Key Points
IL-6 levels in bone marrow predict event-free survival in pediatric AML.
Exogenous IL-6 protects AML blasts from chemotherapy-induced apoptosis.
Abstract
The tumor microenvironment can protect cancer cells from conventional anticancer therapies. Thus, targeting these protective mechanisms could eradicate therapy-resistant cancer cells and improve outcomes. Interleukin-6 (IL-6) provides extrinsic protection for several solid tumors and multiple myeloma. In pediatric acute myeloid leukemia (AML), IL-6–induced STAT3 signaling frequently becomes stronger at relapse, and increases in IL-6–induced STAT3 activity are associated with inferior survival after relapse. These findings suggested that the IL-6–induced STAT3 pathway may promote chemotherapy resistance and disease progression. Thus, we investigated the dysregulation of IL-6 levels in the bone marrow niche in pediatric patients with AML and the association between IL-6 levels and outcome. We measured levels of over 40 cytokines and growth factors in plasma from diagnostic bone marrow aspirates of 45 pediatric AML patients and 7 healthy sibling controls. Of the measured cytokines, only IL-6 levels were associated with event-free survival. Importantly, the effect of elevated IL-6 was most striking among children classified as having a low risk of relapse. In these patients, 5-year event-free survival was 82.5% ± 11% for patients with low IL-6 levels at diagnosis (n = 14) compared with 17.3% ± 11% for patients with elevated IL-6 (n = 13, log-rank P = .0003). In vitro, exogenous IL-6 reduced mitoxantrone-induced apoptosis in cell lines and primary pediatric AML samples. These results suggest that IL-6 levels at diagnosis could be used to help identify children at high risk of relapse, particularly those who are otherwise classified as low risk by current algorithms. Moreover, the IL-6 pathway could represent a target for overcoming environment-mediated chemotherapy resistance.
Introduction
In the United States, 500 children are diagnosed with acute myeloid leukemia (AML) every year, and the best available treatments only cure ∼60% of these children.1,2 In the last 50 years, incremental improvements in cure rates for pediatric AML have been achieved by refinements of treatment regimens, improved supportive care, and optimization of risk-stratification algorithms.2-6 Current algorithms are based on a few cytogenetic changes and mutations and on an individual’s response to the initial treatment.2,7 For pediatric patients with AML, the ability of the algorithm to stratify risk at diagnosis is limited. Few prognostic cytogenetic changes have been identified thus far, and the majority of patients do not have these markers at diagnosis. Currently, 30% of pediatric patients with AML are classified as high risk (HR) and 70% as low risk (LR), yet ∼30% of LR patients relapse.7 Relapsed AML is frequently resistant to chemotherapy, and it is difficult to achieve a second remission.8 Thus, one critical step to improve outcomes in pediatric patients with AML is identifying additional markers that can improve our risk-stratification algorithms.9
Previous studies have investigated the relationship between cytokines and clinical outcome in adult AML, and several cytokines have been shown to be prognostic. For example, elevated levels of interleukin-6 (IL-6) in peripheral blood predicted poorer overall survival (OS), whereas elevated IL-10 correlated with improved outcomes.10 Additionally, elevated levels of some cytokines in the adult AML bone marrow (BM) niche have been described, including IL-6.11 Recently, a study of a small pediatric AML cohort showed that IL-6 levels in peripheral blood were elevated compared with healthy age-matched controls.12 However, the cytokine milieu in the BM niche of pediatric patients with AML has not been investigated previously. This is partially due to a lack of available banked samples.
In a previous study, we used BM samples from pediatric patients with AML to identify a novel functional profile in that predicts prognosis.13 Specifically, we demonstrated that the sensitivity of AML cells to ligand-induced activation of STAT3 predicts both event-free survival (EFS) and OS in pediatric patients with AML. IL-6 is one of the ligands that activates STAT3. IL-6 binds a 4-subunit complex composed of 2 gp130 subunits and 2 IL-6 receptors (IL-6Rα) that activates JAK2. Activated JAK2 phosphorylates STAT3 at tyrosine 705 (pY-STAT3). Phosphorylated STAT3 proteins dimerize and are translocated to the nucleus, where they act as transcription factors regulating expression of prosurvival genes.14,15 Clinically, activation of STAT3 promotes cell survival in the presence of cytotoxic chemotherapy.13,16-18 Increased STAT3 activity been associated with chemotherapy resistance and inferior survival across many malignancies.19,20
The aim of this study was to investigate the role that IL-6 in the BM microenvironment plays in determining long-term clinical responses to cytotoxic chemotherapy. We determined the relationship between IL-6 levels in the BM at diagnosis and subsequent EFS. We further determined the effects of IL-6 on chemotherapy-induced apoptosis and on STAT3 signaling in the critical leukemia stem cell (LSC) population. Our findings identify IL-6 as a potential marker with which to improve risk stratification and a potential therapeutic target in pediatric AML.
Materials and methods
Patient samples
Plasma.
In total, 45 pediatric patients with AML who were treated at Texas Children’s Cancer and Hematology Centers between 2007 and 2016 were included on this study. Selection of patient samples was based on both the availability of specimens and on sufficient follow-up time to ascertain EFS. For this reason, our cohort is enriched for relapses. Plasma samples from the diagnostic BM aspirate were collected and frozen at −80°C. All patients initially received treatment according to Children’s Oncology Group protocols AAML0531 or AAML1031.1,21 The regimens were very similar on these 2 trials. Risk stratification differed between the 2 trials, most notably with the inclusion of minimal residual disease measurement on AAML1031. Thus, for the purposes of our data analysis, all 45 patients were assigned a risk group based on the current standard of care at our institution, following AAML1031 guidelines and also including patients with t(10;11), t(6;11), t(6;9), and antecedent myelodysplastic syndrome as HR.7,22,23 This assignment took into account all established prognostic markers for pediatric AML. Plasma from healthy pediatric sibling donors undergoing BM harvest between 2014 and 2016 were used as controls. Cytokine levels were not related to the sample’s age (supplemental Figure 1).
Viably frozen cells.
Samples used for viable cell assays included both excess pheresis product and BM aspirate samples. Prior to cryopreservation, samples were enriched for mononuclear cells using density centrifugation.24 Viably frozen patient samples were thawed into Iscove modified Dulbecco medium (HyClone, Pittsburg, PA) with 20% fetal bovine serum (FBS; Invitrogen) with 100 U/mL penicillin and 100 μg/mL streptomycin (Invitrogen). Cells were washed once with the medium and then incubated in the same medium for 2 hours. Cell number and viability were assessed using the Trypan blue dye exclusion method.25 Samples used for cell-based assays were confirmed to have at least 80% viability after thawing. All patients or guardians provided written informed consent for the use of tissue for research purposes in accordance with the Declaration of Helsinki. These studies were approved by the institutional review board of Baylor College of Medicine.
Cell lines
The human AML cell lines THP-1 and NB4 were obtained from ATCC (Manassas, VA). They were grown in RPMI medium (ATCC) supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. THP-1 and NB4 cells were grown in a humidified 37°C incubator with 5% CO2.
Quantification of cytokines
We evaluated 41 cytokines using the Milliplex Human Cytokine Magnetic Bead Premixed 41-plex kit (EMD Millipore). The measured cytokines included soluble CD40 ligand, epidermal growth factor, eotaxin/C-C motif chemokine 11, fibroblast growth factor 2, Fms-related tyrosine kinase 3 ligand, fractalkine, granulocyte-colony stimulating factor, granulocyte-macrophage colony-stimulating factor, growth-regulated α, interferon-α2 (IFN-α2), IFN-γ, IL-1α, IL-1β, IL-1RA, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17, IFN-γ–inducible protein 10, monocyte chemotactic protein 1, monocyte chemotactic protein 3, macrophage-derived chemokine/C-C motif chemokine 22, macrophage inflammatory protein 1α, macrophage inflammatory protein 1β, platelet-derived growth factor AA, platelet-derived growth factor AB/BB, RANTES (regulated on activation, normal T-cell expressed and secreted), transforming growth factor α, tumor necrosis factor-α (TNF-α), TNF-β, and vascular endothelial growth factor. Experiments were performed in triplicate and according to the manufacturer’s instructions with overnight incubation. A MAGPIX multiplex array reader was used to detect fluorescent signals, and Milliplex Analyst 5.1 was used to analyze data. Standard curves were generated using reference concentrations provided in each kit, and raw relative fluorescence intensity values were converted to cytokine concentrations in picograms per milliliter. When values for individual samples were outside the quantification limits of the test, the result was recorded as the upper or lower quantifiable concentration. Measurement of soluble IL-6Rα (sIL-6Rα), IL-11, oncostatin M (OSM), and leukemia inhibitory factor (LIF) levels were performed on a subset of patient samples (Table 1). These receptors and cytokines were measured using the protocol described above with beads directed toward these analytes (EMD Millipore). Levels of these analytes were measured in duplicate.
Patient data by unique patient number
| UPN . | Age/sex . | Cytogenetics FLT3-ITD . | Risk group . | Remission analysis . | Expanded analysis . | Event . | Outcome . | IL-6 level (pg/mL) . |
|---|---|---|---|---|---|---|---|---|
| 1 | 6.0/F | t(9;11) | LR | Y | Y | Censored | A | 6.34 |
| 2 | 0.76/F | Normal | HR | N | Y | Relapse | DD | 133.46 |
| 3 | 11.2/F | t(8;21) | LR | N | Y | Relapse | DD | 88.62 |
| 4 | 17.0/F | 4+ | LR | N | N | Censored | A | 3.22 |
| 5 | 0.55/F | t(2;11) | LR | Y | N | Relapse | TD | 71.3 |
| 6 | 0.70/F | ND | LR | N | Y | Relapse | DD | 41.36 |
| 7 | 11.2/M | Normal | HR | N | Y | Refractory | DD | 1.59 |
| 8 | 14.9/M | t(6;9) | HR | N | Y | Censored | A | 8369 |
| 9 | 1.5/F | Complex | LR | N | Y | Relapse | DD | 12.23 |
| 10 | 10.4/M | 13q− | HR | N | Y | Censored | A | 62.21 |
| 11 | 11.1/M | t(9;11) | LR | Y | Y | Censored | A | 15.51 |
| 12 | 7.7/M | t(8;21) | LR | Y | Y | Relapse | TD | 47.97 |
| 13 | 9.7/M | t(1;6;11) | HR | N | Y | Relapse | DD | 6.26 |
| 14 | 3.4/F | t(8;21) | LR | Y | N | Censored | A | 465.47 |
| 15 | 13.3/M | t(10;11) | HR | N | N | Refractory | DD | 19.42 |
| 16 | 14.1/F | t(9;11) | LR | Y | Y | Censored | A | 3.31 |
| 17 | 12.5/M | −7 | HR | N | N | Relapse | A | <3 |
| 18 | 4.8/F | t(8;21) | LR | N | Y | Censored | A | 11.88 |
| 19 | 1.8/F | Tetraploid | LR | N | N | Relapse | A | 13.8 |
| 20 | 3.7/F | 11q23 del | LR | N | N | Relapse | A | 6.62 |
| 21 | 0.6/F | t(1;11) | LR | Y | N | Censored | A | 11.79 |
| 22 | 0.4/F | 11q23 del, FLT3-ITD | HR | N | N | Relapse | DD | 13.08 |
| 23 | 12.4/M | Normal | HR | N | N | Relapse | DD | 4.55 |
| 24 | 8.3/M | −7 | HR | Y | Y | DIR | TD | 10.66 |
| 25 | 14.9/M | t(8;21) | LR | Y | Y | Relapse | DD | 34.45 |
| 26 | 4.4/M | FLT3-ITD | HR | N | N | Relapse | DD | 38.83 |
| 27 | 9.7/M | inv(16) | LR | Y | N | Censored | A | 5.15 |
| 28 | 7.1/M | t(8;21) | LR | N | N | Censored | A | 41.81 |
| 29 | 9.7/M | Normal | HR | N | N | Relapse | DD | 4.86 |
| 30 | 14.0/M | inv(16) | LR | Y | N | Relapse | A | 12059 |
| 31 | 0.4/F | t(10;11) | HR | N | N | Censored | A | 4.4 |
| 32 | 8.5/M | inv(16), 7q− | LR | Y | N | Relapse | A | 3.77 |
| 33 | 0.8/M | inv(16) | LR | Y | Y | Censored | A | 3.15 |
| 34 | 12.7/F | Complex | LR | Y | Y | Censored | A | 10.42 |
| 35 | 3.0/M | t(8;21) | LR | Y | Y | Censored | A | 4.78 |
| 36 | 14.3/F | inv(16) | LR | Y | N | Censored | A | 4.7 |
| 37 | 3.3/M | Normal | LR | Y | N | Relapse | DD | 28.12 |
| 38 | 2.1/F | +8, +21 | LR | Y | N | Censored | A | 4.42 |
| 39 | 1.3/F | Complex, t(10;11) | HR | Y | Y | Censored | A | 52.27 |
| 40 | 16.6/F | t(6;11) | HR | N | N | Relapse | DD | <3 |
| 41 | 17.3/M | Derivative 8 | LR | Y | Y | Relapse | A | 26.96 |
| 42 | 1.3/M | t(10;11) | HR | Y | N | Censored | A | 40.12 |
| 43 | 13.6/F | 8+ | HR | N | N | Relapse | DD | 3.19 |
| 44 | 0.6/F | t(11;15) | LR | Y | N | Censored | A | 6.93 |
| 45 | 17.7/M | 13q− | HR | N | N | Relapse | A | 6.86 |
| NBM1 | 5.8/F | — | — | — | Y | — | — | 2.62 |
| NBM2 | 12.2/M | — | — | — | — | — | — | <1.7 |
| NBM3 | 9.2/F | — | — | — | — | — | — | 4.54 |
| NBM4 | 3.1/F | — | — | — | Y | — | — | <1.53 |
| NBM5 | 13.0/M | — | — | — | Y | — | — | <1.7 |
| NBM6 | 6.7/M | — | — | — | Y | — | — | 5.67 |
| NBM7 | 4.8/M | — | — | — | Y | — | — | <1.53 |
| UPN . | Age/sex . | Cytogenetics FLT3-ITD . | Risk group . | Remission analysis . | Expanded analysis . | Event . | Outcome . | IL-6 level (pg/mL) . |
|---|---|---|---|---|---|---|---|---|
| 1 | 6.0/F | t(9;11) | LR | Y | Y | Censored | A | 6.34 |
| 2 | 0.76/F | Normal | HR | N | Y | Relapse | DD | 133.46 |
| 3 | 11.2/F | t(8;21) | LR | N | Y | Relapse | DD | 88.62 |
| 4 | 17.0/F | 4+ | LR | N | N | Censored | A | 3.22 |
| 5 | 0.55/F | t(2;11) | LR | Y | N | Relapse | TD | 71.3 |
| 6 | 0.70/F | ND | LR | N | Y | Relapse | DD | 41.36 |
| 7 | 11.2/M | Normal | HR | N | Y | Refractory | DD | 1.59 |
| 8 | 14.9/M | t(6;9) | HR | N | Y | Censored | A | 8369 |
| 9 | 1.5/F | Complex | LR | N | Y | Relapse | DD | 12.23 |
| 10 | 10.4/M | 13q− | HR | N | Y | Censored | A | 62.21 |
| 11 | 11.1/M | t(9;11) | LR | Y | Y | Censored | A | 15.51 |
| 12 | 7.7/M | t(8;21) | LR | Y | Y | Relapse | TD | 47.97 |
| 13 | 9.7/M | t(1;6;11) | HR | N | Y | Relapse | DD | 6.26 |
| 14 | 3.4/F | t(8;21) | LR | Y | N | Censored | A | 465.47 |
| 15 | 13.3/M | t(10;11) | HR | N | N | Refractory | DD | 19.42 |
| 16 | 14.1/F | t(9;11) | LR | Y | Y | Censored | A | 3.31 |
| 17 | 12.5/M | −7 | HR | N | N | Relapse | A | <3 |
| 18 | 4.8/F | t(8;21) | LR | N | Y | Censored | A | 11.88 |
| 19 | 1.8/F | Tetraploid | LR | N | N | Relapse | A | 13.8 |
| 20 | 3.7/F | 11q23 del | LR | N | N | Relapse | A | 6.62 |
| 21 | 0.6/F | t(1;11) | LR | Y | N | Censored | A | 11.79 |
| 22 | 0.4/F | 11q23 del, FLT3-ITD | HR | N | N | Relapse | DD | 13.08 |
| 23 | 12.4/M | Normal | HR | N | N | Relapse | DD | 4.55 |
| 24 | 8.3/M | −7 | HR | Y | Y | DIR | TD | 10.66 |
| 25 | 14.9/M | t(8;21) | LR | Y | Y | Relapse | DD | 34.45 |
| 26 | 4.4/M | FLT3-ITD | HR | N | N | Relapse | DD | 38.83 |
| 27 | 9.7/M | inv(16) | LR | Y | N | Censored | A | 5.15 |
| 28 | 7.1/M | t(8;21) | LR | N | N | Censored | A | 41.81 |
| 29 | 9.7/M | Normal | HR | N | N | Relapse | DD | 4.86 |
| 30 | 14.0/M | inv(16) | LR | Y | N | Relapse | A | 12059 |
| 31 | 0.4/F | t(10;11) | HR | N | N | Censored | A | 4.4 |
| 32 | 8.5/M | inv(16), 7q− | LR | Y | N | Relapse | A | 3.77 |
| 33 | 0.8/M | inv(16) | LR | Y | Y | Censored | A | 3.15 |
| 34 | 12.7/F | Complex | LR | Y | Y | Censored | A | 10.42 |
| 35 | 3.0/M | t(8;21) | LR | Y | Y | Censored | A | 4.78 |
| 36 | 14.3/F | inv(16) | LR | Y | N | Censored | A | 4.7 |
| 37 | 3.3/M | Normal | LR | Y | N | Relapse | DD | 28.12 |
| 38 | 2.1/F | +8, +21 | LR | Y | N | Censored | A | 4.42 |
| 39 | 1.3/F | Complex, t(10;11) | HR | Y | Y | Censored | A | 52.27 |
| 40 | 16.6/F | t(6;11) | HR | N | N | Relapse | DD | <3 |
| 41 | 17.3/M | Derivative 8 | LR | Y | Y | Relapse | A | 26.96 |
| 42 | 1.3/M | t(10;11) | HR | Y | N | Censored | A | 40.12 |
| 43 | 13.6/F | 8+ | HR | N | N | Relapse | DD | 3.19 |
| 44 | 0.6/F | t(11;15) | LR | Y | N | Censored | A | 6.93 |
| 45 | 17.7/M | 13q− | HR | N | N | Relapse | A | 6.86 |
| NBM1 | 5.8/F | — | — | — | Y | — | — | 2.62 |
| NBM2 | 12.2/M | — | — | — | — | — | — | <1.7 |
| NBM3 | 9.2/F | — | — | — | — | — | — | 4.54 |
| NBM4 | 3.1/F | — | — | — | Y | — | — | <1.53 |
| NBM5 | 13.0/M | — | — | — | Y | — | — | <1.7 |
| NBM6 | 6.7/M | — | — | — | Y | — | — | 5.67 |
| NBM7 | 4.8/M | — | — | — | Y | — | — | <1.53 |
Age is given in years at the time of initial diagnosis. Risk group is defined as per AAML1031 guidelines with the following additions: mixed lineage leukemia translocations identified as poor risk factors, t(6;9)(p23;q34), and myelodysplastic syndrome evolving into AML were also considered HR characteristics. Outcome column indicates status at last contact.
A, alive at time of last follow-up; DD, death due to disease; DIR, death in remission; F, female; ITD, internal tandem duplication; M, male; NBM, normal bone marrow; ND, not done; TD, death in remission due to toxicity; UPN, unique patient number.
Assessment of IL-6–induced chemoprotection
AML cell lines were plated in standard growth medium (described above) and exposed to exogenous IL-6 and sIL-6Rα (50 ng/mL and 100 ng/mL, respectively) or vehicle for 24 hours. Mitoxantrone, etoposide, or cytarabine was added, and cells were incubated for another 24 hours. Apoptosis was quantified based on Annexin V/fluorescein isothiocyanate and propidium iodide positivity using an LSRII flow cytometer (BD Biosciences). Data were analyzed using FCS Express 5 software (De Novo, Glendale, CA). For primary sample culture, cells were plated in Iscove modified Dulbecco medium with 20% FBS, exposed to IL-6 and sIL-6Rα (5 ng/mL and 10 ng/mL, respectively) or vehicle for 6 hours, and treated with mitoxantrone for 18 hours. Mitoxantrone-induced apoptosis was quantified as described above. We chose mitoxantrone as the representative anthracycline, because its fluorescence spectrum overlaps much less with our analytical fluorochromes than daunorubicin.
IL-6–induced STAT3 activity in LSCs
Diagnostic BM samples from 10 pediatric patients with AML were thawed, and a viability ≥80% was confirmed using a Trypan blue exclusion assay. Primary samples were sorted into LSC-enriched (LSCe) (CD34+/CD38−/Aldefluorint) and non–stem AML cell (CD34+/CD38−/Aldefluorlow) populations using a MoFlo Cell Sorter (Beckman Coulter) as described previously.26 Cells from each subpopulation were divided into 3 aliquots. Two were left untreated to use as an isotype control and determine constitutive pY-STAT3 levels. The remaining aliquot was stimulated for 15 minutes with 50 ng/mL IL-6 and 100 ng/mL sIL-6Rα. Cells were fixed, permeabilized, and stained with hCD45-V450, isotype control/phycoerythrin, and pY-STAT3/phycoerythrin as appropriate (BD Biosciences). Flow cytometry data were collected on an LSRII flow cytometer and analyzed using FCS Express 4. For each subpopulation, pY-STAT3 was measured in untreated and stimulated cells. Data are expressed as the percentage of cells in the positive region, as defined by the isotype control. A Wilcoxon signed-rank test was used to search for significant differences in parameters between subpopulations.
Statistics
For each cytokine, the mean concentration (in picograms per milliliter) was reported. When cytokine level data passed the Shapiro-Wilks normality test, healthy donor and AML patient samples were compared using a Student t test. In cases where data did not pass the normality test, a Wilcoxon nonparametric test was used. To account for multiple comparisons within the multiplex analysis, the Bonferroni method produced an adjusted significance level of 0.0012 to identify significant differences between cytokines in patients vs healthy controls. Only differences meeting this stringent threshold were reported. Cytokine levels were analyzed using cut-point analysis to determine whether levels were associated with clinical outcome. Five cut points for each of the 3 differentially expressed cytokines were tested with the selection interval including the inner 80% of the continuous covariate’s distribution. The 5 cut points tested were the integer values closest to the 10th, 25th, 50th, 75th, and 90th percentiles. The optimal cut point was determined by the minimum P value approach, and clinical outcomes were compared between groups. EFS was defined as the time from diagnosis to the identification of refractory disease, relapse, or toxic death. Estimates of EFS were reported with corresponding Greenwood standard errors. Groups were compared for significant differences using the log-rank test. Adjustment to the significance by the Bonferroni method produced a significance threshold of 0.01. P values falling between 0.05 and 0.01 are notated within the text and within figure legends. All analyses were performed using GraphPad Prism version 6.05 for Windows (GraphPad Software, La Jolla, CA).
Results
Plasma cytokine profiles of pediatric patients with AML compared with those of healthy donors
We analyzed the cytokine profiles of plasma samples collected from the diagnostic BM aspirates of pediatric patients with AML (AML cohort; n = 45) and from normal BM collected from healthy sibling donors (NBM cohort; n = 7). Among the 41 cytokines and chemokines surveyed, 3 had significantly different levels in the AML cohort compared with the NBM cohort. For example, IL-6 levels were significantly elevated in AML patients compared with normal controls (Figure 1A; P = .0001). The median IL-6 level in the AML cohort was 11.79 pg/mL (interquartile range, 4.62-40.74). In the NBM cohort, the median IL-6 level was 1.7 pg/mL (interquartile range, 1.53-4.54). IL-6 levels were below the lower limit of the assay for 3 of the 45 samples in the AML cohort and 4 of the 7 samples in the NBM cohort.
Cytokine levels vary in pediatric AML at diagnosis. Cytokine levels from BM plasma samples taken at the time of diagnosis for patients with untreated AML were compared with those of healthy children. After Bonferroni correction, 3 out of 41 tested cytokines and growth factors had levels at AML diagnosis that were significantly different when compared with normal controls. Levels of IL-6 (A), IL-10 (B), and IL-8 (C) were significantly elevated in a subset of AML patients at diagnosis compared with normal children. ***P < .001 by Mann-Whitney U test. The threshold of significance after Bonferroni correction was P = .0012.
Cytokine levels vary in pediatric AML at diagnosis. Cytokine levels from BM plasma samples taken at the time of diagnosis for patients with untreated AML were compared with those of healthy children. After Bonferroni correction, 3 out of 41 tested cytokines and growth factors had levels at AML diagnosis that were significantly different when compared with normal controls. Levels of IL-6 (A), IL-10 (B), and IL-8 (C) were significantly elevated in a subset of AML patients at diagnosis compared with normal children. ***P < .001 by Mann-Whitney U test. The threshold of significance after Bonferroni correction was P = .0012.
In addition to IL-6, levels of IL-10 and IL-8 were significantly different in the AML cohort compared with the NBM cohort (Figure 1B-C). Importantly, in our patient group, the concentrations of these cytokines were elevated in a subset of AML patient samples. This result suggests that the composition of cytokines within the BM microenvironment may vary substantially between patients. This variability in cytokine levels provided the ability to evaluate for potential relationships between cytokine levels and clinical outcomes.
Cytokine levels at diagnosis and clinical outcome
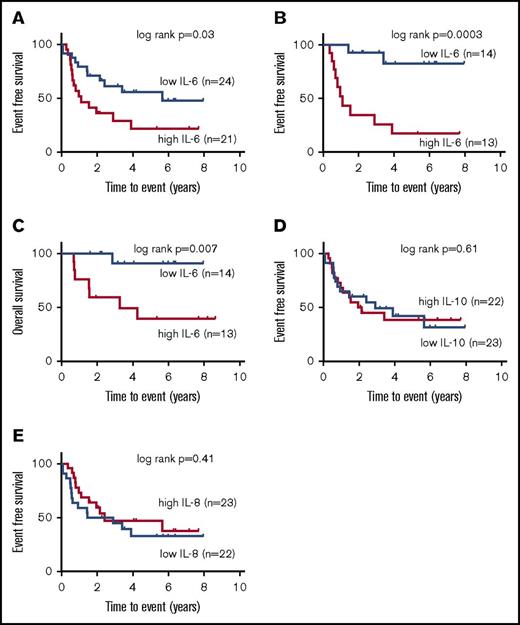
We performed cut-point analyses to determine if clinical outcomes were correlated with cytokine levels. Our previous study showed that increased IL-6–induced pY-STAT3 at relapse compared with diagnosis was a predictor of poor outcomes after relapse.20 Therefore, we first examined whether elevated IL-6 levels at diagnosis were associated with an increased risk of relapse. By cut-point analysis, 12 pg/mL was identified as the most discriminating level of IL-6 by which to group patients by clinical outcome. Using the cut point of 12 pg/mL IL-6 for the entire cohort (n = 45), both EFS and OS were evaluated. The 5-year EFS was 20.6% ± 10% for patients with high IL-6 levels at diagnosis compared with 43.4% ± 12% for those with low IL-6 levels (Figure 2A; log-rank P = .036). Differences in 5-year OS were not significant between groups (supplemental Figure 2A).
Elevated IL-6 levels are associated with inferior clinical outcome. (A) Using a cut point of 12 pg/mL for the entire cohort (n = 45), 5-year EFS was 20.6% ± 10% for those with high IL-6 levels at diagnosis vs 43.4% ± 12% for those with low IL-6 levels (log-rank P = .036). (B) For LR patients (n = 27), a cut point of 12 pg/mL identified patients with inferior EFS (17.3% ± 11% vs 82.5% ± 11% for high vs low IL-6 levels, P = .0003). (C) Inferior OS was also noted for the LR patients (cut point of 12 pg/mL, 39% ± 14.8% vs 90.1% ± 8.7% for high vs low IL-6 levels, P = .007, n = 27). No other cytokines were associated with clinical outcome in our cohort. Notably, levels of IL-8 (D) and IL-10 (E) were not associated with EFS. Estimates of EFS are reported with corresponding Greenwood standard errors. Groups were compared for significant differences by the log-rank test.
Elevated IL-6 levels are associated with inferior clinical outcome. (A) Using a cut point of 12 pg/mL for the entire cohort (n = 45), 5-year EFS was 20.6% ± 10% for those with high IL-6 levels at diagnosis vs 43.4% ± 12% for those with low IL-6 levels (log-rank P = .036). (B) For LR patients (n = 27), a cut point of 12 pg/mL identified patients with inferior EFS (17.3% ± 11% vs 82.5% ± 11% for high vs low IL-6 levels, P = .0003). (C) Inferior OS was also noted for the LR patients (cut point of 12 pg/mL, 39% ± 14.8% vs 90.1% ± 8.7% for high vs low IL-6 levels, P = .007, n = 27). No other cytokines were associated with clinical outcome in our cohort. Notably, levels of IL-8 (D) and IL-10 (E) were not associated with EFS. Estimates of EFS are reported with corresponding Greenwood standard errors. Groups were compared for significant differences by the log-rank test.
Next, we analyzed the subset of patients that would be currently classified as LR. In this subset (n = 27), 12 relapses (43%) were observed, which was a sufficient number of events to evaluate differences in clinical outcome between groups despite the relatively small sample size. Among patients with high IL-6 levels (n = 13), 5-year EFS was 17.3% ± 11% compared with 82.5% ± 11% for those patients with low IL-6 levels (n = 14; P = .0003; Figure 2B). OS for those with high IL-6 levels was 39.4% ± 15% compared with 90.1% ± 9% for those patients with low IL-6 levels (n = 14; P = .007; Figure 2C). There were no toxic deaths prior to relapse among these 27 patients. Therefore, disease-free survival is equivalent to EFS in the LR patients. Patients that were classified as HR had poor OS and EFS regardless of IL-6 levels at diagnosis (supplemental Figure 2B-C).
The other cytokines that were significantly different in diagnostic AML samples compared with normal BM, IL-8 and IL-10, were subjected to cut-point analysis. In contrast to results adult AML,10,11 IL-8 and IL-10 levels were not correlated with EFS (Figure 2D-E).
IL-6 levels and clinical signs of inflammation at diagnosis
Because IL-6 is an inflammatory cytokine that plays a key role in the acute-phase immune response, we performed a chart review to determine the likelihood of a concurrent inflammatory process when the BM plasma sample was collected. Specifically, we looked for documentation of fever or infection at the time of diagnosis as these factors could contribute to elevated IL-6 levels. Fever at diagnosis or parental report of fever at presentation was documented in 29 of 45 patients with AML. Fever correlated weakly with IL-6 levels (Spearman R = 0.30, P = .02) but did not correlate with outcome (supplemental Figure 3A-B). Infection at the time of diagnosis was documented in 6 patients (13%) and did not correlate with IL-6 levels (supplemental Figure 3C).
IL-6 levels and clinical characteristics including body mass index
Due to the known relationship between obesity and outcome in pediatric AML and recent evidence suggesting that IL-6 may have a role in fatty acid metabolism and obesity,27 we further interrogated our data for correlations between clinical characteristics and IL-6 levels. Body mass index did not correlate with IL-6 levels (supplemental Figure 4A; Spearman R = 0.1244, P = .2562, n = 30). Furthermore, age, gender, BM blast percentage on initial biopsy, and white blood cell count at diagnosis did not correlate with IL-6 levels (supplemental Figure 4B-E). Although our cohort was too small for a true multivariate analysis, we interrogated our data for relationships between IL-6 levels and cytogenetic and mutation data and found no significant relationships.
Cytokine levels at diagnosis compared with remission
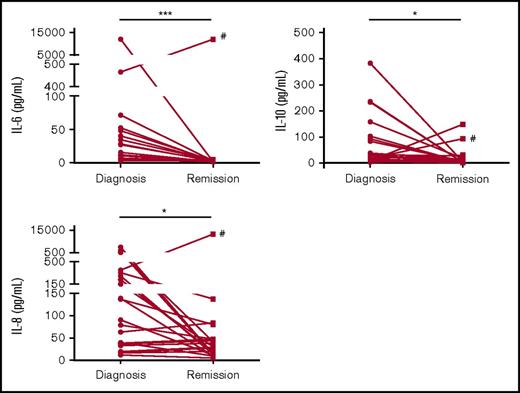
In an exploratory analysis, we compared paired diagnostic and remission plasma samples. In the majority of patients, the levels of differentially expressed cytokines normalized to that of healthy donors at the time of remission (Figure 3). For example, 20 of the 22 patients with plasma samples collected at both diagnosis and remission (remission cohort) had significantly decreased IL-6 levels at remission compared with diagnosis (Figure 3A). The median IL-6 level of the remission samples was 2.8 pg/mL, compared with 11.2 pg/mL at diagnosis (P = .0004). Of note, the one patient with a high IL-6 level at remission (Figure 3A) had no documented signs of infection at either diagnosis or remission.
Alterations in cytokine levels return toward normal levels at remission in the majority of patients. For 22 of the AML patients, matched remission BM plasma samples were available. BM plasma IL-6, IL-10, and IL-8 levels decreased in the majority of patients at remission compared with diagnosis. *P < .05, **P < .01, ***P < .001. #Unique patient number 14.
Alterations in cytokine levels return toward normal levels at remission in the majority of patients. For 22 of the AML patients, matched remission BM plasma samples were available. BM plasma IL-6, IL-10, and IL-8 levels decreased in the majority of patients at remission compared with diagnosis. *P < .05, **P < .01, ***P < .001. #Unique patient number 14.
Evaluation of other IL-6 family cytokines
IL-6 is one of 6 members of the IL-6 family of cytokines. The IL-6 family cytokines signal through receptor complexes containing the transmembrane gp130 receptor subunit. Therefore, we expanded our cytokine analysis to evaluate plasma levels of other IL-6 cytokine family members, IL-11, OSM, and LIF. We also quantified the level of sIL-6Rα in this subset of samples. No differences in the levels of IL-11, LIF, or sIL-6Rα were observed between AML and NBM samples (Figure 4; supplemental Figure 6). The OSM levels in diagnostic samples from the AML patients were significantly higher than those from the normal controls (median of 30.3 pg/mL vs 11.7 pg/mL, respectively; P = .03). No differences were observed between diagnosis and remission, and cut-point analysis did not show an association between OSM level and EFS (Figure 4).
OSM and sIL-6Rα do not predict survival in pediatric AML samples. For a subset of patients with available samples, levels of OSM and sIL-6Rα were evaluated. For OSM, levels between diagnostic AML BM plasma samples and normal controls differed significantly (A), though there was no difference between levels at diagnosis vs remission in a paired sample analysis (B). No cut point was identified that predicted outcome for OSM level (cut point of 50 pg/mL is shown in panel C). sIL-6Rα BM plasma levels were not significantly different between normal controls and AML samples at diagnosis (D) or between diagnostic and remission samples in a paired sample analysis (E). *P < .05; ns, not significant.
OSM and sIL-6Rα do not predict survival in pediatric AML samples. For a subset of patients with available samples, levels of OSM and sIL-6Rα were evaluated. For OSM, levels between diagnostic AML BM plasma samples and normal controls differed significantly (A), though there was no difference between levels at diagnosis vs remission in a paired sample analysis (B). No cut point was identified that predicted outcome for OSM level (cut point of 50 pg/mL is shown in panel C). sIL-6Rα BM plasma levels were not significantly different between normal controls and AML samples at diagnosis (D) or between diagnostic and remission samples in a paired sample analysis (E). *P < .05; ns, not significant.
IL-6 and chemotherapy resistance
To determine how elevated IL-6 levels in the BM niche could contribute to increased relapse risk, we tested the effect of exogenous IL-6 on chemotherapy-induced apoptosis. We evaluated the effect of IL-6 pathway stimulation on apoptosis induced by cytarabine, etoposide, and mitoxantrone in AML cell lines and primary AML cell cultures. We stimulated with IL-6 and sIL-6Rα, because sIL-6Rα is expressed ubiquitously in the BM niche, but the membrane-bound form is variably expressed on blasts (Figure 4). Exogenous IL-6/sIL-6Rα protected AML blasts from mitoxantrone-induced apoptosis in both AML cell lines tested (Figure 5A-B). However, exogenous IL-6 did not significantly affect cytarabine- or etoposide-induced apoptosis in these cell lines (data not shown). These results are consistent with our prior finding that environment-induced mitoxantrone resistance is driven primarily by soluble factors as opposed to stromal contact interactions, whereas resistance to etoposide and cytarabine requires contact interactions.28 When primary AML samples (n = 7) were treated with IL-6 and sIL-6Rα for 6 hours and then exposed to mitoxantrone for 18 hours, apoptosis was significantly reduced in cultures. A representative patient sample (Figure 5C) and composite data from the seven tested samples are shown (Figure 5D).
Exposure to IL-6 reduces chemotherapy-induced apoptosis. The AML cell lines THP-1 (A) and NB4 (B) had less mitoxantrone-induced apoptosis when pretreated for 24 hours with IL-6 and sIL-6Rα at 50 ng/mL and 100 ng/mL, respectively (red), than vehicle-treated cells (blue) (THP-1, n = 5; NB4, n = 8; *P < .05, analysis of variance). (C) Representative primary sample demonstrating decreased mitoxantrone-induced apoptosis with IL-6 pretreatment, and (D) mean apoptosis rates of 7 primary AML samples with vs without IL-6 pretreatment. Duration of mitoxantrone exposure was 24 hours for cell lines and 18 hours for primary samples. Values shown are mean ± standard error of the mean; P < .05 by Wilcoxon signed-rank test. FITC, fluorescein isothiocyanate.
Exposure to IL-6 reduces chemotherapy-induced apoptosis. The AML cell lines THP-1 (A) and NB4 (B) had less mitoxantrone-induced apoptosis when pretreated for 24 hours with IL-6 and sIL-6Rα at 50 ng/mL and 100 ng/mL, respectively (red), than vehicle-treated cells (blue) (THP-1, n = 5; NB4, n = 8; *P < .05, analysis of variance). (C) Representative primary sample demonstrating decreased mitoxantrone-induced apoptosis with IL-6 pretreatment, and (D) mean apoptosis rates of 7 primary AML samples with vs without IL-6 pretreatment. Duration of mitoxantrone exposure was 24 hours for cell lines and 18 hours for primary samples. Values shown are mean ± standard error of the mean; P < .05 by Wilcoxon signed-rank test. FITC, fluorescein isothiocyanate.
IL-6 and the LSC population of AML
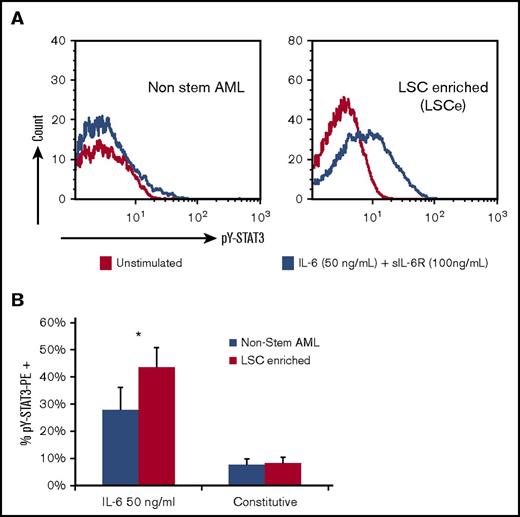
Our previous study showed that IL-6–induced pY-STAT3 is frequently increased at relapse, even in patients with minimal measurable IL-6–induced pY-STAT3 at diagnosis.20 LSCs are described to be relatively quiescent and thus more likely to survive upfront chemotherapy. Furthermore, LSCs retain the ability to generate a clone that emerges at relapse. Therefore, we investigated the effect of IL-6 on LSCs. Diagnostic AML samples (n = 8) were sorted into LSCe (CD34+/CD38−/Aldefluorint) and non–stem AML populations (CD34+/CD38−/Aldefluorlow),26 and the levels of constitutive and IL-6–induced STAT3 phosphorylation were measured by flow cytometry (see supplemental Figure 6 for the gating strategy used). The LSCe population comprised 1.2% to 12% of sorted cells (mean, 5.6%). The LSCe populations had a higher percentage of cells with IL-6–induced pY-STAT3 compared with the paired non–stem AML populations (44% ± 7.3% vs 28% ± 8.3%, respectively; P = .039, n = 8). There were no differences in levels of constitutive pY-STAT3 between LSCe and non–stem AML populations (8.2% ± 2.1% vs 7.7% ± 2.1%; Figure 6). These data show that IL-6–induced STAT3 signaling pathways are frequently more robust in the LSCe population than the non–stem AML population in pediatric patients with AML. Together, our results suggest that IL-6 in the microenvironment promotes relapse by supporting LSCs and augmenting chemotherapy resistance.
IL-6–induced pY-STAT3 is increased in the leukemia stem cell enriched (LSCe) subpopulation. (A) Histograms of sorted subpopulations of a representative diagnostic AML BM sample. Constitutive (yellow) and IL-6–induced (blue) pY-STAT3 are shown for the non–stem AML population (left) and the LSCe population (right). (B) A significant difference in the mean percentage of cells with IL-6–induced pY-STAT3 in the LSCe subpopulation compared with the non–stem AML subpopulation was found. No differences were seen in constitutive pY-STAT3 between sorted populations (n = 8). Bar graphs show mean ± standard error of the mean. *P < .05. PE, phycoerythrin.
IL-6–induced pY-STAT3 is increased in the leukemia stem cell enriched (LSCe) subpopulation. (A) Histograms of sorted subpopulations of a representative diagnostic AML BM sample. Constitutive (yellow) and IL-6–induced (blue) pY-STAT3 are shown for the non–stem AML population (left) and the LSCe population (right). (B) A significant difference in the mean percentage of cells with IL-6–induced pY-STAT3 in the LSCe subpopulation compared with the non–stem AML subpopulation was found. No differences were seen in constitutive pY-STAT3 between sorted populations (n = 8). Bar graphs show mean ± standard error of the mean. *P < .05. PE, phycoerythrin.
Discussion
Accurate risk stratification is critical for determining the intensity of therapy. Clinicians need to select a treatment that is aggressive enough to cure the disease while limiting treatment-related toxicity. Additionally, they need to know when it is appropriate to incorporate targeted agents. To address this need, we investigated various cytokines in plasma from BM aspirates to determine their potential as prognostic markers and biological factors contributing to treatment failure. In this study, IL-6, IL-10, and IL-8 levels were significantly different in the AML cohort compared with the NBM cohort. These data are similar to several previous studies that showed elevated levels in peripheral blood and BM of IL-6, IL-10, IL-8, IL-4, TNF-α, and IL-17A in adult patients with AML.10,11 Among the various cytokines tested, only elevated IL-6 levels predicted lower survival in our cohort. The significance of this relationship was emphasized when data were limited to patients classified as LR by current criteria. Importantly, based on extensive clinical records, the elevated IL-6 levels were not strongly correlated with clinical signs of inflammation or infection. Thus, IL-6 may be valuable as a marker of HR disease and as a target for new therapies for pediatric patients with AML.
Our data generate the question of the source of the elevated IL-6 within the BM niche. One potential source is AML blasts autosecreting IL-6. IL-6 autosecretion has been demonstrated previously in solid tumors and in adults with AML.29-32 An alternative source of IL-6 production is normal stromal cells or monocytes within the BM. It is possible that through paracrine signals, AML blasts are driving IL-6 production by these normal cells.33 Our observation of decreased IL-6 levels in plasma collected during remission would be compatible with either explanation but the lack of correlation between IL-6 levels, and BM blast percentage at diagnosis supports a paracrine process. Additional studies are planned to better delineate the source of elevated IL-6 within the BM niche at diagnosis.
Next, we investigated potential biological mechanisms by which elevated IL-6 levels could reduce survival in pediatric patients with AML. Since STAT3 signaling is known to regulate antiapoptosis gene expression, one possible mechanism could be by promoting chemoresistance. In this study, we showed that exogenous IL-6 reduced mitoxantrone-induced apoptosis in AML cell lines and primary cultures. Importantly, this effect was seen only with mitoxantrone. This is consistent with our prior work demonstrating that soluble factors are most important in mediating chemoresistance to mitoxantrone while adhesion mediated factors are important for resistance to etoposide and cytarabine.28 Since mitoxantrone is used in current upfront pediatric AML treatment protocols in North America, this finding is potentially clinically significant.
Another possible mechanism by which IL-6 promotes relapse could be by supporting the LSCs. To this point, we showed that a higher proportion of cells in the LSCe subpopulation had increased pY-STAT3 levels following treatment with exogenous IL-6 compared with non–stem AML cells. Based on these results, we hypothesize that IL-6–induced pY-STAT3 activity could potentially help cancer cells, particularly the critical LSC subpopulation, survive initial rounds of chemotherapy and allow them to subsequently expand, leading to relapsed disease. This hypothesis is supported by our previous study showing increased levels of IL-6–induced pY-STAT3 at relapse compared with those at diagnosis.20 The small sample size and lack of inclusion of patients from different institutions are limitations of our study. Additionally, our cohort is enriched for relapses with 43% of LR patients experiencing a relapse, a higher rate than is seen within children currently classified as LR.7 A large validation study utilizing peripheral blood samples from multiple sites and a high sensitivity assay is currently underway. Determining whether this potential predictive marker will have sufficient positive predictive value when tested in a cohort with fewer events will be important in the further evaluation of the clinical utility of IL-6. Despite these limitations of our study, we have identified a potential mechanism of chemoresistance in pediatric AML. Importantly, the IL-6–induced STAT3 pathway can be targeted with the US Food and Drug Administration–approved sIL-6 receptor monoclonal antibody tocilizumab. Studies are underway to evaluate the feasibility of incorporating this agent into existing treatment regimens as a chemosensitizing agent.
In summary, our data support the prospective validation of IL-6 levels in plasma from BM as a factor in risk stratification algorithms. In addition, treatments that target the IL-6 signaling pathway could potentially eradicate chemoresistant LSCs and prevent relapse. However, additional studies are needed. Among these, we need to investigate agents that target the IL-6–induced STAT3 pathway to determine which patients are most likely to respond to the intervention and the clinical feasibility of targeting this pathway.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the Cytometry and Cell Sorting Core at Baylor College of Medicine and acknowledge the expert assistance of Tatiana Gotslova.
The Cytometry and Cell Sorting Core at Baylor College of Medicine is funded by the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (grant P30 AI036211), NIH, National Cancer Institute (grant P30 CA125123), and NIH, National Center for Research Resources (grant S10 RR024574). Collection and storage of human specimens by the Children’s Oncology Group is supported through funding from the NIH, National Cancer Institute (grant U24CA114766). This work was supported by grants from the Thrasher Research Fund Early Career Award (A.M.S.), Children’s Leukemia Research Association (A.M.S.), a gift from the Turn it Gold Fund (A.M.S.), and NIH, National Cancer Institute grants R01CA17026 (M.S.R.) and K12CA090433 (A.M.S.).
Authorship
Contribution: A.M.S. designed and performed experiments, analyzed and interpreted data, collected clinical data, and wrote the manuscript; J.M.M. collected clinical data; J.O.M. conducted experiments; A.S.G. assisted with performing experiments; and M.S.R. designed experiments, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alexandra M. Stevens, Texas Children’s Cancer and Hematology Centers, 1102 Bates St, Suite 750, Houston, TX 77030; e-mail: amsteven@txch.org.