CAR-T product (axicabtagene ciloleucel [axi-cel] vs tisagenlecleucel), age (in years), CAR-HEMATOTOX score (0-1 vs ≥2), and response to Positron Emission Tomography (PET) (complete response/partial response vs stable disease/progressive disease). Notably, although these variables were already balanced at baseline, patients were matched 1:1 for CAR-T products to minimize potential bias. The final 1:1 matched population consisted of 254 patients (127 in the LVT-no group and 127 in the LVT-yes group), with major variables balanced between the 2 groups (Table 2; Figure 1A).
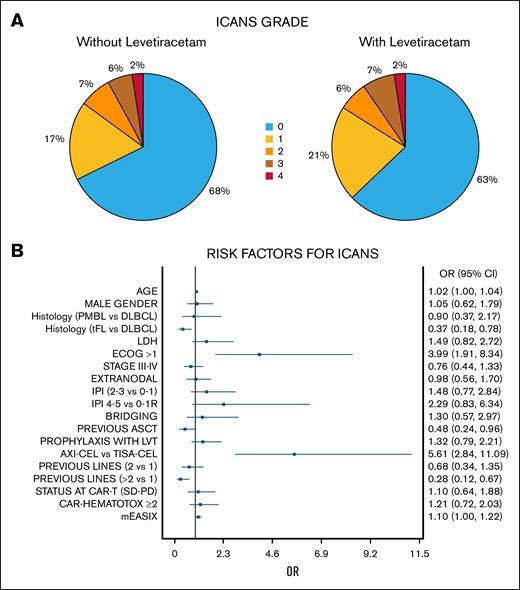
Risk factors for ICANS. (A) Incidence and grading of ICANS according to the different prophylactic strategies (with or without LVT). (B) Risk factors for ICANS of any grade in univariate analysis (forest plot). ASCT, autologous hematopoietic stem cell transplantation; CR, complete response; DLBCL, diffuse large B-cell lymphoma; IPI, International Prognostic Index; ECOG, Eastern Cooperative Oncology Group performance status scale; PD, progressive disease; PMBL, primary mediastinal BCL; PR, partial response; SD, stable disease; tFL, transformed follicular lymphoma; tisa-cel, tisagenlecleucel.
Risk factors for ICANS. (A) Incidence and grading of ICANS according to the different prophylactic strategies (with or without LVT). (B) Risk factors for ICANS of any grade in univariate analysis (forest plot). ASCT, autologous hematopoietic stem cell transplantation; CR, complete response; DLBCL, diffuse large B-cell lymphoma; IPI, International Prognostic Index; ECOG, Eastern Cooperative Oncology Group performance status scale; PD, progressive disease; PMBL, primary mediastinal BCL; PR, partial response; SD, stable disease; tFL, transformed follicular lymphoma; tisa-cel, tisagenlecleucel.
ICANS and prophylaxis with LVT
ICANS occurred in 87 patients (35%), with grade 2 to 4 events observed in 15% of cases and grade 3 to 4 events in 8.7%. No significant difference was found in the occurrence of any-grade ICANS when comparing the LVT-no group (32.3%) and the LVT-yes group (37.1%; odds ratio [OR], 1.32; 95% confidence interval [CI], 0.79-2.21; P = .29; Figure 1A).
Risk factors for developing any-grade ICANS were diffuse LBCL vs transformed follicular lymphoma, an ECOG PS score of >1, receiving axi-cel, receiving CAR-T therapy in earlier lines with no previous autologous stem cells transplant, and a higher baseline mEASIX. Notably, in multivariate analysis, only axi-cel and higher ECOG PS score remained significant (Figure 1B; supplemental Materials).
We then moved to consider risk factors for higher ICANS, which was similar in LVT-no/-yes groups when considering grades 2 to 4 (15.1% vs 16.1%; P = .80), grades 3 to 4 (7.9% vs 9.7%; P = .71), or grade 4 (2.4% in both groups). Among patients treated with tisagenlecleucel, only 2 developed grade 2 to 4 ICANS. Therefore, we conducted the risk analysis on treatment-requiring ICANS (grade 0-1 vs 2-4) only in the axi-cel patients.
Among the 166 axi-cel patients, risk factors for grade 2 to 4 ICANS were ECOG PS score of >1, International Prognostic Index score of >1, and higher baseline mEASIX. In multivariate analysis, ECOG PS score of >1 (OR, 5.32; 95% CI, 1.43-19.78) and mEASIX (OR, 1.30; 95% CI, 1.07-1-58), remained the only prognostic factors (supplemental Materials).
CRS and intensive treatments
The incidence of CRS was 88.5%, and it was similar between the 2 groups, with a slightly higher incidence observed in patients receiving LVT (85% vs 92.1%; P = .08). This difference was mainly due to a higher incidence of treatment-requiring grade 2 to 4 CRS in the LVT group (26.8% vs 57.9%; P < .001). However, the occurrence of severe CRS (grade 3-4) was similar regardless of LVT prophylaxis (7.9% vs 9.7%; P = .20).
Tocilizumab was administered at least once in 113 patients (44.8%), with a significantly higher rate of administration in the LVT-yes group (20.5% vs 69.6%; P < .001). Dexamethasone was administered to 73 patients (28.9%), more frequently in those receiving LVT as prophylaxis (18.9% vs 39.2%; P < .001). Regarding the use of prednisone boluses, typically reserved for refractory or worsening ICANS, we observed more steroid use in the LVT group (3.2% vs 8.1%; P = .031).
Overall, 54 patients (21.2%) were transferred to the ICU during the acute phase after CAR-T infusion, mainly due to CRS or ICANS. Patients not receiving LVT had a significantly higher ICU admission rate (29.9% vs 12.6%; P < .001).
Myelotoxicity and ICAHT and prophylaxis with LVT
Early ICAHT was defined based on the depth and time to recovery of neutropenia from CAR-T infusion to day 30 after infusion. Among the 245 evaluable patients, 218 (88.9%) experienced early ICAHT. Of these, 123 patients (50%) developed grade 2 to 4 early ICAHT, with 18.7% of patients experiencing grade 3 to 4 ICAHT. Grade 2 to 4 early ICAHT was more frequent in patients receiving LVT prophylaxis, with nearly double the incidence in the LVT-yes group (37.3% vs 63.9%; OR, 2.97; 95% CI, 1.77-5.00). In multivariate analysis, risk factors for grade 2 to 4 ICAHT were receiving prophylaxis LVT (OR, 8.27; 95% CI, 3.49-19.61), receiving axi-cel (OR, 2.48; 95% CI, 1.05-5.87), and a baseline CAR-HEMATOTOX score of >1 (OR, 2.69; 95% CI, 1.16-6.24). Univariate and multivariate analysis are detailed in the supplemental Materials.
Outcomes of CAR-Ts according to prophylaxis with LVT
Overall survival at 1 and 2 years was 70.5% and 60.7%, respectively, and was not different according to LVT prophylaxis (P = .337).
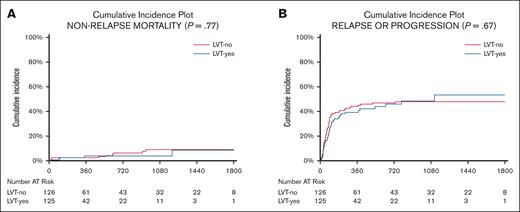
The cumulative incidence of relapse was 42% and 46% at 1 and 2 years, respectively, with no difference between LVT-no and LVT-yes groups (the Gray test, P = .67; Figure 2).
Non-relapse mortality and relapse. Cumulative incidence of NRM (A) and relapse (B) after CAR-T therapy by antiepileptic prophylaxis with LVT. The type of strategy does not influence NRM or relapse rate.
Non-relapse mortality and relapse. Cumulative incidence of NRM (A) and relapse (B) after CAR-T therapy by antiepileptic prophylaxis with LVT. The type of strategy does not influence NRM or relapse rate.
Nonrelapse mortality (NRM) was 2.9% and 5.7% at 1 and 2 years after CAR-Ts, respectively, with no significant differences for LVT-no and LVT-yes patients (Gray test, P = .77; Figure 2; supplemental Materials).
The causes of NRM were infections (4% of patients), CRS/ICANS (1.2%), or miscellaneous (3%, mainly secondary cancers or cardiovascular events).
Discussion
The baseline characteristics of the starting populations differed between patients treated with CAR-Ts in Rome and those treated in Paris, with patients in the 2 Italian centers having a lower median age (56 vs 63 years) and a higher proportion of patients with ECOG PS score of >1 at infusion (17% vs 8%). CAR-T therapy was approved earlier in France than in Italy, both for third-line and second-line treatments, and without age restrictions. These differences in age and ECOG PS may reflect discrepancies in approval criteria, because patients treated in later lines may more likely experience a rapid decline in ECOG PS between inclusion and infusion. However, major discrepancies were corrected through propensity score matching, ensuring comparability between the groups.
Once the LVT-no and LVT-yes populations were balanced, we found that LVT prophylaxis was ineffective in reducing the incidence of ICANS, whether considering any-grade ICANS or more severe, treatment-requiring events.
The pathogenesis of ICANS is increasingly being explored, with a focus on encephalopathy, damage to the blood-brain barrier (BBB), and involvement of both neuronal and glial cells.15,16 Overall, peripheral blood and cerebral spinal fluid markers point toward neuroinflammation as a major driver of ICANS, with BBB disruption playing a central role in ICANS. Endothelial activation and tight junction breakdown, driven by cytokine release and off-tumor targeting of CD19+ pericytes, increase BBB permeability.17 This allows immune cells to infiltrate and causes neuroinflammation. Neuropathological studies have shown multifocal white matter injury, oligodendrocyte loss, and demyelination in severe ICANS cases. Microglial activation and CD8+ T-cell infiltration further contribute to CNS pathology.18,19 Additionally, elevated pretreatment neurofilament light chain levels suggest underlying neuronal or microvascular weakness that may make a person more prone to ICANS after CAR-T therapy.20,21 In 2022, a large study involving 135 patients monitored with EEG was reported, in which daily ICANS was compared with EEG findings. The investigators developed a visual EEG method that showed a strong association with clinical ICANS (referred to as VE-ICANS)22; in that study, the most predictive alterations in EEG were slowing of delta waves, low voltages, and/or θ slowing, which are not considered typical of epileptic patterns. Other EEG patterns more typical of epilepsy (generalized periodic discharges, and generalized rhythmic delta activity), were rarely documented (in 8.8% and 3.1% of patients with grade ≥2 ICANS, respectively). Notably, only 6.4% of patients with grade ≥2 ICANS (or 3.2% of the total patient population) developed seizures.22
Similar findings, with a prevalence of nonepileptic EEG abnormalities during ICANS, mainly slowing of waves, and a very low incidence of seizures, have also been reported by other researchers.1,23 This observation holds true even in high-risk populations, such as CAR-T recipients treated for mantle cell lymphoma24 or CNS/ocular lymphoma (with epileptic EEG findings in 8% of total patients and 18% of patients with ICANS).25 Notably, around one-third of patients showed EEG abnormalities at baseline, which may confer a higher risk of developing ICANS.1
Overall, current data suggest that in most cases, ICANS is not associated with epileptic findings or seizures but is more frequently linked to EEG signs of slowing and reduced voltages. In this context, it is not surprising that an antiseizure medication such as LVT may not be effective as prophylaxis for ICANS. Our findings provide clinical evidence that LVT prophylaxis did not reduce the incidence or severity of ICANS in a real-world, multicenter population of patients treated with CD19-directed CAR-T therapy, when compared with a homogenized control population. The risk of poststroke epilepsy among adults has been considered relatively low in literature.26 In our opinion, prophylactic LVT may be a reasonable strategy to mitigate neurotoxicity risk independently from a preexisting antiepileptic therapy only in selected patients with specific risk factors, namely CNS disease involvement, structural brain abnormalities, epilepsy, elevated neuronal injury, previous cerebrovascular events with cortical ischemic or hemorrhagic lesions, CRS-driven neuroinflammation, older age, high-risk mEASIX, or high-risk CAR-T products. Several studies have investigated alternative strategies to reduce the incidence and severity of ICANS, reporting encouraging results with a lower overall rate of neurotoxicity than standard CAR-T recipients. These approaches target specific inflammatory pathways and include the prophylactic use of anakinra (anti–interleukin-1 receptor),27 defibrotide,28 siltuximab (anti–interleukin-6),29 or early corticosteroids.30 Although these strategies appear promising and seem to reduce the incidence and severity of ICANS, the available evidence is limited to nonrandomized trials, and their actual clinical benefit, as well as the potential for combining different interventions, remain to be fully elucidated.
The use of LVT may not be without undesired side effects. LVT has been associated with behavioral changes, such as depressive symptoms or suicidal ideation. Because patients treated with CAR-T therapy are often exposed to triggering factors, such as prolonged hospitalization or corticosteroid treatment, the indiscriminate administration of LVT may contribute to neuropsychiatric symptoms, which are reported in up to 33% of CAR-T recipients overall (anxiety, 18%; depression, 12%).31
Antiepileptic drugs such as LVT can be associated with lower peripheral blood counts. Although this was not the primary end point, we observed that the indiscriminate administration of LVT resulted in worsened early ICAHT in the LVT-yes group, based on the severity and duration of neutropenia, as defined by current guidelines.14 Given that the drivers of early infections are primarily bacterial or fungal agents,32 a cautious approach to LVT prophylaxis may be warranted.
Late ICAHT were not considered in this analysis, because standard LVT deescalation typically leads to the withdrawal of prophylaxis by week 8 to 10 after CAR-T infusion. As a note, patients in the French cohort were also exposed to granulocyte colony-stimulating factor (G-CSF). However, prophylactic G-CSF has been shown not to be associated with reduced or shortened neutropenia,33,34 and we did not consider G-CSF exposure as a confounding factor.
We also observed that patients receiving LVT prophylaxis seemed to experience more CRS and required higher doses of tocilizumab and steroids yet were less likely to be hospitalized in an ICU. These observations should be considered within the context of different ICU management strategies: Italian centers are more likely to perform in-service management of acute patients, possibly leading to higher administration of specific anti-inflammatory drugs, whereas French organizations tend to provide early ICU admission (ICU admission from registry data: 11% in Italy vs 27% in France).35,36 In any case, early administration of tocilizumab or steroids seems not to reduce efficacy, as observed in cohorts 4 and 6 of the ZUMA-1 study.30 Similarly, in our experience, the LVT strategy did not affect the efficacy or NRM of CAR-T treatment.
We acknowledge several limitations of this study, including the involvement of only 3 centers and the use of center-specific interventional strategies, which may have contributed to differences between cohorts and in toxicity management. However, the potential impact of center-related variability was mitigated through propensity score matching.
Conclusions
This study is, to our knowledge, the first to investigate the utility and toxicity of LVT prophylaxis in CAR-T therapy for LBCL, comparing 2 propensity-matched patient populations. Our findings show that indiscriminate LVT prophylaxis does not reduce the incidence of any-grade or grade 2 to 4 ICANS. Additionally, the administration of LVT may be linked to increased myelosuppression in the early weeks after CAR-T infusion. Based on these results, we conclude that antiepileptic prophylaxis should not be routinely prescribed in patients with LBCL. However, its role may still be considered in selected populations or once ICANS has already developed. With regard to CAR-T therapies administered in patients with other cancers, such as acute leukemia, multiple myeloma, or mantle cell lymphoma, or with autoimmune diseases, data on effectiveness of LVT are still lacking and further studies are required.
Acknowledgments
The authors acknowledge the research funding support of the Centro di Ricerca sulle Cellule Staminali Emopoietiche e le Terapie Cellulari, Università Cattolica del Sacro Cuore, Roma, Italy. The authors thank Diana Giannarelli for the developing the propensity score matching and her support in statistics. E.G. received a research grant from Gruppo Italiano Trapianto Midollo Osseo.
Authorship
Contribution: E.G., A.M., and R.D.B. conceived and designed the study; I.P., A.C., C.T., A.D.R., and E.G. collected data; E.G. analyzed data and drafted the manuscript, M.V., A.D.R., E.G., S.S., C.B., I.D.B., C.C., P.C., F.S., and M.M. managed patients; and S.H., S.S., C.T., and A.M. critically revised the manuscript.
Conflict-of-interest disclosure: E.G. received travel grants from, and/or participated in speakers bureau for, Novartis, Bristol Myers Squibb (BMS), and Kite/Gilead. C.B. has received advisory fees from Johnson and Johnson and BMS. R.D.B. reports honoraria for board membership and conference attendance and travel grants from Roche, Kite/Gilead, Novartis, and BMS. C.T. reports honoraria for board membership and conference attendance and travel grants from Roche, Kite/Gilead, Novartis, and BMS. The remaining authors declare no competing financial interests.
Correspondence: Eugenio Galli, UOC Ematologia e Trapianto di Cellule Staminali Emopoietiche, Dipartimento di Scienze di Laboratorio ed Ematologiche, Fondazione Policlinico Universitario A. Gemelli IRCCS, Largo A. Gemelli 8, 00184 Rome, Italy; email: eugenio.galli@policlinicogemelli.it.
References
Author notes
S.S. and C.T. contributed equally to this study.
Data are available on request from the corresponding author, Eugenio Galli (eugenio.galli@policlinicogemelli.it).
The full-text version of this article contains a data supplement.