Key Points
Transplantation-associated thrombotic microangiopathy is associated with complement activation in vitro.
This data further supports the use of eculizumab for the treatment of patients with TA-TMA.
Introduction
Hematopoietic stem cell transplantation (HSCT)–associated thrombotic microangiopathy (TA-TMA) significantly affects transplantation-related morbidity and mortality.1-5 The cause of TA-TMA has remained unknown over the past several decades, limiting treatment options to nonspecific therapies adapted from other diseases.6,7 The role of complement activation in TA-TMA is controversial; moreover, the magnitude of any role is debated. Recent data demonstrating remission of TA-TMA after use of the anti-C5 monoclonal antibody eculizumab suggest a central pathophysiologic role of the complement cascade.8-10 The modified Ham test uses PIGA-null TF-1 cells, which lack expression of the complement regulatory proteins CD55 and CD59, rendering them increasingly vulnerable to complement-mediated cell death.11 This test has been used in the research setting to distinguish microangiopathic diseases based on the degree of complement activation.11,12 We aimed to determine if TA-TMA is associated with complement activation in vitro using a modified Ham test.
Methods
After obtaining institutional review board approval, we assayed 5 samples of pooled normal human serum (NHS; Sigma-Aldrich, St Louis, MO), serum samples from 6 patients diagnosed with TA-TMA, and serum samples from 5 patients post-HSCT without TA-TMA. This study was conducted in accordance with the Declaration of Helsinki. Six replicates of each assay were performed. Patient samples had been collected at weekly intervals during HSCT and stored at −80°C in the Cincinnati Children’s Hospital HSCT repository. Samples from patients with TA-TMA were obtained after a diagnosis of TA-TMA was made2 but before any anticomplement therapy was administered.
PIGA-null TF1 cells were maintained in culture as previously described.11,13 PIGA-null TF-1 cells lack expression of the complement regulatory proteins CD55 and CD59, rendering them increasingly vulnerable to complement-mediated cell death.11 We performed the modified Ham test essentially as described by Gavriilaki et al.11 Briefly, we assayed PIGA-null TF-1 cells via flow cytometry to confirm they lacked CD59 expression. We plated cells in 96-well V-bottom plates (Corning, New York, NY) until confluent and then washed with phosphate-buffered saline. Serum was diluted in gelatin veronal buffer (Sigma-Aldrich) at a concentration of 1:5 to a total volume of 100 μL. Cells were incubated with serum for 30 minutes at 37°C. Cells were washed with phosphate-buffered saline and incubated with the cell proliferation reagent WST-1 (Roche, Basel, Switzerland) for 2 hours at 37°C. We diluted WST-1 in the cell-culture medium at a concentration of 1:10 and added 100 μL of WST-1 solution per well. The colorimetric assay is based on cleavage of WST-1 by mitochondrial dehydrogenases in viable cells.11,14 We measured absorbance in a SpectraMax 190 Reader (Molecular Devices, Sunnyvale, CA) at 440 nm with a reference wavelength at 600 nm.
Heat-inactivated serum was used as a negative control by taking the same patient’s serum and incubating at 56°C for 30 minutes. We used lipopolysaccharide 1 mg/mL (Sigma-Aldrich), a potent activator of the complement cascade,15,16 as a positive control. We incubated the serum solution at a concentration of 1:100 with lipopolysaccharide at 37°C for 10 minutes, then at a minimum of 5 minutes on ice before incubation with cells.
We normalized absorbance values of each sample after subtraction of the absorbance value of a blank cell containing 100 μL of the WST-1 solution. Percentage of viable cells was expressed as a ratio of the absorbance of each sample multiplied by 100 to the absorbance of the same sample’s heat-inactivated control. We determined cell nonviability using the following equation: (100% − [sample absorbance/heat-inactivated sample absorbance]). We exported data to Prism (GraphPad Software, La Jolla, CA) for analysis. We compared groups using a Wilcoxon rank sum test.
Results and discussion
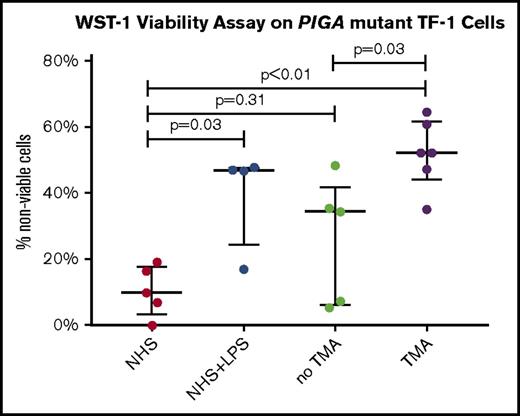
Samples were obtained a mean of 39.6 days post-HSCT (Table 1; supplemental Table 1). Patients with TA-TMA had higher median levels of lactate dehydrogenase (408 vs 218 U/L; P = .02) at the time of sample procurement. Patients with TA-TMA were receiving a median of 3 antihypertensive medications, whereas those without TA-TMA were receiving a median of 1 antihypertensive. Four of 5 patients with TA-TMA were eventually treated with eculizumab (after sample procurement). We identified a greater percentage of nonviable PIGA-negative cells after incubation with serum from patients with TA-TMA than patients post-HSCT without TA-TMA (median, 52.3% vs 34.5%; P = .03) or pooled NHS (median, 52.3% vs 9.9%; P < .01; Figure 1). Patients without TA-TMA did not significantly differ from pooled NHS (median, 34.5% vs 9.9%, P = .31).
Patient characteristics
| Patient . | TA-TMA . | Day post-HSCT . | LDH (normal range), U/L . | Haptoglobin (normal range, 16-200), mg/dL . | sC5b-9 (normal range, 72-244), ng/mL . | Serum creatinine (current-to-baseline ratio) . | Schistocytes . | Urine protein-to-creatinine ratio . | Quantity of antihypertensives . | Treated with eculizumab . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Yes | +96 | 436 (135-395) | <7 | 301 | 0.5 | None | 7.2 | 4 | Yes, day +100 |
| 2 | Yes | +6 | 1293 (155-450) | 9 | 196 | 0.9 | 1+ | 3.2 | 2 | Yes, day +49 |
| 3 | Yes | +1 (auto 2 of 2) | 573 (135-395) | 114 | 243 | 2.3 | None | 7.9 | 1 | Yes, day +98 |
| 4 | Yes | +44 | 377 (100-325) | 23 | NA | 2.1 | None | 0.3 | 1 | No |
| 5 | Yes | +61 | 437 (135-395) | 112 | 369 | 0.8 | None | 5.3 | 4 | Yes, day +133 |
| 6 | Yes | +38 | 328 (87-246) | 22 | 338 | 1.9 | 1+ | 18.7 | 4 | Yes, day +128 |
| 7 | No | +28 | 218 (135-395) | <7 | 68 | 0.9 | None | 0.4 | 2 | No |
| 8 | No | +25 (auto 2 of 2) | NA | NA | NA | 0.9 | None | NA | 0 | No |
| 9 | No | +82 | NA | NA | NA | 1.2 | None | NA | 1 | No |
| 10 | No | +26 | 203 (155-450) | 149 | NA | 0.8 | None | 3.8 | 1 | No |
| 11 | No | +28 (auto 1 of 4) | 264 (100-325) | 145 | NA | 1.3 | None | 1.2 | 1 | No |
| Patient . | TA-TMA . | Day post-HSCT . | LDH (normal range), U/L . | Haptoglobin (normal range, 16-200), mg/dL . | sC5b-9 (normal range, 72-244), ng/mL . | Serum creatinine (current-to-baseline ratio) . | Schistocytes . | Urine protein-to-creatinine ratio . | Quantity of antihypertensives . | Treated with eculizumab . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Yes | +96 | 436 (135-395) | <7 | 301 | 0.5 | None | 7.2 | 4 | Yes, day +100 |
| 2 | Yes | +6 | 1293 (155-450) | 9 | 196 | 0.9 | 1+ | 3.2 | 2 | Yes, day +49 |
| 3 | Yes | +1 (auto 2 of 2) | 573 (135-395) | 114 | 243 | 2.3 | None | 7.9 | 1 | Yes, day +98 |
| 4 | Yes | +44 | 377 (100-325) | 23 | NA | 2.1 | None | 0.3 | 1 | No |
| 5 | Yes | +61 | 437 (135-395) | 112 | 369 | 0.8 | None | 5.3 | 4 | Yes, day +133 |
| 6 | Yes | +38 | 328 (87-246) | 22 | 338 | 1.9 | 1+ | 18.7 | 4 | Yes, day +128 |
| 7 | No | +28 | 218 (135-395) | <7 | 68 | 0.9 | None | 0.4 | 2 | No |
| 8 | No | +25 (auto 2 of 2) | NA | NA | NA | 0.9 | None | NA | 0 | No |
| 9 | No | +82 | NA | NA | NA | 1.2 | None | NA | 1 | No |
| 10 | No | +26 | 203 (155-450) | 149 | NA | 0.8 | None | 3.8 | 1 | No |
| 11 | No | +28 (auto 1 of 4) | 264 (100-325) | 145 | NA | 1.3 | None | 1.2 | 1 | No |
Laboratory values are from the day the tested patient sample was collected. Lactate dehydrogenase (LDH) normal range values vary by age.
Auto, autologous transplantation; NA, not applicable (laboratory value not obtained in patient at given time).
WST-1 viability assay on PIGA-mutant TF-1 cells. Percentage of nonviable cells (circles), with median (wide horizontal line) and interquartile range (narrow horizontal line). LPS, lipopolysaccharide.
WST-1 viability assay on PIGA-mutant TF-1 cells. Percentage of nonviable cells (circles), with median (wide horizontal line) and interquartile range (narrow horizontal line). LPS, lipopolysaccharide.
Our data further demonstrate that TA-TMA is associated with complement activation in vitro. Our assay results corroborate the role of complement in TA-TMA and support the clinical use of eculizumab for the treatment of patients with TA-TMA. Despite our findings, the mechanism of complement activation in TA-TMA remains unclear. Further investigation into the genetic underpinnings and clinical causes of complement activation post-HSCT may improve risk stratification for TA-TMA and is ongoing in our laboratory.10 This report is somewhat limited by its small sample size. Future refinement of this assay may aid in the diagnosis of TA-TMA, but it is not yet ready for clinical use on the basis of this limited sample size. Moreover, comparison with flow cytometry– and immunohistochemistry-based assays in development for atypical hemolytic uremic syndrome may also be helpful.11,17 Additionally, we were unable to stratify samples for this assay based on TA-TMA risk because of small sample size. Additional studies including patients with TA-TMA of varying severity (based on levels of sC5b-9 and urine protein)2 may help better define who may benefit from complement blockade therapy.
The full-text version of this article contains a data supplement.
Authorship
Contribution: S.J.R., S.J., C.E.D., S.M.D., and B.P.D. designed research; S.J.R. and N.L. performed research; R.A.B., E.G., and B.P.D. contributed vital new reagents or analytical tools; S.J.R. and N.L. collected data; S.J.R., N.L., and C.E.D. analyzed and interpreted data; S.J.R. and N.L. performed statistical analysis; and S.J.R., N.L., S.J., and S.M.D. wrote the manuscript.
Conflict-of-interest disclosure: S.M.D. discloses a consultancy with Novartis AG. S.J. and S.M.D. have a US provisional patent application for methods and compositions related to transplantation-associated thrombotic microangiopathy. E.G. is supported by a European Haematology Association clinical research grant. R.A.B. serves on the advisory boards of Alexion Pharmaceuticals, Apellis Pharmaceuticals, and Achillion Pharmaceuticals. (R.A.B. has not received any honorarium from them in the past year. R.A.B. does receive grant funding for his laboratory from Alexion Pharmaceuticals.) B.P.D. received honoraria from Alexion Pharmaceuticals and has been in consultancy for both Alexion Pharmaceuticals and Achillion Pharmaceuticals and has provided paid expert testimony within the past 2 years. The remaining authors declare no competing financial interests.
Correspondence: Seth J. Rotz, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: rotzs@ccf.org.