Skip Nav Destination
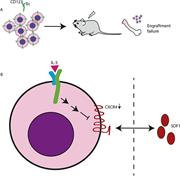
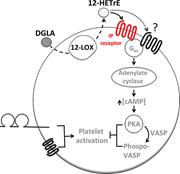
Lymphadenopathy driven by TCR-Vγ8Vδ1 T-cell expansion in FAS-related autoimmune lymphoproliferative syndrome
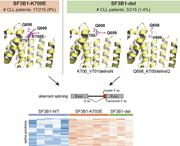
Novel SF3B1 in-frame deletions result in aberrant RNA splicing in CLL patients
Inotuzumab ozogamicin in adults with relapsed or refractory CD22-positive acute lymphoblastic leukemia: a phase 1/2 study
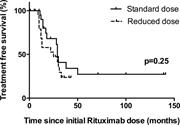
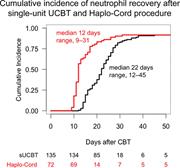
Single umbilical cord blood with or without CD34+ cells from a third-party donor in adults with leukemia
Issue Archive
Table of Contents
EXCEPTIONAL CASE REPORTS
Lymphadenopathy driven by TCR-Vγ8Vδ1 T-cell expansion in FAS-related autoimmune lymphoproliferative syndrome
Clinical Trials & Observations
Stefano Vavassori,Jacob D. Galson,Johannes Trück,Anke van den Berg,Rienk Y. J. Tamminga,Aude Magerus-Chatinet,Olivier Pellé,Ulrike Camenisch Gross,Ewerton Marques Maggio,Seraina Prader,Lennart Opitz,Ursina Nüesch,Andrea Mauracher,Benjamin Volkmer,Oliver Speer,Luzia Suda,Benno Röthlisberger,Dieter Robert Zimmermann,Rouven Müller,Arjan Diepstra,Lydia Visser,Eugenia Haralambieva,Bénédicte Neven,Frédéric Rieux-Laucat,Jana Pachlopnik Schmid
Novel SF3B1 in-frame deletions result in aberrant RNA splicing in CLL patients
Clinical Trials & Observations
Anant A. Agrawal,Michael Seiler,Lindsey T. Brinton,Rose Mantel,Rosa Lapalombella,Jennifer A. Woyach,Amy J. Johnson,Ping Zhu,Markus Warmuth,Lihua Yu,John C. Byrd,Peter G. Smith,James S. Blachly,Silvia Buonamici
CLINICAL TRIALS AND OBSERVATIONS
Inotuzumab ozogamicin in adults with relapsed or refractory CD22-positive acute lymphoblastic leukemia: a phase 1/2 study
Clinical Trials & Observations
Daniel J. DeAngelo,Wendy Stock,Anthony S. Stein,Andrei Shustov,Michaela Liedtke,Charles A. Schiffer,Erik Vandendries,Katherine Liau,Revathi Ananthakrishnan,Joseph Boni,A. Douglas Laird,Luke Fostvedt,Hagop M. Kantarjian,Anjali S. Advani
HEMATOPOIESIS AND STEM CELLS
IMMUNOBIOLOGY
LYMPHOID NEOPLASIA
A novel CXCR4 antagonist IgG1 antibody (PF-06747143) for the treatment of hematologic malignancies
Shu-Hui Liu,Yin Gu,Bernadette Pascual,Zhengming Yan,Max Hallin,Cathy Zhang,Conglin Fan,Wenlian Wang,Justine Lam,Mary E. Spilker,Rolla Yafawi,Eileen Blasi,Brett Simmons,Nanni Huser,Wei-Hsien Ho,Kevin Lindquist,Thomas-Toan Tran,Jyothirmayee Kudaravalli,Jing-Tyan Ma,Gretchen Jimenez,Ishita Barman,Colleen Brown,Sherman Michael Chin,Maria J. Costa,David Shelton,Tod Smeal,Valeria R. Fantin,Flavia Pernasetti
MYELOID NEOPLASIA
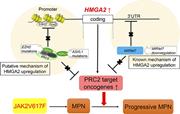
Hmga2 collaborates with JAK2V617F in the development of myeloproliferative neoplasms
Koki Ueda,Kazuhiko Ikeda,Takayuki Ikezoe,Kayo Harada-Shirado,Kazuei Ogawa,Yuko Hashimoto,Takahiro Sano,Hiroshi Ohkawara,Satoshi Kimura,Akiko Shichishima-Nakamura,Yuichi Nakamura,Yayoi Shikama,Tsutomu Mori,Philip J. Mason,Monica Bessler,Soji Morishita,Norio Komatsu,Kotaro Shide,Kazuya Shimoda,Shuhei Koide,Kazumasa Aoyama,Motohiko Oshima,Atsushi Iwama,Yasuchika Takeishi
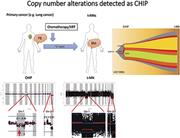
Copy number alterations detected as clonal hematopoiesis of indeterminate potential
Koichi Takahashi,Feng Wang,Hagop Kantarjian,Xingzhi Song,Keyur Patel,Sattva Neelapu,Curtis Gumbs,Latasha Little,Samantha Tippen,Rebecca Thornton,Courtney D. DiNardo,Farhad Ravandi,Carlos Bueso-Ramos,Jianhua Zhang,Xifeng Wu,Guillermo Garcia-Manero,P. Andrew Futreal
RED CELLS, IRON, AND ERYTHROPOIESIS
THROMBOSIS AND HEMOSTASIS
Variable phenotypic penetrance of thrombosis in adult mice after tissue-selective and temporally controlled Thbd gene inactivation
Thijs E. van Mens,Hai-Po H. Liang,Sreemanti Basu,Irene Hernandez,Mark Zogg,Jennifer May,Min Zhan,Qiuhui Yang,Jamie Foeckler,Shawn Kalloway,Rashmi Sood,Caren Sue Karlson,Hartmut Weiler
TRANSFUSION MEDICINE
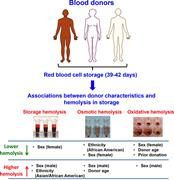
Ethnicity, sex, and age are determinants of red blood cell storage and stress hemolysis: results of the REDS-III RBC-Omics study
Tamir Kanias,for the National Heart, Lung, and Blood Institute Recipient Epidemiology Donor Evaluation Study III (REDS-III) Program,Marion C. Lanteri,for the National Heart, Lung, and Blood Institute Recipient Epidemiology Donor Evaluation Study III (REDS-III) Program,Grier P. Page,for the National Heart, Lung, and Blood Institute Recipient Epidemiology Donor Evaluation Study III (REDS-III) Program,Yuelong Guo,for the National Heart, Lung, and Blood Institute Recipient Epidemiology Donor Evaluation Study III (REDS-III) Program,Stacy M. Endres,for the National Heart, Lung, and Blood Institute Recipient Epidemiology Donor Evaluation Study III (REDS-III) Program,Mars Stone,for the National Heart, Lung, and Blood Institute Recipient Epidemiology Donor Evaluation Study III (REDS-III) Program,Sheila Keating,for the National Heart, Lung, and Blood Institute Recipient Epidemiology Donor Evaluation Study III (REDS-III) Program,Alan E. Mast,for the National Heart, Lung, and Blood Institute Recipient Epidemiology Donor Evaluation Study III (REDS-III) Program,Ritchard G. Cable,for the National Heart, Lung, and Blood Institute Recipient Epidemiology Donor Evaluation Study III (REDS-III) Program,Darrell J. Triulzi,for the National Heart, Lung, and Blood Institute Recipient Epidemiology Donor Evaluation Study III (REDS-III) Program,Joseph E. Kiss,for the National Heart, Lung, and Blood Institute Recipient Epidemiology Donor Evaluation Study III (REDS-III) Program,Edward L. Murphy,for the National Heart, Lung, and Blood Institute Recipient Epidemiology Donor Evaluation Study III (REDS-III) Program,Steve Kleinman,for the National Heart, Lung, and Blood Institute Recipient Epidemiology Donor Evaluation Study III (REDS-III) Program,Michael P. Busch,for the National Heart, Lung, and Blood Institute Recipient Epidemiology Donor Evaluation Study III (REDS-III) Program,Mark T. Gladwin,for the National Heart, Lung, and Blood Institute Recipient Epidemiology Donor Evaluation Study III (REDS-III) Program
TRANSPLANTATION
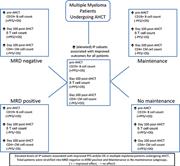
Immune signatures associated with improved progression-free and overall survival for myeloma patients treated with AHSCT
Christine M. Ho,Philip L. McCarthy,Paul K. Wallace,Yali Zhang,Ahmad Fora,Patrick Mellors,Joseph D. Tario,Benjamin L. S. McCarthy,George L. Chen,Sarah A. Holstein,Sophia R. Balderman,Xuefang Cao,Bruno Paiva,Theresa Hahn
Single umbilical cord blood with or without CD34+ cells from a third-party donor in adults with leukemia
Clinical Trials & Observations
Jaime Sanz,on behalf of Grupo Español de Trasplante Hematopoyético (GETH),Mi Kwon,on behalf of Grupo Español de Trasplante Hematopoyético (GETH),Guiomar Bautista,on behalf of Grupo Español de Trasplante Hematopoyético (GETH),Miguel A. Sanz,on behalf of Grupo Español de Trasplante Hematopoyético (GETH),Pascual Balsalobre,on behalf of Grupo Español de Trasplante Hematopoyético (GETH),José Luis Piñana,on behalf of Grupo Español de Trasplante Hematopoyético (GETH),Carlos Solano,on behalf of Grupo Español de Trasplante Hematopoyético (GETH),Rafael Duarte,on behalf of Grupo Español de Trasplante Hematopoyético (GETH),Christelle Ferrá,on behalf of Grupo Español de Trasplante Hematopoyético (GETH),Ignacio Lorenzo,on behalf of Grupo Español de Trasplante Hematopoyético (GETH),Carmen Martín,on behalf of Grupo Español de Trasplante Hematopoyético (GETH),Pere Barba,on behalf of Grupo Español de Trasplante Hematopoyético (GETH),María Jesús Pascual,on behalf of Grupo Español de Trasplante Hematopoyético (GETH),Rodrigo Martino,on behalf of Grupo Español de Trasplante Hematopoyético (GETH),Jorge Gayoso,on behalf of Grupo Español de Trasplante Hematopoyético (GETH),Ismael Buño,on behalf of Grupo Español de Trasplante Hematopoyético (GETH),Carmen Regidor,on behalf of Grupo Español de Trasplante Hematopoyético (GETH),Almudena de la Iglesia,on behalf of Grupo Español de Trasplante Hematopoyético (GETH),Juan Montoro,on behalf of Grupo Español de Trasplante Hematopoyético (GETH),José Luis Díez-Martín,on behalf of Grupo Español de Trasplante Hematopoyético (GETH),Guillermo F. Sanz,on behalf of Grupo Español de Trasplante Hematopoyético (GETH),Rafael Cabrera,on behalf of Grupo Español de Trasplante Hematopoyético (GETH)
COMMENTARIES
-
Cover Image
Cover Image
![issue cover]()
COVER FIGURE
Model of evolution from clonal hematopoiesis of indeterminate potential (CHIP) to therapy-related myeloid neoplasms. A CHIP clone with a DNMT3A mutation acquired deletion 7, and then 2 subclones acquired a PTPN11 mutation and trisomy 22, an NRAS mutation. See the article by Takahashi et al. - PDF Icon Front MatterFront Matter
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Advertisement intended for health care professionals