Key Points
Specific immune phenotypes were predictive of long-term survival for MM patients undergoing transplantation.
MRD status and use of maintenance therapy were associated with unique immune profiles predictive of outcome.
Abstract
Multiple therapeutic options exist for multiple myeloma (MM), including autologous hematopoietic stem cell transplantation (AHSCT). Measurement of minimal residual disease (MRD) and immune reconstitution is rapidly becoming an integral part of the care of MM patients. We investigated comprehensive immune profiling (IP) associated with progression-free survival (PFS) and overall survival (OS). From August 2007 to January 2014, 101 consecutive MM patients underwent peripheral blood IP and marrow MRD testing before and approximately 100 days after AHSCT. Higher pre-AHSCT CD19+ B-cell counts correlated with improved 2-year PFS (83% [highest quartile] vs 53% [lowest quartile]; P = .01) and OS (93% [highest quartile] vs 63% [lowest quartile]; P = .0003). This effect was seen primarily in patients with MRD-positive marrow tests. Higher γδ T-cell counts post-AHSCT correlated with improved 2-year PFS (65% [highest quartile] vs 45% [lowest quartile]; P = .02) and OS (89% [highest quartile] vs 65% [lowest quartile]; P = .01). Higher CD4+ central memory (CM) cell counts post-AHSCT were associated with improved 2-year OS (95% [upper quartile] vs 47% [lowest quartile]; P = .0003) but not PFS. The higher γδ T-cell and CD4+ CM-cell count associations were primarily observed in MRD-negative patients post-AHSCT and in patients not receiving maintenance therapy. This proof-of-concept study demonstrates that IP before and after AHSCT can be of complementary prognostic value for depth of response. Maintenance therapy seems to overcome negative IP. IP and MRD should be measured in clinical trials of maintenance therapy with novel agents post-AHSCT for MM to confirm their utility for prognosis and management.
Introduction
Multiple myeloma (MM) is an incurable disease that is often treated with induction therapy and autologous hematopoietic stem cell transplantation (AHSCT), followed by consolidation and/or maintenance therapy.1 The goal of initial treatment is to induce a response and prolong the time to disease progression and the need for re-treatment. Because most MM patients will eventually experience disease progression, new strategies must be developed to prolong response and delay time to disease progression. Identifying those patients who have prolonged responses to initial therapy and their clinical characteristics may facilitate the understanding of MM biology and the development of novel strategies to prolong control of this disease.
The immune system is an area of interest for understanding control of MM and other malignancies. Immune cell populations have been profiled before, during, or after therapy to identify immune profiles that are associated with improved outcome. Diagnostic factors associated with improved progression-free survival (PFS) and/or overall survival (OS) include elevated peripheral blood (PB) CD4+ count,2 PB absolute lymphocyte count (ALC) >3000 cells per μL,3 PB ALC ≥1400 cells per μL,4 higher PB CD4+ and CD19+ cell count,5,6 and higher PB total CD4+, CD4+ naive, and CD19+ cell counts.7 After initial therapy, improved PFS and/or OS were seen with PB ALC >800 cells per μL8 and higher PB CD4+, CD4+ naive, and CD19+ cell counts.7 Day +15 post-AHSCT ALC recovery (PB >500 lymphocytes per μL) correlates with improved PFS and OS.9 A higher autograft ALC infused is associated with early ALC recovery, improved OS, and improved time to disease progression.10 Another study examined T cells (CD3+/CD8+ or CD3+/CD4+), natural killer (NK) cells (CD3–/CD56+), and B cells (CD19+) and found that increased PB NK cells on day 30 post-AHSCT were associated with improved PFS but not OS.11 When comparing 20 symptomatic MM patients who had survived >10 years from diagnosis with age-matched MM patients who were <10 years from diagnosis, the patients who had survived >10 years had increased proliferative cytotoxic T-cell clones, decreased regulatory T cells (Tregs), and increased proinflammatory Th17 cells.12
Earlier studies did not examine detailed PB subsets before and after AHSCT. We hypothesized that a comprehensive immune profiling (IP) of PB T-cell, NK-cell, B-cell, and dendritic cell (DC) subpopulations would identify immune cell phenotypes associated with prolonged PFS and OS after AHSCT. Examining IP after AHSCT would facilitate our understanding of immune reconstitution post-AHSCT and its correlation with outcome. Developing strategies to enhance selected populations after AHSCT could improve outcomes for MM patients.
Methods
Patient population
This is a retrospective cohort study of 101 consecutive adult (older than age 18 years) MM patients who received a planned single AHSCT at Roswell Park Cancer Institute from August 2007 to January 2014. This study was reviewed and approved by the RPCI institutional review board and was conducted in accordance with the Declaration of Helsinki. Grafts consisted of PB hematopoietic stem cells mobilized with granulocyte colony-stimulating factor (G-CSF) (n = 23), G-CSF plus plerixafor (n = 77), or G-CSF plus plerixafor followed by cyclophosphamide plus G-CSF remobilization (n = 1). Patients underwent apheresis for a minimum of 2 days, regardless of cell dose collected on the first day, to reach a target CD34+ cell dose of 4 to 8 × 106/kg. Extra cells were cryopreserved for potential future use. The minimum CD34+ cell dose infused was 2 × 106/kg. All patients received a high-dose regimen of melphalan in a single dose of 200 mg/m2 (n = 85) or, if comorbidities were present, 120 mg/m2 (n = 16).
Immune profiling panels
Comprehensive IP was performed on fresh PB samples at a median of 17 days before AHSCT (n = 101) and at a median of 99 days (n = 80) after AHSCT. Table 1 defines the IP panels. Eighty patients had an immunophenotyping assessment for abnormal plasma cells (minimal residual disease [MRD]) performed on bone marrow samples around day +100 post-AHSCT, regardless of disease response (complete response [CR] vs not in CR). Briefly, by using standard flow cytometry immunophenotyping techniques, 3 tubes of 4 fluorochromes per tube were tested for the expression of CD38, CD138, CD45, CD56, CD19, CD117, CD28, and cytoplasmic κ and λ; the majority of samples (>75%) had 250 000 to 2 million events acquired.
Immunophenotypingpanels
| Immune cell phenotype . | Epitopes tested . |
|---|---|
| T cells | |
| CD3+ total | CD3+ |
| CD4+ total | CD4+ |
| CD8+ total | CD8+ |
| T-cell subsets | |
| CD3+CD4+ | CD3+CD4+ |
| CD4+CD8+ double positive | CD3+CD4+CD8+ |
| CD4–CD8– double negative | CD3+CD4–CD8– |
| CD4+ naive | CD3+CD4+CD45RA+CD45RO–CD27+ |
| CD4+ central memory | CD3+CD4+CD45RA–CD45RO+CD27+ |
| CD4+ effector memory | CD3+CD4+CD45RA–CD45RO+CD27– |
| CD4+ effector | CD3+CD4+CD45RA+CD45RO–CD27– |
| CD4+ recent thymic emigrants | CD3+CD4+CD45RA+CD31+CD45RO– |
| CD4– recent thymic emigrants | CD3+CD4–CD45RA+CD31+CD45RO– |
| Tregs bright (CD4+) | CD3+CD4+CD25 (br) |
| Tregs dim (CD4+) | CD3+CD4+CD25+CD127 (d) |
| Tregs DR+ (CD4+) | CD3+CD4+CD25+CD127 (d)HLADr+ |
| Tregs dim (CD4–) | CD3+CD4–CD25+CD127 (d) |
| Tregs DR– (CD4–) | CD3+CD4–CD25+CD127–HLADr– |
| CD3+CD8+ | CD3+CD8+ |
| CD8+ naive | CD3+CD8+CD45RA+CD45RO–CD27+ |
| CD8+ primed (effector) | CD3+CD8+CD45RA+CD45RO–CD27– |
| CD8+ CM | CD3+CD8+CD45RA–CD45RO+CD27+ |
| CD8+ effector memory | CD3+CD8+CD45RA–CD45RO+CD27– |
| γδ T cells | CD3+ γδ+ |
| B cells | |
| CD19+ total | CD19+ |
| CD20+ total | CD20+ |
| CD19+ naive | CD19+CD27– |
| Naive (Bm 1) | CD19+CD38–IgD+CD27–CD20+/− |
| Naive (Bm 2) | CD19+CD38+IgD+CD27–CD20+/− |
| CD19+ memory | CD19+CD27+ |
| Memory pre-switch | CD19+CD38+/−IgD+CD27+CD20+/− |
| Memory post-switch | CD19+CD38+/−IgD–CD27+CD20+/− |
| NK cells | |
| Total NK cells | CD56+CD16+ |
| CD16/56+ γδ+/− CD8− CD3+ CD4+ | |
| CD16/56+ γδ+/− CD8+ CD3+ CD4− | |
| DCs | |
| Myeloid CD11c+ | HLADr+CD123+/− Dump– CD11c+ |
| Plasmacytoid CD123+ | HLADr+ CD123+ Dump– CD11c– |
| Immune cell phenotype . | Epitopes tested . |
|---|---|
| T cells | |
| CD3+ total | CD3+ |
| CD4+ total | CD4+ |
| CD8+ total | CD8+ |
| T-cell subsets | |
| CD3+CD4+ | CD3+CD4+ |
| CD4+CD8+ double positive | CD3+CD4+CD8+ |
| CD4–CD8– double negative | CD3+CD4–CD8– |
| CD4+ naive | CD3+CD4+CD45RA+CD45RO–CD27+ |
| CD4+ central memory | CD3+CD4+CD45RA–CD45RO+CD27+ |
| CD4+ effector memory | CD3+CD4+CD45RA–CD45RO+CD27– |
| CD4+ effector | CD3+CD4+CD45RA+CD45RO–CD27– |
| CD4+ recent thymic emigrants | CD3+CD4+CD45RA+CD31+CD45RO– |
| CD4– recent thymic emigrants | CD3+CD4–CD45RA+CD31+CD45RO– |
| Tregs bright (CD4+) | CD3+CD4+CD25 (br) |
| Tregs dim (CD4+) | CD3+CD4+CD25+CD127 (d) |
| Tregs DR+ (CD4+) | CD3+CD4+CD25+CD127 (d)HLADr+ |
| Tregs dim (CD4–) | CD3+CD4–CD25+CD127 (d) |
| Tregs DR– (CD4–) | CD3+CD4–CD25+CD127–HLADr– |
| CD3+CD8+ | CD3+CD8+ |
| CD8+ naive | CD3+CD8+CD45RA+CD45RO–CD27+ |
| CD8+ primed (effector) | CD3+CD8+CD45RA+CD45RO–CD27– |
| CD8+ CM | CD3+CD8+CD45RA–CD45RO+CD27+ |
| CD8+ effector memory | CD3+CD8+CD45RA–CD45RO+CD27– |
| γδ T cells | CD3+ γδ+ |
| B cells | |
| CD19+ total | CD19+ |
| CD20+ total | CD20+ |
| CD19+ naive | CD19+CD27– |
| Naive (Bm 1) | CD19+CD38–IgD+CD27–CD20+/− |
| Naive (Bm 2) | CD19+CD38+IgD+CD27–CD20+/− |
| CD19+ memory | CD19+CD27+ |
| Memory pre-switch | CD19+CD38+/−IgD+CD27+CD20+/− |
| Memory post-switch | CD19+CD38+/−IgD–CD27+CD20+/− |
| NK cells | |
| Total NK cells | CD56+CD16+ |
| CD16/56+ γδ+/− CD8− CD3+ CD4+ | |
| CD16/56+ γδ+/− CD8+ CD3+ CD4− | |
| DCs | |
| Myeloid CD11c+ | HLADr+CD123+/− Dump– CD11c+ |
| Plasmacytoid CD123+ | HLADr+ CD123+ Dump– CD11c– |
Bm, B mature; br, bright; d, dim; DR, antigen D–related.
Statistical analysis
Univariable analyses of patient and clinical characteristics were conducted by using the Pearson χ2 test or Fisher’s exact text for categorical variables and Wilcoxon 2-sample test or Kruskal-Wallis 1-way test for continuous variables, as appropriate. OS was calculated from date of AHSCT to the date of death as a result of any cause; patients were censored if they were alive at last follow-up. PFS time was calculated from date of AHSCT to date of first disease progression/relapse or death as a result of any cause; patients were censored at last follow-up in the absence of disease progression/relapse. Cell marker categories were defined as >25%, 25% to 75%, and <75% of the absolute cell count for each cell type. The middle group was included if it was significantly differentiated from the high and low groups. If the middle group was not significantly differentiated, it was combined with the upper quartile. Kaplan-Meier survival curves were constructed; significance was tested by using the log-rank statistic. The following patient characteristics were tested for association with PFS and OS and were not statistically significant: Karnofsky performance status, race, plerixafor use, CD34+ cell dose infused, number of pre-AHSCT lines of therapy, remission status at AHSCT, ALC recovery by day +15, any infection between day 0 and day +100 post-AHSCT, lenalidomide-containing regimen pre-AHSCT, bortezomib-containing regimen pre-AHSCT, thalidomide-containing regimen pre-AHSCT, body mass index, AHSCT regimen, bisphosphonates pre-AHSCT, and 25-hydroxy vitamin D level pre-AHSCT. Age at AHSCT was the only patient-, disease-, or treatment-related variable that was associated with OS or PFS in the univariable analysis. Therefore, multivariable Cox proportional hazards models were used to estimate the hazard ratio of OS or PFS, adjusting only for age at AHSCT, which was significant in the univariable analysis. Multiple comparisons were considered in the setting of a small sample size, with P < .01 set as the threshold for statistical significance. All statistical analyses were performed by using SAS V9.4 (SAS Institute, Cary, NC).
Results
The characteristics of 101 patients undergoing a single AHSCT are provided in Table 2. The following responses were seen pre- and post-AHSCT: for patients in CR (n = 16) pre-AHSCT, all remained in CR at day 100 post-AHSCT. For patients with a very good partial response (VGPR; n = 39) pre-AHSCT, 20 (51%) attained a CR, 11 (28%) remained in VGPR, and 8 (21%) were less than VGPR but did not fulfill the criteria for progressive disease. For patients with less than a VGPR (or partial response or better) (n = 43) pre-AHSCT, 13 (30%) attained a CR, 8 (19%) attained a VGPR, and 22 (51%) remained at less than VGPR but did not fulfill the criteria for progressive disease. All patients survived to day +100.
Patient characteristics
| Characteristic . | No. . | % . | Median . | Range . |
|---|---|---|---|---|
| Total no. of patients | 101 | |||
| Age, years | 60 | 28-75 | ||
| Female sex | 55 | 54 | ||
| Myeloma subtype | ||||
| Serum | ||||
| IgG κ | 40 | 40 | ||
| IgG λ | 16 | 16 | ||
| IgA κ | 14 | 14 | ||
| IgA λ | 12 | 12 | ||
| IgM κ | 2 | 2 | ||
| IgD κ | 2 | 2 | ||
| IgD λ | 1 | 1 | ||
| Light chain (urine) | ||||
| κ only | 6 | 6 | ||
| λ only | 7 | 7 | ||
| Biclonal (IgG, IgA, κ) | 1 | 1 | ||
| Nonsecretory | 0 | 0 | ||
| ISS at diagnosis | ||||
| I | 34 | 34 | ||
| II | 29 | 29 | ||
| III | 27 | 27 | ||
| Missing | 11 | 11 | ||
| Durie-Salmon staging at diagnosis | ||||
| I | 20 | 20 | ||
| II | 14 | 14 | ||
| III | 67 | 66 | ||
| Lines of therapy pre-AHSCT | ||||
| 1 | 44 | 44 | ||
| 2 | 35 | 35 | ||
| 3 | 15 | 15 | ||
| >3 | 7 | 7 | ||
| Pre-AHSCT treatment | ||||
| PI/IMiD | 71 | 71 | ||
| PI | 16 | 16 | ||
| IMiD | 12 | 12 | ||
| PI/IMiD/other | 1 | 1 | ||
| Other | 1 | 1 | ||
| Mobilization regimen | ||||
| G-CSF | 23 | 23 | ||
| G-CSF + plerixafor | 77 | 76 | ||
| G-CSF + plerixafor, then cyclophosphamide + G-CSF remobilization | 1 | 1 | ||
| KPS pre-AHSCT | ||||
| 70 | 20 | 19 | ||
| 80 | 52 | 51 | ||
| 90 | 29 | 29 | ||
| Time from diagnosis to AHSCT, months | 10.2 | 0.5-62.1 | ||
| CD34 dose infused, 106/kg | 3.12 | 1.7-9.93 | ||
| TNC dose infused, 108/kg | 4.83 | 1.8-44.14 |
| Characteristic . | No. . | % . | Median . | Range . |
|---|---|---|---|---|
| Total no. of patients | 101 | |||
| Age, years | 60 | 28-75 | ||
| Female sex | 55 | 54 | ||
| Myeloma subtype | ||||
| Serum | ||||
| IgG κ | 40 | 40 | ||
| IgG λ | 16 | 16 | ||
| IgA κ | 14 | 14 | ||
| IgA λ | 12 | 12 | ||
| IgM κ | 2 | 2 | ||
| IgD κ | 2 | 2 | ||
| IgD λ | 1 | 1 | ||
| Light chain (urine) | ||||
| κ only | 6 | 6 | ||
| λ only | 7 | 7 | ||
| Biclonal (IgG, IgA, κ) | 1 | 1 | ||
| Nonsecretory | 0 | 0 | ||
| ISS at diagnosis | ||||
| I | 34 | 34 | ||
| II | 29 | 29 | ||
| III | 27 | 27 | ||
| Missing | 11 | 11 | ||
| Durie-Salmon staging at diagnosis | ||||
| I | 20 | 20 | ||
| II | 14 | 14 | ||
| III | 67 | 66 | ||
| Lines of therapy pre-AHSCT | ||||
| 1 | 44 | 44 | ||
| 2 | 35 | 35 | ||
| 3 | 15 | 15 | ||
| >3 | 7 | 7 | ||
| Pre-AHSCT treatment | ||||
| PI/IMiD | 71 | 71 | ||
| PI | 16 | 16 | ||
| IMiD | 12 | 12 | ||
| PI/IMiD/other | 1 | 1 | ||
| Other | 1 | 1 | ||
| Mobilization regimen | ||||
| G-CSF | 23 | 23 | ||
| G-CSF + plerixafor | 77 | 76 | ||
| G-CSF + plerixafor, then cyclophosphamide + G-CSF remobilization | 1 | 1 | ||
| KPS pre-AHSCT | ||||
| 70 | 20 | 19 | ||
| 80 | 52 | 51 | ||
| 90 | 29 | 29 | ||
| Time from diagnosis to AHSCT, months | 10.2 | 0.5-62.1 | ||
| CD34 dose infused, 106/kg | 3.12 | 1.7-9.93 | ||
| TNC dose infused, 108/kg | 4.83 | 1.8-44.14 |
ISS, International Staging System; KPS, Karnofsky performance status; PI, proteasome inhibitor (only); TNC, total nucleated cells.
Clinical characteristics and correlation with outcomes
Univariable analysis was performed to evaluate correlation between clinical characteristics and PFS/OS. Patients in CR or stringent CR (sCR) before AHSCT did not have significantly improved PFS (P = .15) or OS (P = .07) when compared with those not in CR or sCR. There was no correlation between age (younger than vs older than age 60 years) and PFS (2-year PFS, 70% vs 61%; P = .1), but there was an improved OS for patients younger than age 60 years at the time of AHSCT (2-year OS, 93% vs 77%; P = .004). We found no PFS or OS association with bisphosphonate use, normal serum immunoglobulin (Ig) levels (IgG, IgA, IgM), pre-AHSCT CD34+ dose infused (<4 × 106, 4-6 × 106, and >6 × 106 cells per kg), vitamin D deficiency pre-AHSCT, Karnofsky performance status, sex, or type of induction therapy (immunomodulatory drug [IMiD] and/or proteasome inhibitor–containing regimen). No IP subset was associated with Ig levels (IgG, IgA, IgM) tested pre-AHSCT or at day 100 post-AHSCT. No IP subpopulations at day +100 post-AHSCT were associated with attaining or maintaining a CR or sCR at day +100 post-AHSCT. Prior exposure to lenalidomide, bortezomib, or thalidomide as single agents or in combination as part of induction or the most recent salvage regimen before AHSCT was not associated with any particular IP before AHSCT or at day +100 post-AHSCT.
ALC recovery by day +15 following AHSCT: correlation with posttransplant outcomes
Day +15 ALC recovery post-AHSCT was not significantly associated with PFS or OS (supplemental Figure 1A-B). The 2-year PFS was 71% (95% CI, 58% to 83%) for early ALC recovery vs 56% (95% CI, 40% to 72%) for late ALC recovery (P = .25). The 2-year OS was 89% (95% CI, 80% to 98%) for early ALC recovery and 76% (95% CI, 61% to 92%) for late ALC recovery (P = .14). After adjusting for multiple comparisons, there were no significant associations between early ALC recovery and day +100 post-AHSCT immune cell subpopulations (data not shown).
Pre-AHSCT PB immune cell populations: association with PFS/OS/MRD
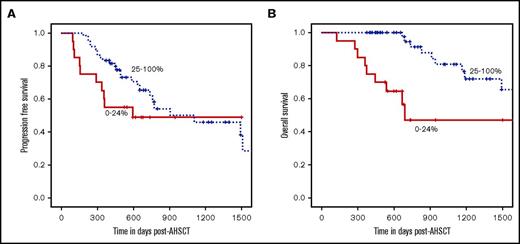
An elevated pre-AHSCT total PB CD19+ B-cell count was significantly associated with improved 2-year PFS (53% for the lowest quartile [0% to 24%], 59% for middle quartiles [25% to 74%], and 83% for the highest quartile [75% to 100%]; note that percentages used here to define lowest, middle, and highest quartiles apply throughout, including the figure legends, unless otherwise stated; P = .01) (Figure 1A) and OS (63% for the lowest, 90% for middle, and 93% for the highest quartile; P = .0003) (Figure 1B). After adjustment for age, CD19+ B-cell count remained a significant predictor of PFS and OS. This association was observed in patients whose bone marrow had detectable MRD (MRD-positive) by flow cytometry before AHSCT (n = 49) (supplemental Figure 2C-D). There was no association of pre-AHSCT total PB CD19+ B-cell count with PFS or OS for those patients whose bone marrow did not have detectable MRD (MRD-negative) (n = 47) (supplemental Figure 2A-B). Improved outcomes were associated with elevated pre-AHSCT CD19+ B-cell count most significantly in those patients who did not receive post-AHSCT maintenance therapy (n = 29) (supplemental Figure 3A-B). For those patients who received post-AHSCT maintenance therapy (n = 71), elevated pre-AHSCT CD19+ B-cell count was significantly associated with improved OS but not PFS (supplemental Figure 3C-D). Higher PB total CD20+, naive, and memory B-cell counts were the only other immune subpopulations associated with a significantly improved 2-year PFS and OS (Table 3).
Elevated pre-AHSCT absolute total CD19+B-cell count is significantly associated with improved PFS and OS. For all patients pre-AHSCT, patients with absolute total CD19+ B-cell counts in the highest quartile had improved PFS (P = .01) and OS (P = .0003) compared those in the middle and lowest quartiles.
Elevated pre-AHSCT absolute total CD19+B-cell count is significantly associated with improved PFS and OS. For all patients pre-AHSCT, patients with absolute total CD19+ B-cell counts in the highest quartile had improved PFS (P = .01) and OS (P = .0003) compared those in the middle and lowest quartiles.
Two-year PFS and OS
| Marker . | Percentile . | Before AHSCT . | Day +100 after AHSCT . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2-y PFS (%) . | 95% CI . | P . | 2-y OS (%) . | 95% CI . | P . | 2-y PFS (%) . | 95% CI . | P . | 2-y OS (%) . | 95% CI . | P . | ||
| CD3+ total | <25 | 56 | 35-77 | 72 | 49-94 | 64 | 43-86 | 74 | 49-98 | ||||
| 25-75 | 65 | 51-79 | NS | 85 | 74-95 | NS | 60 | 44-77 | NS | 84 | 71-97 | NS | |
| >75 | 72 | 54-90 | 94 | 84-105 | 62 | 39-85 | 88 | 71-104 | |||||
| CD4+ total | <25 | 47 | 26-68 | 53 | 27-79 | 75 | 56-94 | 69 | 41-96 | ||||
| 25-75 | 69 | 55-83 | NS | 92 | 85-100 | .04 | 49 | 32-66 | NS | 86 | 74-98 | NS | |
| >75 | 72 | 54-90 | 95 | 85-105 | 72 | 50-94 | 87 | 70-104 | |||||
| CD8+ total | <25 | 64 | 44-85 | 83 | 68-98 | 60 | 38-81 | 81 | 62-101 | ||||
| 25-75 | 63 | 49-77 | NS | 82 | 70-95 | NS | 64 | 47-80 | NS | 84 | 71-97 | NS | |
| >75 | 68 | 49-86 | 90 | 76-104 | 59 | 33-84 | 80 | 58-102 | |||||
| CD3+CD4+ | <25 | 47 | 26-68 | 53 | 27-79 | 70 | 50-90 | 65 | 38-92 | ||||
| 25-75 | 69 | 55-83 | NS | 92 | 85-100 | .04 | 52 | 35-69 | NS | 88 | 77-99 | NS | |
| >75 | 72 | 54-90 | 95 | 85-105 | 72 | 50-94 | 87 | 70-104 | |||||
| CD4+CD8+ double positive | <25 | 51 | 32-71 | 76 | 57-95 | 75 | 55-94 | 85 | 65-105 | ||||
| 25-75 | 64 | 49-79 | .04 | 84 | 72-96 | NS | 51 | 33-68 | NS | 79 | 64-93 | NS | |
| >75 | 77 | 60-93 | 92 | 82-103 | 67 | 45-89 | 87 | 71-104 | |||||
| CD4–CD8– double negative | <25 | 50 | 29-72 | 67 | 43-91 | 41 | 17-65 | 82 | 62-101 | ||||
| 25-75 | 67 | 53-81 | NS | 92 | 84-100 | NS | 60 | 42-78 | .05 | 81 | 66-97 | NS | |
| >75 | 72 | 54-90 | 84 | 70-98 | 80 | 62-98 | 84 | 67-101 | |||||
| CD4+ naive | <25 | 60 | 39-81 | 59 | 29-90 | 75 | 56-94 | 80 | 62-98 | ||||
| 25-75 | 65 | 51-78 | NS | 88 | 78-97 | NS | 61 | 44-78 | NS | 77 | 60-94 | NS | |
| >75 | 69 | 49-89 | 91 | 78-103 | 53 | 30-75 | 94 | 84-105 | |||||
| CD4+ central memory | <25 | 48 | 26-69 | 65 | 42-88 | 49 | 26-71 | 47 | 21-73 | ||||
| 25-75 | 66 | 52-80 | NS | 89 | 79-98 | NS | 67 | 51-84 | NS | 100 | 100-100 | .001 | |
| >75 | 80 | 65-96 | 93 | 81-106 | 61 | 38-84 | 85 | 66-104 | |||||
| CD4+ effector memory | <25 | 42 | 19-65 | 79 | 60-99 | 65 | 43-86 | 71 | 49-93 | ||||
| 25-75 | 71 | 58-84 | .01 | 84 | 73-95 | NS | 61 | 44-78 | NS | 82 | 67-97 | NS | |
| >75 | 72 | 54-90 | 90 | 76-104 | 57 | 32-83 | 95 | 85-105 | |||||
| CD4+ recent thymic emigrants | <25 | 67 | 48-86 | 59 | 33-86 | 63 | 41-85 | 64 | 36-92 | ||||
| 25-75 | 56 | 41-71 | NS | 90 | 81-98 | NS | 69 | 54-85 | NS | 84 | 71-97 | NS | |
| >75 | 80 | 65-96 | 92 | 81-103 | 49 | 25-73 | 93 | 81-106 | |||||
| CD4– recent thymic emigrants | <25 | 64 | 45-83 | 73 | 50-95 | 66 | 43-89 | 77 | 51-103 | ||||
| 25-75 | 66 | 52-81 | NS | 85 | 73-96 | .03 | 61 | 45-78 | NS | 87 | 75-99 | NS | |
| >75 | 62 | 42-82 | 92 | 81-103 | 56 | 32-80 | 76 | 55-97 | |||||
| Tregs bright (CD4+) | <25 | 61 | 41-82 | 77 | 59-95 | 55 | 33-77 | 77 | 57-97 | ||||
| 25-75 | 64 | 50-79 | NS | 88 | 77-98 | NS | 53 | 34-72 | NS | 85 | 73-98 | NS | |
| >75 | 72 | 55-90 | 84 | 68-101 | 80 | 62-98 | 87 | 70-104 | |||||
| Tregs dim (CD4+) | <25 | 46 | 25-68 | 61 | 40-83 | 59 | 37-81 | 66 | 43-89 | ||||
| 25-75 | 68 | 54-83 | NS | 96 | 90-101 | .04 | 54 | 37-72 | NS | 87 | 74-99 | NS | |
| >75 | 77 | 60-93 | 85 | 69-101 | 77 | 57-98 | 92 | 78-107 | |||||
| Tregs DR+ (CD4+) | <25 | 53 | 30-75 | 86 | 70-101 | 47 | 24-70 | 89 | 74-104 | ||||
| 25-75 | 62 | 48-76 | NS | 81 | 68-93 | NS | 60 | 43-76 | NS | 76 | 61-91 | NS | |
| >75 | 85 | 71-98 | 90 | 76-104 | 83 | 64-101 | 89 | 68-109 | |||||
| Tregs dim (CD4–) | <25 | 49 | 26-72 | 85 | 68-101 | 60 | 38-82 | 77 | 56-98 | ||||
| 25-75 | 72 | 59-85 | NS | 81 | 69-93 | NS | 62 | 45-79 | NS | 84 | 71-97 | NS | |
| >75 | 64 | 45-83 | 90 | 76-103 | 63 | 40-85 | 86 | 68-104 | |||||
| Tregs DR– (CD4–) | <25 | 50 | 28-72 | 85 | 68-101 | 65 | 43-86 | 74 | 51-98 | ||||
| 25-75 | 68 | 54-82 | NS | 78 | 65-91 | NS | 58 | 41-75 | NS | 81 | 67-95 | NS | |
| >75 | 71 | 53-90 | 95 | 86-104 | 68 | 46-89 | 93 | 79-106 | |||||
| CD3+CD8+ | <25 | 61 | 41-81 | 74 | 53-95 | 59 | 37-81 | 74 | 49-98 | ||||
| 25-75 | 66 | 52-80 | NS | 86 | 75-97 | NS | 64 | 48-80 | NS | 87 | 76-99 | NS | |
| >75 | 67 | 47-86 | 90 | 76-104 | 58 | 33-84 | 80 | 58-102 | |||||
| CD8+ naive | <25 | 66 | 47-86 | 76 | 55-96 | 80 | 62-98 | 80 | 62-98 | ||||
| 25-75 | 59 | 44-74 | NS | 83 | 71-95 | .03 | 54 | 36-71 | NS | 78 | 61-94 | NS | |
| >75 | 74 | 56-92 | 94 | 84-105 | 54 | 30-78 | 94 | 82-106 | |||||
| CD8+ primed (effector) | <25 | 57 | 36-78 | 76 | 54-99 | 70 | 50-90 | 80 | 58-102 | ||||
| 25-75 | 68 | 54-81 | NS | 85 | 74-96 | NS | 63 | 47-80 | NS | 78 | 62-94 | NS | |
| >75 | 66 | 47-86 | 88 | 75-101 | 46 | 21-71 | 89 | 75-103 | |||||
| CD8+ central memory | <25 | 41 | 18-63 | 76 | 57-96 | 53 | 28-78 | 90 | 76-103 | ||||
| 25-75 | 74 | 61-87 | NS | 88 | 78-98 | NS | 50 | 33-68 | .003 | 74 | 58-90 | NS | |
| >75 | 69 | 51-87 | 84 | 68-101 | 89 | 75-103 | 91 | 74-108 | |||||
| CD8+ effector memory | <25 | 58 | 35-80 | 87 | 72-101 | 56 | 32-80 | 90 | 77-103 | ||||
| 25-75 | 69 | 55-82 | NS | 84 | 72-95 | NS | 58 | 41-75 | NS | 79 | 64-94 | NS | |
| >75 | 63 | 43-83 | 85 | 69-101 | 72 | 50-93 | 80 | 57-102 | |||||
| γδ T cells | <25 | 52 | 31-73 | 70 | 48-91 | 45 | 23-67 | 65 | 42-88 | ||||
| 25-75 | 62 | 47-77 | NS | 87 | 77-98 | NS | 59 | 40-77 | .05 | 90 | 79-101 | .04 | |
| >75 | 80 | 65-96 | 92 | 81-103 | 78 | 59-97 | 87 | 71-104 | |||||
| CD19+ total | <25 | 53 | 33-74 | 63 | 40-86 | 64 | 42-85 | 69 | 46-92 | ||||
| 25-75 | 59 | 44-75 | .01 | 90 | 81-98 | .0003 | 66 | 50-82 | NS | 86 | 73-99 | NS | |
| >75 | 83 | 69-98 | 93 | 81-106 | 50 | 22-77 | 90 | 77-103 | |||||
| CD20+ total | <25 | 39 | 17-61 | 63 | 40-86 | 63 | 41-85 | 68 | 43-92 | ||||
| 25-75 | 69 | 55-83 | .001 | 90 | 81-98 | .01 | 66 | 50-82 | NS | 87 | 74-99 | NS | |
| >75 | 78 | 61-95 | 94 | 82-106 | 50 | 22-77 | 90 | 77-103 | |||||
| CD19+ naive | <25 | 53 | 33-74 | 71 | 50-91 | 63 | 41-85 | 68 | 43-92 | ||||
| 25-75 | 62 | 48-77 | .01 | 86 | 76-97 | .002 | 65 | 49-80 | NS | 87 | 74-99 | NS | |
| >75 | 78 | 60-95 | 93 | 81-106 | 52 | 24-81 | 89 | 76-103 | |||||
| CD19+ naive (Bm 2) | <25 | 53 | 33-74 | 67 | 46-87 | 63 | 41-85 | 68 | 43-92 | ||||
| 25-75 | 60 | 45-75 | .01 | 85 | 73-97 | .0002 | 67 | 51-83 | NS | 87 | 74-99 | NS | |
| >75 | 82 | 66-98 | 1 | 49 | 21-76 | 89 | 76-103 | ||||||
| CD19+ memory | <25 | 51 | 31-71 | 65 | 43-87 | 56 | 33-80 | 82 | 62-101 | ||||
| 25-75 | 62 | 47-77 | .05 | 89 | 79-98 | .01 | 68 | 52-84 | NS | 82 | 67-97 | NS | |
| >75 | 84 | 69-98 | 94 | 82-106 | 59 | 37-81 | 83 | 64-101 | |||||
| CD19+ memory pre-switch | <25 | 58 | 38-78 | 73 | 52-94 | 54 | 30-78 | 82 | 63-101 | ||||
| 25-75 | 63 | 48-77 | NS | 89 | 80-98 | NS | 67 | 51-83 | NS | 88 | 76-99 | NS | |
| >75 | 75 | 57-93 | 84 | 66-102 | 64 | 42-85 | 72 | 47-97 | |||||
| CD10+ memory post-switch | <25 | 43 | 22-65 | 61 | 38-84 | 80 | 58-102 | 94 | 84-105 | ||||
| 25-75 | 69 | 55-83 | NS | 91 | 82-100 | .01 | 57 | 40-74 | NS | 76 | 60-92 | NS | |
| >75 | 76 | 59-93 | 94 | 83-105 | 54 | 31-76 | 85 | 69-101 | |||||
| Total NK cells | <25 | 63 | 40-86 | 85 | 69-101 | 60 | 35-85 | 78 | 59-97 | ||||
| 25-75 | 59 | 45-73 | NS | 79 | 66-92 | NS | 65 | 49-80 | NS | 82 | 68-97 | NS | |
| >75 | 76 | 59-93 | 94 | 83-105 | 49 | 23-75 | 90 | 77-103 | |||||
| CD16/56+ γδ+/− CD8− CD3+ CD4+ | <25 | 62 | 42-82 | 77 | 54-100 | 54 | 32-76 | 72 | 47-97 | ||||
| 25-75 | 61 | 46-75 | NS | 81 | 69-93 | NS | 60 | 43-77 | NS | 88 | 77-99 | NS | |
| >75 | 75 | 57-92 | 95 | 85-105 | 72 | 50-94 | 80 | 60-101 | |||||
| CD16/56+ γδ+/− CD8+ CD3+ CD4− | <25 | 75 | 58-92 | 88 | 74-101 | 69 | 48-90 | 75 | 46-104 | ||||
| 25-75 | 61 | 46-75 | NS | 82 | 70-95 | NS | 67 | 51-82 | .05 | 91 | 80-101 | NS | |
| >75 | 64 | 45-83 | 87 | 73-101 | 43 | 17-69 | 68 | 44-92 | |||||
| Myeloid CD11c+ | <25 | 62 | 43-82 | 68 | 46-90 | 54 | 32-76 | 75 | 53-98 | ||||
| 25-75 | 62 | 47-77 | NS | 87 | 78-97 | NS | 59 | 43-76 | NS | 81 | 67-95 | NS | |
| >75 | 76 | 58-93 | 94 | 82-106 | 74 | 52-97 | 95 | 85-105 | |||||
| Plasmacytoid CD123+ | <25 | 68 | 47-88 | 70 | 43-96 | 56 | 32-79 | 89 | 74-104 | ||||
| 25-75 | 56 | 42-70 | NS | 85 | 74-95 | NS | 59 | 42-75 | NS | 76 | 61-92 | NS | |
| >75 | 85 | 71-98 | 89 | 75-104 | 74 | 54-94 | 90 | 77-103 | |||||
| Marker . | Percentile . | Before AHSCT . | Day +100 after AHSCT . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2-y PFS (%) . | 95% CI . | P . | 2-y OS (%) . | 95% CI . | P . | 2-y PFS (%) . | 95% CI . | P . | 2-y OS (%) . | 95% CI . | P . | ||
| CD3+ total | <25 | 56 | 35-77 | 72 | 49-94 | 64 | 43-86 | 74 | 49-98 | ||||
| 25-75 | 65 | 51-79 | NS | 85 | 74-95 | NS | 60 | 44-77 | NS | 84 | 71-97 | NS | |
| >75 | 72 | 54-90 | 94 | 84-105 | 62 | 39-85 | 88 | 71-104 | |||||
| CD4+ total | <25 | 47 | 26-68 | 53 | 27-79 | 75 | 56-94 | 69 | 41-96 | ||||
| 25-75 | 69 | 55-83 | NS | 92 | 85-100 | .04 | 49 | 32-66 | NS | 86 | 74-98 | NS | |
| >75 | 72 | 54-90 | 95 | 85-105 | 72 | 50-94 | 87 | 70-104 | |||||
| CD8+ total | <25 | 64 | 44-85 | 83 | 68-98 | 60 | 38-81 | 81 | 62-101 | ||||
| 25-75 | 63 | 49-77 | NS | 82 | 70-95 | NS | 64 | 47-80 | NS | 84 | 71-97 | NS | |
| >75 | 68 | 49-86 | 90 | 76-104 | 59 | 33-84 | 80 | 58-102 | |||||
| CD3+CD4+ | <25 | 47 | 26-68 | 53 | 27-79 | 70 | 50-90 | 65 | 38-92 | ||||
| 25-75 | 69 | 55-83 | NS | 92 | 85-100 | .04 | 52 | 35-69 | NS | 88 | 77-99 | NS | |
| >75 | 72 | 54-90 | 95 | 85-105 | 72 | 50-94 | 87 | 70-104 | |||||
| CD4+CD8+ double positive | <25 | 51 | 32-71 | 76 | 57-95 | 75 | 55-94 | 85 | 65-105 | ||||
| 25-75 | 64 | 49-79 | .04 | 84 | 72-96 | NS | 51 | 33-68 | NS | 79 | 64-93 | NS | |
| >75 | 77 | 60-93 | 92 | 82-103 | 67 | 45-89 | 87 | 71-104 | |||||
| CD4–CD8– double negative | <25 | 50 | 29-72 | 67 | 43-91 | 41 | 17-65 | 82 | 62-101 | ||||
| 25-75 | 67 | 53-81 | NS | 92 | 84-100 | NS | 60 | 42-78 | .05 | 81 | 66-97 | NS | |
| >75 | 72 | 54-90 | 84 | 70-98 | 80 | 62-98 | 84 | 67-101 | |||||
| CD4+ naive | <25 | 60 | 39-81 | 59 | 29-90 | 75 | 56-94 | 80 | 62-98 | ||||
| 25-75 | 65 | 51-78 | NS | 88 | 78-97 | NS | 61 | 44-78 | NS | 77 | 60-94 | NS | |
| >75 | 69 | 49-89 | 91 | 78-103 | 53 | 30-75 | 94 | 84-105 | |||||
| CD4+ central memory | <25 | 48 | 26-69 | 65 | 42-88 | 49 | 26-71 | 47 | 21-73 | ||||
| 25-75 | 66 | 52-80 | NS | 89 | 79-98 | NS | 67 | 51-84 | NS | 100 | 100-100 | .001 | |
| >75 | 80 | 65-96 | 93 | 81-106 | 61 | 38-84 | 85 | 66-104 | |||||
| CD4+ effector memory | <25 | 42 | 19-65 | 79 | 60-99 | 65 | 43-86 | 71 | 49-93 | ||||
| 25-75 | 71 | 58-84 | .01 | 84 | 73-95 | NS | 61 | 44-78 | NS | 82 | 67-97 | NS | |
| >75 | 72 | 54-90 | 90 | 76-104 | 57 | 32-83 | 95 | 85-105 | |||||
| CD4+ recent thymic emigrants | <25 | 67 | 48-86 | 59 | 33-86 | 63 | 41-85 | 64 | 36-92 | ||||
| 25-75 | 56 | 41-71 | NS | 90 | 81-98 | NS | 69 | 54-85 | NS | 84 | 71-97 | NS | |
| >75 | 80 | 65-96 | 92 | 81-103 | 49 | 25-73 | 93 | 81-106 | |||||
| CD4– recent thymic emigrants | <25 | 64 | 45-83 | 73 | 50-95 | 66 | 43-89 | 77 | 51-103 | ||||
| 25-75 | 66 | 52-81 | NS | 85 | 73-96 | .03 | 61 | 45-78 | NS | 87 | 75-99 | NS | |
| >75 | 62 | 42-82 | 92 | 81-103 | 56 | 32-80 | 76 | 55-97 | |||||
| Tregs bright (CD4+) | <25 | 61 | 41-82 | 77 | 59-95 | 55 | 33-77 | 77 | 57-97 | ||||
| 25-75 | 64 | 50-79 | NS | 88 | 77-98 | NS | 53 | 34-72 | NS | 85 | 73-98 | NS | |
| >75 | 72 | 55-90 | 84 | 68-101 | 80 | 62-98 | 87 | 70-104 | |||||
| Tregs dim (CD4+) | <25 | 46 | 25-68 | 61 | 40-83 | 59 | 37-81 | 66 | 43-89 | ||||
| 25-75 | 68 | 54-83 | NS | 96 | 90-101 | .04 | 54 | 37-72 | NS | 87 | 74-99 | NS | |
| >75 | 77 | 60-93 | 85 | 69-101 | 77 | 57-98 | 92 | 78-107 | |||||
| Tregs DR+ (CD4+) | <25 | 53 | 30-75 | 86 | 70-101 | 47 | 24-70 | 89 | 74-104 | ||||
| 25-75 | 62 | 48-76 | NS | 81 | 68-93 | NS | 60 | 43-76 | NS | 76 | 61-91 | NS | |
| >75 | 85 | 71-98 | 90 | 76-104 | 83 | 64-101 | 89 | 68-109 | |||||
| Tregs dim (CD4–) | <25 | 49 | 26-72 | 85 | 68-101 | 60 | 38-82 | 77 | 56-98 | ||||
| 25-75 | 72 | 59-85 | NS | 81 | 69-93 | NS | 62 | 45-79 | NS | 84 | 71-97 | NS | |
| >75 | 64 | 45-83 | 90 | 76-103 | 63 | 40-85 | 86 | 68-104 | |||||
| Tregs DR– (CD4–) | <25 | 50 | 28-72 | 85 | 68-101 | 65 | 43-86 | 74 | 51-98 | ||||
| 25-75 | 68 | 54-82 | NS | 78 | 65-91 | NS | 58 | 41-75 | NS | 81 | 67-95 | NS | |
| >75 | 71 | 53-90 | 95 | 86-104 | 68 | 46-89 | 93 | 79-106 | |||||
| CD3+CD8+ | <25 | 61 | 41-81 | 74 | 53-95 | 59 | 37-81 | 74 | 49-98 | ||||
| 25-75 | 66 | 52-80 | NS | 86 | 75-97 | NS | 64 | 48-80 | NS | 87 | 76-99 | NS | |
| >75 | 67 | 47-86 | 90 | 76-104 | 58 | 33-84 | 80 | 58-102 | |||||
| CD8+ naive | <25 | 66 | 47-86 | 76 | 55-96 | 80 | 62-98 | 80 | 62-98 | ||||
| 25-75 | 59 | 44-74 | NS | 83 | 71-95 | .03 | 54 | 36-71 | NS | 78 | 61-94 | NS | |
| >75 | 74 | 56-92 | 94 | 84-105 | 54 | 30-78 | 94 | 82-106 | |||||
| CD8+ primed (effector) | <25 | 57 | 36-78 | 76 | 54-99 | 70 | 50-90 | 80 | 58-102 | ||||
| 25-75 | 68 | 54-81 | NS | 85 | 74-96 | NS | 63 | 47-80 | NS | 78 | 62-94 | NS | |
| >75 | 66 | 47-86 | 88 | 75-101 | 46 | 21-71 | 89 | 75-103 | |||||
| CD8+ central memory | <25 | 41 | 18-63 | 76 | 57-96 | 53 | 28-78 | 90 | 76-103 | ||||
| 25-75 | 74 | 61-87 | NS | 88 | 78-98 | NS | 50 | 33-68 | .003 | 74 | 58-90 | NS | |
| >75 | 69 | 51-87 | 84 | 68-101 | 89 | 75-103 | 91 | 74-108 | |||||
| CD8+ effector memory | <25 | 58 | 35-80 | 87 | 72-101 | 56 | 32-80 | 90 | 77-103 | ||||
| 25-75 | 69 | 55-82 | NS | 84 | 72-95 | NS | 58 | 41-75 | NS | 79 | 64-94 | NS | |
| >75 | 63 | 43-83 | 85 | 69-101 | 72 | 50-93 | 80 | 57-102 | |||||
| γδ T cells | <25 | 52 | 31-73 | 70 | 48-91 | 45 | 23-67 | 65 | 42-88 | ||||
| 25-75 | 62 | 47-77 | NS | 87 | 77-98 | NS | 59 | 40-77 | .05 | 90 | 79-101 | .04 | |
| >75 | 80 | 65-96 | 92 | 81-103 | 78 | 59-97 | 87 | 71-104 | |||||
| CD19+ total | <25 | 53 | 33-74 | 63 | 40-86 | 64 | 42-85 | 69 | 46-92 | ||||
| 25-75 | 59 | 44-75 | .01 | 90 | 81-98 | .0003 | 66 | 50-82 | NS | 86 | 73-99 | NS | |
| >75 | 83 | 69-98 | 93 | 81-106 | 50 | 22-77 | 90 | 77-103 | |||||
| CD20+ total | <25 | 39 | 17-61 | 63 | 40-86 | 63 | 41-85 | 68 | 43-92 | ||||
| 25-75 | 69 | 55-83 | .001 | 90 | 81-98 | .01 | 66 | 50-82 | NS | 87 | 74-99 | NS | |
| >75 | 78 | 61-95 | 94 | 82-106 | 50 | 22-77 | 90 | 77-103 | |||||
| CD19+ naive | <25 | 53 | 33-74 | 71 | 50-91 | 63 | 41-85 | 68 | 43-92 | ||||
| 25-75 | 62 | 48-77 | .01 | 86 | 76-97 | .002 | 65 | 49-80 | NS | 87 | 74-99 | NS | |
| >75 | 78 | 60-95 | 93 | 81-106 | 52 | 24-81 | 89 | 76-103 | |||||
| CD19+ naive (Bm 2) | <25 | 53 | 33-74 | 67 | 46-87 | 63 | 41-85 | 68 | 43-92 | ||||
| 25-75 | 60 | 45-75 | .01 | 85 | 73-97 | .0002 | 67 | 51-83 | NS | 87 | 74-99 | NS | |
| >75 | 82 | 66-98 | 1 | 49 | 21-76 | 89 | 76-103 | ||||||
| CD19+ memory | <25 | 51 | 31-71 | 65 | 43-87 | 56 | 33-80 | 82 | 62-101 | ||||
| 25-75 | 62 | 47-77 | .05 | 89 | 79-98 | .01 | 68 | 52-84 | NS | 82 | 67-97 | NS | |
| >75 | 84 | 69-98 | 94 | 82-106 | 59 | 37-81 | 83 | 64-101 | |||||
| CD19+ memory pre-switch | <25 | 58 | 38-78 | 73 | 52-94 | 54 | 30-78 | 82 | 63-101 | ||||
| 25-75 | 63 | 48-77 | NS | 89 | 80-98 | NS | 67 | 51-83 | NS | 88 | 76-99 | NS | |
| >75 | 75 | 57-93 | 84 | 66-102 | 64 | 42-85 | 72 | 47-97 | |||||
| CD10+ memory post-switch | <25 | 43 | 22-65 | 61 | 38-84 | 80 | 58-102 | 94 | 84-105 | ||||
| 25-75 | 69 | 55-83 | NS | 91 | 82-100 | .01 | 57 | 40-74 | NS | 76 | 60-92 | NS | |
| >75 | 76 | 59-93 | 94 | 83-105 | 54 | 31-76 | 85 | 69-101 | |||||
| Total NK cells | <25 | 63 | 40-86 | 85 | 69-101 | 60 | 35-85 | 78 | 59-97 | ||||
| 25-75 | 59 | 45-73 | NS | 79 | 66-92 | NS | 65 | 49-80 | NS | 82 | 68-97 | NS | |
| >75 | 76 | 59-93 | 94 | 83-105 | 49 | 23-75 | 90 | 77-103 | |||||
| CD16/56+ γδ+/− CD8− CD3+ CD4+ | <25 | 62 | 42-82 | 77 | 54-100 | 54 | 32-76 | 72 | 47-97 | ||||
| 25-75 | 61 | 46-75 | NS | 81 | 69-93 | NS | 60 | 43-77 | NS | 88 | 77-99 | NS | |
| >75 | 75 | 57-92 | 95 | 85-105 | 72 | 50-94 | 80 | 60-101 | |||||
| CD16/56+ γδ+/− CD8+ CD3+ CD4− | <25 | 75 | 58-92 | 88 | 74-101 | 69 | 48-90 | 75 | 46-104 | ||||
| 25-75 | 61 | 46-75 | NS | 82 | 70-95 | NS | 67 | 51-82 | .05 | 91 | 80-101 | NS | |
| >75 | 64 | 45-83 | 87 | 73-101 | 43 | 17-69 | 68 | 44-92 | |||||
| Myeloid CD11c+ | <25 | 62 | 43-82 | 68 | 46-90 | 54 | 32-76 | 75 | 53-98 | ||||
| 25-75 | 62 | 47-77 | NS | 87 | 78-97 | NS | 59 | 43-76 | NS | 81 | 67-95 | NS | |
| >75 | 76 | 58-93 | 94 | 82-106 | 74 | 52-97 | 95 | 85-105 | |||||
| Plasmacytoid CD123+ | <25 | 68 | 47-88 | 70 | 43-96 | 56 | 32-79 | 89 | 74-104 | ||||
| 25-75 | 56 | 42-70 | NS | 85 | 74-95 | NS | 59 | 42-75 | NS | 76 | 61-92 | NS | |
| >75 | 85 | 71-98 | 89 | 75-104 | 74 | 54-94 | 90 | 77-103 | |||||
NS, not significant.
Day 100 post-AHSCT immune cell populations: association with PFS/OS/MRD and maintenance therapy
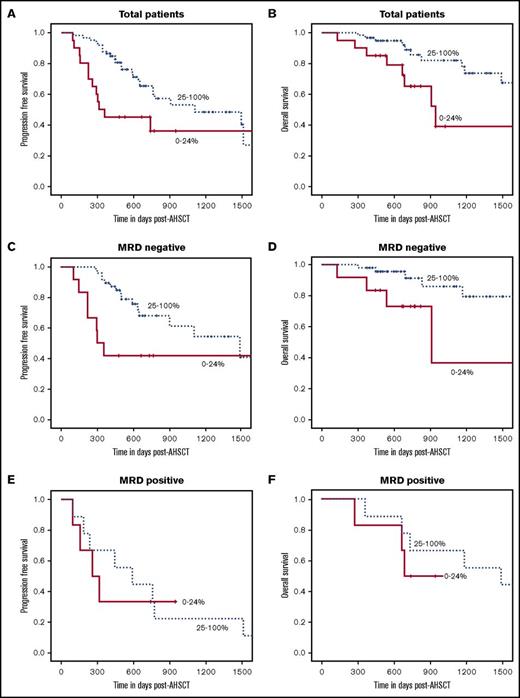
IP at day 100 post-AHSCT and the association with PFS and OS is shown in Table 3. Higher PB γδ T-cell counts correlated with improved 2-year PFS: 45% for the lowest and 65% for the highest quartile (P = .02) and OS: 65% for the lowest and 89% for the highest quartile (P = .01) (Figure 2A-B). After adjustment by age, γδ T-cell count remained a significant predictor of PFS and OS. Unlike the pre-AHSCT PB CD19+ B-cell population, this association was observed in those patients with MRD-negative bone marrow tests at day 100 post-AHSCT (n = 60) (Figure 2C-D) and not in those patients with MRD-positive bone marrow tests (n = 15) (Figure 2E-F). Day +100 AHSCT PB CD4+ central memory (CM) cell counts were significantly associated with improved OS: 47% for the lowest and 95% for the highest quartile (P = .0003) (Figure 3B), but there was no association with PFS (Figure 3A). After adjustment for age, CD4+ CM T-cell count remained a significant predictor of OS. This association was observed in those patients with MRD-negative bone marrow tests at day 100 post-AHSCT (n = 60) (supplemental Figure 4A-B) and was not observed in patients with MRD-positive bone marrow tests (n = 15) (supplemental Figure 4C-D).
Elevated day +100 post-AHSCT absolute γδ T-cell count is significantly correlated with improved PFS and OS in all patients and in those who are MRD-negative. (A-B) For all patients at day +100 post-AHSCT, those with absolute γδ T-cell counts in the top 3 quartiles combined had improved PFS (P = .02) and OS (P = .01) compared with those in the lowest quartile. (C-D) For patients who had no detectable MRD (n = 60) by flow cytometry at day +100 post-AHSCT elevated absolute γδ T-cell count was significantly correlated with improved PFS (P = .02) and OS (P = .02). (E-F) For patients who had detectable MRD (n = 15) by flow cytometry at day +100 post-AHSCT, there was no significant correlation with elevated absolute γδ T-cell counts and PFS (P = .8) and OS (P = .5).
Elevated day +100 post-AHSCT absolute γδ T-cell count is significantly correlated with improved PFS and OS in all patients and in those who are MRD-negative. (A-B) For all patients at day +100 post-AHSCT, those with absolute γδ T-cell counts in the top 3 quartiles combined had improved PFS (P = .02) and OS (P = .01) compared with those in the lowest quartile. (C-D) For patients who had no detectable MRD (n = 60) by flow cytometry at day +100 post-AHSCT elevated absolute γδ T-cell count was significantly correlated with improved PFS (P = .02) and OS (P = .02). (E-F) For patients who had detectable MRD (n = 15) by flow cytometry at day +100 post-AHSCT, there was no significant correlation with elevated absolute γδ T-cell counts and PFS (P = .8) and OS (P = .5).
Elevated day +100 post-AHSCT absolute CD4+CM cell count is significantly associated with improved OS but not PFS. (A-B) For all patients at day +100 post-AHSCT, those with absolute CD4+ CM T-cell counts in the top 3 quartiles combined had improved OS (P = .0003) but not PFS (P = .2) compared with those in the lowest quartile.
Elevated day +100 post-AHSCT absolute CD4+CM cell count is significantly associated with improved OS but not PFS. (A-B) For all patients at day +100 post-AHSCT, those with absolute CD4+ CM T-cell counts in the top 3 quartiles combined had improved OS (P = .0003) but not PFS (P = .2) compared with those in the lowest quartile.
Improved outcomes were associated with elevated γδ T cells (supplemental Figure 5A-B) and elevated CD4+ CM T cells (supplemental Figure 6A-B), most significantly in patients who did not receive any post-AHSCT maintenance therapy (n = 22); improved outcomes were not observed in patients who received post-AHSCT maintenance therapy (n = 58) (supplemental Figures 5C-D and 6C-D).
Discussion
This was a proof-of-concept study that highlighted the feasibility of performing IP in MM patients pre- and post-AHSCT and the potential prognostic significance of IP in these patients. It also illustrates the benefit of IP and the importance of incorporating it into future clinical trials. Our modern cohort of patients had improved responses to novel combination therapies with median OS with lenalidomide maintenance of up to 10 years. Early surrogate end points are necessary for clinical trial development.
This report did not find a correlation with ALC recovery (a surrogate for immune recovery) and PFS or OS, unlike previous studies.3,4,8 This may be a result of the higher ALCs infused in the stem cell products in this study when compared with other studies. Other factors that may account for this difference were found in previous reports that included a greater proportion of patients with refractory disease, more patients receiving bone marrow grafts (20% vs 0%), and induction regimens without IMiDs and proteasome inhibitors and no plerixafor use.9 In comparison with other studies, this analysis did not demonstrate any significance between number of Tregs and PFS or OS. The studies that found that decreased Tregs were associated with long-term disease control performed IP in the bone marrow.13,14 In contrast, our study performed IP in the PB, likely accounting for the difference in findings. We plan to include bone marrow IP in future studies.
This analysis centered on comprehensive IP in the PB of MM patients undergoing AHSCT to determine whether more defined immune populations were associated with outcome. Higher CD19+ B-cell populations before AHSCT were associated with improved PFS and OS. This may represent a return to B-cell immune functionality and would be a surrogate for robust immune reconstitution after antimyeloma induction therapy. These findings are evocative of the observation that higher levels of CD19+ B cells at diagnosis predict for response and longer event-free survival and OS.6 Patients whose bone marrow was MRD-positive with low PB B-cell numbers had inferior outcomes, whereas patients who were MRD-negative were not impacted by the level of PB B cells. This would suggest that myeloma cytoreduction during induction is important for predicting outcome, but those with MRD benefit from an enhanced immune profile. Improved outcomes were significantly associated with elevated pre-AHSCT CD19+ B-cell counts for patients who did not receive any post-AHSCT maintenance therapy. This reinforces the concept that immune competence, as manifested by improved B-cell counts, is correlated with improved outcomes.
This is the first report demonstrating that higher PB γδ T-cell numbers at day +100 post-AHSCT are associated with improved PFS and OS. γδ T cells respond to infections and tumor antigens as components of innate and adaptive immunity.15 The pleiotropic functions of γδ T cells include antigen presentation to, co-stimulation of, and triggering of αβ T-cell proliferation and differentiation, eliciting antitumor cells by CD8+ and CD4+ T cells as well as being directly cytotoxic.16,17 Other γδ T-cell functions include macrophage recruitment, DC maturation, cytokine and chemokine secretion, and regulation of B-cell function.16 CD4+ CM cells have been associated with lymph node homing, DC stimulation, and differentiation into CD4+ effector cells.18
Improved PFS and OS with elevated γδ T cells (and OS in the case of CD4+ CM cells) were seen in patients who were MRD-negative at day 100 post-AHSCT and not in those who were MRD-positive. This would imply that a higher tumor cell burden would not be controlled only by a favorable IP. Indeed a favorable IP exerted a beneficial effect primarily in patients who did not receive maintenance therapy, although the applicability of this finding is limited given the small number of patients who did not receive maintenance therapy. Thus maintenance therapy seems to overcome negative IP, and we need to further understand the pleiotropic effects of lenalidomide on immune function.19,20 Because the majority of patients received lenalidomide, it is not possible to determine whether the same effect would be seen with bortezomib.
Bisphosphonates, including zoledronate and pamidronate, can stimulate γδ T-cell proliferation resulting in cytotoxic activity against myeloma cell lines and decreased survival of malignant plasma cells from MM patients’ bone marrow.21,22 Lenalidomide enhances γδ T-cell proliferation and activity against the malignant B cells.23 Many MM patients receive bisphosphonates and/or lenalidomide as part of initial therapy. In our patient population, because of the combined use of both drugs, we could not differentiate whether there was an effect of lenalidomide or zoledronate on γδ T-cell subpopulations and outcome before and after AHSCT. Bisphosphonate and lenalidomide administration are standard treatments after transplantation. Thus, future investigation should focus on how elevated γδ T-cell levels are attained after transplantation and whether this elevation leads to improved outcome.
This study was limited by retrospective design and a relatively small MM patient population; however, the patient population was representative of a standard transplantation center. The lack of association between induction regimen and IP likely reflects patient and treatment heterogeneity. The detailed IP is an advantage for determining the effect of lymphocyte populations on outcome. Although this study focused on cellular immune profiles, it remains a critical question whether any major cytokines and chemokines from these immune cells contribute to the patients’ outcome. Although previous studies have addressed the significance of a few cytokines and chemokines in MM,24,25 it will require a separate study to define how soluble factors from these major types of effector cells (eg, γδ T cells, CD3+/CD4+/CD8+ T cells, and NK cells) affect the 2-year PFS and OS in patients. This has implications for emerging immunotherapies such as daratumumab, which has been shown to have profound immune effects,26 and elotuzumab, which has synergistic action with lenalidomide.27
It is well established that IMiDs have immunomodulatory effects as well.28 Robust recovery of specific immune cell subpopulations may represent a protective effect for the MM patient after AHSCT. The role of prolonged therapy to maintain response will be the subject of future studies. Understanding how immune subpopulations trigger and regulate the immune response to tumor may enable long-term control of MM.
Presented at the 55th annual meeting of the American Society of Hematology, New Orleans, LA, 7-10 December 2013, and at the 58th annual meeting of the American Society of Hematology, San Diego, CA, 3-6 December 2016.
The full-text version of this article contains a data supplement.
Acknowledgments
This work was partially supported by National Institutes of Health National Cancer Institute grant P30CA016056 involving the use of the Roswell Park Cancer Institute Flow and Imaging Cytometry Shared Resource.
Authorship
Contribution: P.L.M., P.K.W., and T.H. designed the research; C.M.H., P.L.M., P.K.W., A.F., P.M., B.L.S.M., B.P., and T.H. performed research and collected data; C.M.H., Y.Z., P.K.W., and T.H. analyzed data; C.M.H., P.L.M., Y.Z., P.K.W., J.D.T, G.L.C., S.A.H., X.C., B.P., and T.H. interpreted results; C.M.H., P.L.M., Y.Z., P.K.W., P.M., G.L.C., S.A.H., S.R.B., X.C., B.P., and T.H. wrote the paper; and all authors gave final approval of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christine M. Ho, Department of Medicine, Blood and Marrow Transplantation, Roswell Park Cancer Institute, Elm and Carlton Streets, Buffalo, NY 14263; e-mail: christine.ho@roswellpark.org.