Key Points
Nonrelapse mortality remains high in MDS patients who underwent haploidentical transplant before 2015.
Haplotransplantation using PT-CY and reduced-intensity conditioning seems an acceptable option in MDS patients lacking HLA-matched donors.
Abstract
The only curative treatment in patients with intermediate or high-risk myelodysplastic syndrome (MDS) is allogeneic hematopoietic stem cell transplantation (HSCT), which usually results in a long-term, disease-free survival rate of between 30% and 50%, depending on the disease risk and the type of donor. In patients without an HLA-matched sibling donor, a family haploidentical donor is an alternative option. The present study reports the European Group for Blood and Marrow Transplantation activity for haploidentical transplantation in MDS patients. A total of 228 patients transplanted from a mismatched HLA-related donor between 2007 and 2014 were studied. The median age at transplant was 56 years. Eighty-four (37%) patients had MDS transformed into acute myeloid leukemia at the time of transplant. Ex vivo T-cell depletion was used in 34 patients. One hundred ninety-four patients received a T-cell replete transplant and 102 patients received posttransplant cyclophosphamide (PT-CY) as graft-versus-host disease (GVHD) prophylaxis. The cumulative incidences of acute and chronic GVHD in PT-CY vs other patients were 25% vs 37% and 37% vs 24%, respectively. The cumulative incidence of nonrelapse mortality was 55% in patients who did not receive PT-CY (no PT-CY) and 41% in patients who did receive PT-CY. Three-year overall survival was 28% in no PT-CY patients and 38% in PT-CY patients. In multivariable analysis, the main risk factors were the intensity of the conditioning regimen and the use of PT-CY. In conclusion, the outcomes of MDS patients who received an haploidentical transplant are close to the results other transplantations from HLA-mismatched donors with approximately one-third of patients alive and free of disease 3 years after transplant, and the use of PT-CY may improve their outcomes.
Introduction
Allogeneic hematopoietic stem cell transplant (HSCT) is a potential curative treatment in patients with poor-prognosis myelodysplastic syndrome (MDS).1,2 Less than 30% of these patients will have an HLA-matched sibling donor available. For patients lacking an HLA-matched sibling donor, an HLA-matched unrelated donor can be recruited in >50% of patients, but this is strongly dependent on the ethnic background, because most of the volunteer donors are of Caucasian origin. So far, no major difference regarding overall survival (OS) or disease-free survival (DFS) has been reported in patients undergoing either HLA-matched sibling or unrelated donor transplantation, and both types are currently used for patients with MDS.3 Unfortunately, ∼20% of patients lack a suitable HLA-matched donor. Historically, HLA-mismatched unrelated donors have been successfully used, and unrelated cord blood (UCB) transplantation emerged as a feasible option at the beginning of the 1990s. With respect to HLA-mismatched related donors and especially HLA haploidentical–related donors (haplos), results before the end of the 1990s were disappointing, but substantial improvements have been made, and haplos are currently considered to be a reasonable option for alternative donor stem cell transplantation. Aversa et al4 have reported that the use of a T cell–depleted graft with CD34+ megadose, and the myeloablative conditioning regimen gave encouraging results. More recently, several methods using T-cell–replete transplant approaches with intensive graft-versus-host disease (GVHD) prophylaxis have been reported, including the use of posttransplant cyclophosphamide (PT-CY).5-7 One prospective phase II study compared UCB donors and haplos with PT-CY and showed similar results, with a progression-free survival (PFS) rate at 1 year was 46% in UCB transplant recipients and 48% in haplo transplant recipients, with more relapse but less nonrelapse mortality (NRM) in haplo transplant recipients.8 The European Group for Blood and Marrow Transplantation (EBMT) has previously reported that HLA-mismatched unrelated donors can give similar results to UCB donors in MDS patients, but worse outcomes than HLA-matched unrelated donors.9 A recent Center for International Blood and Marrow Transplant Research (CIBMTR) study reported similar results in acute myeloid leukemia (AML) patients transplanted with haplo with PT-CY vs HLA-matched unrelated donors.10 A similar comparison in patients with acute leukemia, MDS, chronic leukemia, or lymphoma has been done by Luo et al11 showing equivalent outcomes for matched-related donors, HLA mismatched-unrelated donors, and haplos according to the Beijing protocol.12 The purpose of the present study is to report the outcomes of MDS patients registered in the EBMT registry in the last decade transplanted from HLA-mismatched related donors and to identify the main risk factors associated with outcome.
Methods
Population selection
Adult patients (≥18 years of age) with MDS who received a first allogeneic HSCT from a HLA-mismatched related donor from 2007 to 2014 were included in the study. When the disease was not classified according to French-American-British or World Health Organization classifications, and when HLA was not available, patients were excluded. For patients with missing data regarding the bone marrow blast percentage, it was assumed that patients in complete remission (CR) at the time of transplant had <5% blasts. Regarding HLA, the minimum information required was if there was 1 HLA mismatch or ≥2 HLA mismatches between recipients and donors among 10 antigens (molecular level). Because one of the aims of the study was to report EBMT activity, all kinds of transplants responding to the criteria above were studied. This retrospective study was approved by the Chronic Malignancies Working Party of the EBMT; all patients registered in the study gave their consent for registry analysis, and the study was conducted in accordance with the Declaration of Helsinki.
Statistics
All time-to-event outcomes were counted from the date of transplant to the date of event (death or relapse) or the date of last follow-up, except acute GVHD, which was arbitrarily censored at 120 days. NRM was considered as death by any cause occurring before disease relapse or progression. Death and second transplant were considered as competing events for GVHD. NRM and relapse or progression were considered to be mutually competing risks. Event-free survival was defined as the time until occurrence of the first of acute GVHD grade III-IV, extensive chronic GVHD, relapse or progression, or second transplant or death.
Data were analyzed by using multivariable Cox proportional hazards (OS and PFS) and cause-specific hazards (NRM) models. Predictors were defined a priori, and no variable selection was performed. The proportional hazards assumption was checked by examination of Schoenfeld residuals and Grambsch and Therneau’s lack-of-fit test.13
Missing data were handled through multiple imputations by chained equations methods.14,15 The number of imputations was fixed as the percentage of patients with missing data as recommended,15 rounded to the nearest 5%. Consequently, 20 independent imputed data sets were generated and analyzed separately. The variables used for multiple imputation were not limited. Estimates of model parameters and discrimination indexes were then pooled over the imputations in accordance with Rubin’s rule.14 All multiple analyses were adjusted for center effect because a center effect was found. Indeed, a center effect was investigated by a permutation test grouping centers with <3 patients transplanted per country (there were many centers and few patients per center).16 Since center was found significantly associated with outcome, all analyses were adjusted by using a random center effect in the model.
All tests were two-sided. Analyses were performed by using the R statistical software version 3.2.1 (R Core Team, http://www.R-project.org).
Results
Patient characteristics
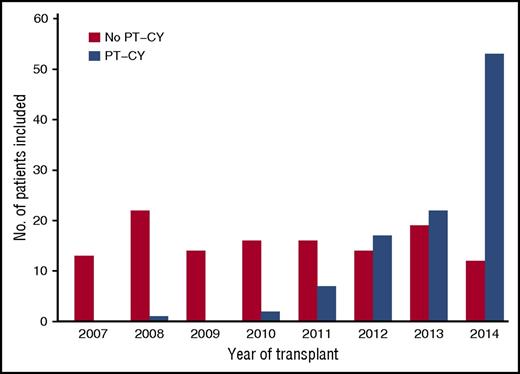
A total of 228 patients receiving a transplant from a HLA-mismatched related donor between 2007 and 2014 fulfilled the inclusion criteria. The baseline characteristics are presented in Table 1. The median age at transplant was 56 years. Eighty-four (37%) patients had MDS transformed into AML at the time of transplant. Among the 186 patients who received a treatment before HSCT, 69 (37%) were in CR. Most recipients received stem cells from a donor with ≥2 HLA mismatches (79%). Ex vivo T-cell depletion (TCD) was used in 34 patients regularly between 2007 and 2014 (minimum, 3/year; maximum, 7/year; 5 in 2014). In vivo TCD was used in 106 patients. PT-CY was used in 102 patients mainly after 2010 (for 99 patients), whereas transplant without PT-CY was regularly used over time (Figure 1). Bone marrow and peripheral blood were used in 73 and 155 patients, respectively. When we compare the patient characteristics of those who received PT-CY to those patients who did not receive PT-CY (no PT-CY), the main differences were that the PT-CY patients were older (median age, 60 years vs 54 years), more frequently had ≥2 HLAs mismatched with the donor (99% vs 62%), and had been transplanted more recently. For patients transplanted with an unmanipulated graft (n = 194), 169 received calcineurin inhibitors for GVHD prophylaxis and 18 received mycophenolate and sirolimus, whereas 7 patients had incomplete data regarding GVHD prophylaxis.
Patient and transplantation characteristics
| Characteristics . | All patients . | No PT-CY . | PT-CY . |
|---|---|---|---|
| Patients, n | 228 | 126 | 102 |
| Female, n (%) | 95 (42) | 50 (40) | 45 (44) |
| Median age at transplant, y (IQR*) | 56 (46-64) | 54 (45 – 63) | 60 (50-65) |
| Year of transplant, n (%) | |||
| 2007-2011 | 91 (40) | 81 (64) | 10 (10) |
| 2012-2014 | 137 (60) | 45 (36) | 92 (90) |
| World Health Organization classification at transplant, n (%) | |||
| RCMD | 31 (13) | 17 (13) | 14 (14) |
| RA/RARS/del5q | 12 (5.2) | 7 (6) | 5 (5) |
| RAEB-1 | 35 (15) | 21 (17) | 14 (14) |
| RAEB-2 | 66 (29) | 34 (27) | 32 (32) |
| Transformed in AML | 84 (36) | 47 (37) | 37 (36) |
| Median time from diagnosis to transplant, mo (IQR) | 11 (6-26) | 10 (6-21) | 13 (7-28) |
| Percent blast at time of transplant, n (%) | |||
| <5% | 106 (47) | 60 (49) | 46 (45) |
| ≥5% | 118 (53) | 62(51) | 56 (55) |
| Missing | 4 | 4 | 0 |
| Status at transplant, n (%) | |||
| Untreated | 2 (1) | 2 (2) | 0 |
| Treated and in CR | 69 (36) | 39 (39) | 30 (34) |
| Treated and not in CR | 119 (62) | 59 (59) | 58 (66) |
| Missing | 40 | 26 | 14 |
| Donor/recipient sex match, n (%) | |||
| Female/male | 52 (23) | 32 (25) | 20 (20) |
| Other combination | 178 (77) | 94 (75) | 82 (80) |
| Number of HLA mismatches, n (%) | |||
| 1 | 49 (21) | 48 (38) | 1 (1) |
| ≥2 | 179 (78) | 78 (62) | 101 (99) |
| Donor/recipient CMV match, n (%) | |||
| Negative/negative | 33 (15) | 21 (18) | 12 (12) |
| Positive/negative | 12 (5) | 6 (5) | 6 (6) |
| Negative/positive | 41 (18) | 25 (21) | 16 (16) |
| Positive/positive | 133 (61) | 66 (56) | 67 (66) |
| Source of stem cells, n (%) | |||
| Bone marrow | 73 (32) | 29 (23) | 44 (43) |
| Peripheral blood | 155 (68) | 97 (77) | 58 (57) |
| Conditioning regimen, n (%) | |||
| Reduced intensity | 118 (52) | 64 (51) | 54 (53) |
| Myeloablative | 110 (48) | 62 (49) | 48 (47) |
| Total body irradiation, n (%) | 62 (27) | 31 (25) | 31 (30) |
| Ex vivo TCD*, n (%) | 34 (15) | 33 (26) | 1 (1) |
| In vivo TCD, n (%) | |||
| Antithymoglobulin | 98 (43) | 96 (76) | 2 (2) |
| Alemtuzumab | 8 (3) | 8 (6) | 0 |
| Characteristics . | All patients . | No PT-CY . | PT-CY . |
|---|---|---|---|
| Patients, n | 228 | 126 | 102 |
| Female, n (%) | 95 (42) | 50 (40) | 45 (44) |
| Median age at transplant, y (IQR*) | 56 (46-64) | 54 (45 – 63) | 60 (50-65) |
| Year of transplant, n (%) | |||
| 2007-2011 | 91 (40) | 81 (64) | 10 (10) |
| 2012-2014 | 137 (60) | 45 (36) | 92 (90) |
| World Health Organization classification at transplant, n (%) | |||
| RCMD | 31 (13) | 17 (13) | 14 (14) |
| RA/RARS/del5q | 12 (5.2) | 7 (6) | 5 (5) |
| RAEB-1 | 35 (15) | 21 (17) | 14 (14) |
| RAEB-2 | 66 (29) | 34 (27) | 32 (32) |
| Transformed in AML | 84 (36) | 47 (37) | 37 (36) |
| Median time from diagnosis to transplant, mo (IQR) | 11 (6-26) | 10 (6-21) | 13 (7-28) |
| Percent blast at time of transplant, n (%) | |||
| <5% | 106 (47) | 60 (49) | 46 (45) |
| ≥5% | 118 (53) | 62(51) | 56 (55) |
| Missing | 4 | 4 | 0 |
| Status at transplant, n (%) | |||
| Untreated | 2 (1) | 2 (2) | 0 |
| Treated and in CR | 69 (36) | 39 (39) | 30 (34) |
| Treated and not in CR | 119 (62) | 59 (59) | 58 (66) |
| Missing | 40 | 26 | 14 |
| Donor/recipient sex match, n (%) | |||
| Female/male | 52 (23) | 32 (25) | 20 (20) |
| Other combination | 178 (77) | 94 (75) | 82 (80) |
| Number of HLA mismatches, n (%) | |||
| 1 | 49 (21) | 48 (38) | 1 (1) |
| ≥2 | 179 (78) | 78 (62) | 101 (99) |
| Donor/recipient CMV match, n (%) | |||
| Negative/negative | 33 (15) | 21 (18) | 12 (12) |
| Positive/negative | 12 (5) | 6 (5) | 6 (6) |
| Negative/positive | 41 (18) | 25 (21) | 16 (16) |
| Positive/positive | 133 (61) | 66 (56) | 67 (66) |
| Source of stem cells, n (%) | |||
| Bone marrow | 73 (32) | 29 (23) | 44 (43) |
| Peripheral blood | 155 (68) | 97 (77) | 58 (57) |
| Conditioning regimen, n (%) | |||
| Reduced intensity | 118 (52) | 64 (51) | 54 (53) |
| Myeloablative | 110 (48) | 62 (49) | 48 (47) |
| Total body irradiation, n (%) | 62 (27) | 31 (25) | 31 (30) |
| Ex vivo TCD*, n (%) | 34 (15) | 33 (26) | 1 (1) |
| In vivo TCD, n (%) | |||
| Antithymoglobulin | 98 (43) | 96 (76) | 2 (2) |
| Alemtuzumab | 8 (3) | 8 (6) | 0 |
Twenty-nine patients received ex vivo and in vivo TCD.
IQR, interquartile range; RA, refractory anemia; RAEB, refractory anemia with excess blast according to World Health Organization definition; RARS, refractory anemia with ring sideroblasts; RCMD, refractory cytopenia with myelodysplasia.
Frequency of haplo transplant for patients with MDS. Blue bars represent PT-CY patients, and yellow bars represent no PT-CY patients.
Frequency of haplo transplant for patients with MDS. Blue bars represent PT-CY patients, and yellow bars represent no PT-CY patients.
Outcome
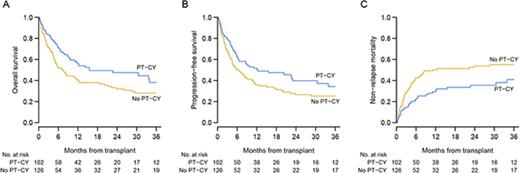
The median follow-up was 26 months (interquartile range: 12 to 49 months): 18 months for PT-CY patients and 47 months for no PT-CY patients. Twenty-one patients had primary or secondary rejections: 10 PT-CY patients and 11 no PT-CY patients. The cumulative incidences for acute grade II-IV and III-IV GVHD were 32% (95% confidence interval [CI]: 26-38) and 12% (95% CI, 8-16), respectively. Incidence of grade II-IV acute GVHD was 25% (95% CI, 17-34) in PT-CY patients vs 37% (95% CI, 29-46) in no PT-CY patients. The incidence of chronic GVHD was 37% (95% CI, 26-48) in PT-CY patients vs 24% (95% CI, 17-33) in no PT-CY patients and was not influenced by stem cell source (data not shown). A total of 131 patients died, and the main causes of death were relapse or progression from the primary disease, infection without GVHD, GVHD, or graft failure (Table 2). Of note, GVHD was a rare cause of death in PT-CY (4% of all death causes). The cumulative incidence for NRM was 49% (95% CI, 41-56) at 3 years, whereas the relapse rate was 22% (95% CI, 16-28). Three-year OS and DFS were 32% (95% CI, 26-41) and 29% (95% CI, 23-37). For PT-CY patients, 3-year OS, DFS, and NRM were 38% (95% CI, 27-53), 34% (95% CI, 24-49), and 41% (95% CI, 29-53), respectively, whereas no PT-CY patients had 3-year OS, DFS, and NRM rates of 28% (95% CI, 20-39), 25% (95% CI, 19-36), and 55% (95% CI, 45-64), respectively (Figure 2). Patients who received a reduced-intensity conditioning regimen had better outcomes with 3-year OS, PFS, and NRM rates of 40% (95% CI, 31-52), 37% (95% CI, 28-48), and 41% (95% CI, 31-51), respectively, vs 23% (95% CI, 14-37), 20% (95% CI, 12-34), and 59% (95% CI, 45-70), respectively, for patients who received a myeloablative conditioning regimen (MAC). Patients who had MDS without blast excess also had better outcomes, with 3-year OS, DFS, and NRM rates of 60% (95% CI, 45-78), 51% (95% CI, 36-71), and 34% (95% CI, 19-49).
Primary cause of deaths
| Cause of death . | No PT-CY . | With PT-CY . |
|---|---|---|
| Any cause, n | 81 | 50 |
| Relapse/progression from primary disease, n (%) | 19 (23) | 16 (32) |
| GVHD, n (%) | 19 (23) | 2 (4) |
| Infection, n (%) | 21 (26) | 14 (28) |
| Rejection, n (%) | 15 (18) | 10 (20) |
| Heart failure, n (%) | 1 (1) | 2 (4) |
| Respiratory failure/lung disease, n (%) | 2 (2) | 1 (2) |
| Hemorrhage, n (%) | 1 (1) | 1 (2) |
| Multiorgan failure, n (%) | 1 (1) | 1 (2) |
| Central nervous system disease, n (%) | 1 (1) | 0 |
| Veno-occlusive disease, n (%) | 1 (1) | 0 |
| Suicide, n (%) | 0 (1) | 1 (2) |
| Unknown, n (%) | 0 | 2 (4) |
| Cause of death . | No PT-CY . | With PT-CY . |
|---|---|---|
| Any cause, n | 81 | 50 |
| Relapse/progression from primary disease, n (%) | 19 (23) | 16 (32) |
| GVHD, n (%) | 19 (23) | 2 (4) |
| Infection, n (%) | 21 (26) | 14 (28) |
| Rejection, n (%) | 15 (18) | 10 (20) |
| Heart failure, n (%) | 1 (1) | 2 (4) |
| Respiratory failure/lung disease, n (%) | 2 (2) | 1 (2) |
| Hemorrhage, n (%) | 1 (1) | 1 (2) |
| Multiorgan failure, n (%) | 1 (1) | 1 (2) |
| Central nervous system disease, n (%) | 1 (1) | 0 |
| Veno-occlusive disease, n (%) | 1 (1) | 0 |
| Suicide, n (%) | 0 (1) | 1 (2) |
| Unknown, n (%) | 0 | 2 (4) |
Outcomes according to the use of PT-CY in GVHD prophylaxis. (A-C) Shown are the OS, PFS, and NRM rates. Blue curves indicate PT-CY patients, and yellow curves indicate no PT-CY patients.
Outcomes according to the use of PT-CY in GVHD prophylaxis. (A-C) Shown are the OS, PFS, and NRM rates. Blue curves indicate PT-CY patients, and yellow curves indicate no PT-CY patients.
Multivariable models
All multivariable analyses were adjusted with a potential center effect as described in “Methods.” For the whole group, the multivariable models showed the following risk factors for PFS, OS, and NRM: transformation into AML, non-CR at the time of transplant, a female donor for a male recipient, and the use of myeloablative regimen were poor prognostic factors (Table 3). The use of PT-CY seemed protective for PFS, OS, and NRM; although the P value did not reach the significance, the trend was reproduced for the 3 outcomes. Recipient age, number of HLA mismatches, in vivo or ex vivo TCD, source of stem cells, cytomegalovirus (CMV) serology, and the transplant period were not associated with the outcomes.
Multivariable analysis for the whole group
| . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| OS | ||
| Transplant in 2012-2014 | 0.90 (0.57-1.42) | .65 |
| Age, per year | 1.00 (0.99-1.02) | .49 |
| RAEB-1 or –2 | 1.54 (0.82-2.89) | .18 |
| Transformation to AML | 2.00 (1.07-3.74) | .031 |
| Non-CR at transplant | 1.88 (1.24-2.86) | .003 |
| Bone marrow vs peripheral blood* | 0.90 (0.56-1.47) | .68 |
| Female donor to male recipient | 1.61 (1.06-2.44) | .026 |
| CMV-positive recipient | 1.53 (0.95-2.46) | .078 |
| ≥2 HLA loci mismatch | 1.22 (0.69-2.16) | .49 |
| MAC | 1.65 (1.05-2.60) | .032 |
| TBI | 1.20 (0.75-1.92) | .44 |
| In vivo TCD | 0.96 (0.48-1.92) | .91 |
| Ex vivo TCD | 0.74 (0.32-1.71) | .48 |
| PT-CY | 0.45 (0.20-1.01) | .054 |
| PFS | ||
| Transplant in 2012-2014 | 0.92 (0.59-1.42) | .70 |
| Age, per year | 1.01 (0.99-1.02) | .40 |
| RAEB-1 or −2 | 1.35 (0.76-2.40) | .31 |
| Transformation to AML | 1.74 (0.98-3.09) | .060 |
| Non-CR at transplant | 1.91 (1.28-2.84) | .001 |
| Bone marrow vs peripheral blood* | 1.12 (0.72-1.72) | .62 |
| Female donor to male recipient | 1.50 (1.00-2.24) | .049 |
| CMV=positive recipient | 1.35 (0.87-2.10) | .18 |
| ≥2 HLA loci mismatch | 1.13 (0.66-1.92) | .66 |
| MAC | 1.55 (1.01-2.39) | .045 |
| TBI | 1.12 (0.72-1.75) | .61 |
| In vivo TCD | 0.89 (0.47-1.69) | .73 |
| Ex vivo TCD | 0.75 (0.34-1.66) | .48 |
| PT-CY | 0.50 (0.24-1.02) | .056 |
| NRM | ||
| Transplant in 2012-2014 | 0.91 (0.53-1.54) | .72 |
| Age, per year | 1.01 (0.99-1.03) | .22 |
| RAEB-1 or −2 | 1.12 (0.57-2.22) | .74 |
| Transformation to AML | 1.62 (0.83-3.17) | .16 |
| Non-CR at transplant | 1.88 (1.24-2.86) | .003 |
| Bone marrow vs peripheral blood* | 0.90 (0.56-1.47) | .68 |
| Female donor to male recipient | 1.61 (1.06-2.44) | .026 |
| CMV-positive recipient | 1.53 (0.95-2.46) | .078 |
| ≥2 HLA loci mismatch | 1.22 (0.69-2.16) | .49 |
| MAC | 1.65 (1.05-2.60) | .032 |
| TBI | 1.20 (0.75-1.92) | .44 |
| In vivo TCD | 0.96 (0.48-1.92) | .91 |
| Ex vivo TCD | 0.74 (0.32-1.71) | .48 |
| PT-CY | 0.46 (0.21-1.01) | .054 |
| . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| OS | ||
| Transplant in 2012-2014 | 0.90 (0.57-1.42) | .65 |
| Age, per year | 1.00 (0.99-1.02) | .49 |
| RAEB-1 or –2 | 1.54 (0.82-2.89) | .18 |
| Transformation to AML | 2.00 (1.07-3.74) | .031 |
| Non-CR at transplant | 1.88 (1.24-2.86) | .003 |
| Bone marrow vs peripheral blood* | 0.90 (0.56-1.47) | .68 |
| Female donor to male recipient | 1.61 (1.06-2.44) | .026 |
| CMV-positive recipient | 1.53 (0.95-2.46) | .078 |
| ≥2 HLA loci mismatch | 1.22 (0.69-2.16) | .49 |
| MAC | 1.65 (1.05-2.60) | .032 |
| TBI | 1.20 (0.75-1.92) | .44 |
| In vivo TCD | 0.96 (0.48-1.92) | .91 |
| Ex vivo TCD | 0.74 (0.32-1.71) | .48 |
| PT-CY | 0.45 (0.20-1.01) | .054 |
| PFS | ||
| Transplant in 2012-2014 | 0.92 (0.59-1.42) | .70 |
| Age, per year | 1.01 (0.99-1.02) | .40 |
| RAEB-1 or −2 | 1.35 (0.76-2.40) | .31 |
| Transformation to AML | 1.74 (0.98-3.09) | .060 |
| Non-CR at transplant | 1.91 (1.28-2.84) | .001 |
| Bone marrow vs peripheral blood* | 1.12 (0.72-1.72) | .62 |
| Female donor to male recipient | 1.50 (1.00-2.24) | .049 |
| CMV=positive recipient | 1.35 (0.87-2.10) | .18 |
| ≥2 HLA loci mismatch | 1.13 (0.66-1.92) | .66 |
| MAC | 1.55 (1.01-2.39) | .045 |
| TBI | 1.12 (0.72-1.75) | .61 |
| In vivo TCD | 0.89 (0.47-1.69) | .73 |
| Ex vivo TCD | 0.75 (0.34-1.66) | .48 |
| PT-CY | 0.50 (0.24-1.02) | .056 |
| NRM | ||
| Transplant in 2012-2014 | 0.91 (0.53-1.54) | .72 |
| Age, per year | 1.01 (0.99-1.03) | .22 |
| RAEB-1 or −2 | 1.12 (0.57-2.22) | .74 |
| Transformation to AML | 1.62 (0.83-3.17) | .16 |
| Non-CR at transplant | 1.88 (1.24-2.86) | .003 |
| Bone marrow vs peripheral blood* | 0.90 (0.56-1.47) | .68 |
| Female donor to male recipient | 1.61 (1.06-2.44) | .026 |
| CMV-positive recipient | 1.53 (0.95-2.46) | .078 |
| ≥2 HLA loci mismatch | 1.22 (0.69-2.16) | .49 |
| MAC | 1.65 (1.05-2.60) | .032 |
| TBI | 1.20 (0.75-1.92) | .44 |
| In vivo TCD | 0.96 (0.48-1.92) | .91 |
| Ex vivo TCD | 0.74 (0.32-1.71) | .48 |
| PT-CY | 0.46 (0.21-1.01) | .054 |
Comparison of bone marrow or peripheral blood as the source of stem cells, the reference is peripheral blood.
Because the 2 transplant procedures (with or without PT-CY) are very different, 2 different multivariable models are also displayed (Table 4). The differential effect between PT-CY and no PT-CY for a variable has been measured, and shows how risk factors can be different according to the transplant strategy.
Separate multivariable models according to PT-CY receipt
| . | No PT-CY . | PT-CY . | . | ||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) . | P . | Hazard ratio (95% CI) . | P . | P (differential effect) . | |
| OS | |||||
| Age, per year | 1.00 (0.99-1.02) | .72 | 1.00 (.98-1.03) | .82 | .97 |
| RAEB-1 or −2 | 2.78 (1.11-6.96) | .028 | .81 (.33-2.00) | .65 | .060 |
| Transformation to AML | 3.69 (1.49-9.16) | .005 | 1.08 (.43-2.67) | .88 | .060 |
| Non-CR at transplant | 2.47 (1.44-4.25) | .001 | 1.32 (.70-2.49) | .39 | .14 |
| Bone marrow vs peripheral blood* | 1.17 (0.58-2.36) | .51 | .72 (.39-1.32) | .29 | .31 |
| Female donor to male recipient | 1.34 (0.78-2.28) | .29 | 2.02 (.97-4.21) | .059 | .37 |
| CM-positive recipient | 1.89 (1.04-3.45) | .038 | 1.19 (.49-2.86) | .70 | .39 |
| ≥2 HLA loci mismatch | 1.26 (0.68-2.31) | .46 | — | — | — |
| MAC | 1.52 (0.79-2.92) | .21 | 3.12 (1.21-8.04) | .018 | .22 |
| TBI | 0.90 (0.48-1.66) | .73 | 2.66 (.95-7.47) | .062 | .075 |
| In vivo TCD | 0.86 (0.45-1.65) | .60 | — | — | — |
| Ex-vivo TCD | 0.78 (0.31-1.96) | .60 | — | — | — |
| PFS | |||||
| Age, per year | 1.00 (0.99-1.02) | .76 | 1.01 (.99-1.04) | .29 | .51 |
| RAEB-1 or −2 | 2.58 (1.13-5.88) | .025 | .66 (.27-1.60) | .36 | .028 |
| Transformation to AML | 3.14 (1.38-7.15) | .007 | .87 (.36-2.14) | .77 | .040 |
| Non-CR at transplant | 2.31 (1.38-3.88) | .002 | 1.55 (.84-2.87) | .16 | .33 |
| Bone marrow vs peripheral blood* | 1.43 (0.73-2.78) | .30 | .91 (.50-1.63) | .74 | .32 |
| Female donor to male recipient | 1.22 (0.73-2.04) | .45 | 2.02 (1.00-4.08) | .050 | .26 |
| CMV-positive recipient | 1.62 (0.92-2.87) | .095 | 1.20 (.53-2.74) | .66 | .56 |
| ≥2 HLA loci mismatch | 1.28 (0.72-2.25) | .40 | — | — | — |
| MAC | 1.46 (0.76-2.80) | .25 | 2.71 (1.13-6.51) | .026 | .27 |
| TBI | 0.81 (0.45-1.44) | .47 | 2.58 (.99-6.69) | .052 | .041 |
| In vivo TCD | 0.87 (0.45-1.68) | .68 | — | — | — |
| Ex vivo TCD | 0.89 (0.37-2.15) | .79 | — | — | — |
| NRM | |||||
| Age, per year | 1.01 (0.99-1.03) | .47 | 1.02 (.99-1.05) | .31 | .68 |
| RAEB-1 or −2 | 1.72 (0.67-4.43) | .26 | .60 (.20-1.82) | .37 | .16 |
| Transformation to AML | 2.50 (0.98-6.35) | .055 | 1.00 (.33-2.98) | >.99 | .21 |
| Non-CR at transplant | 2.36 (1.28-4.35) | .006 | 1.16 (.54-2.50) | .70 | .16 |
| Bone marrow vs peripheral blood* | 0.86 (0.38-1.96) | .73 | .72 (.33-1.57) | .42 | .76 |
| Female donor to male recipient | 1.39 (0.77-2.51) | .28 | 2.27 (.95-5.39) | .064 | .36 |
| CMV-positive recipient | 1.96 (0.99-3.89) | .053 | 1.42 (.45-4.50) | .56 | .63 |
| ≥2 HLA loci mismatch | 1.24 (0.62-2.49) | .54 | — | — | — |
| MAC | 2.03 (0.95-4.36) | .068 | 2.01 (.75-5.39) | .16 | .99 |
| TBI | 0.99 (0.38-1.97) | .98 | 1.53 (.49-4.81) | .46 | .52 |
| In vivo TCD | 0.84 (0.41-1.75) | .74 | — | — | — |
| Ex vivo TCD | 0.58 (0.21-1.64) | .31 | — | — | — |
| . | No PT-CY . | PT-CY . | . | ||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) . | P . | Hazard ratio (95% CI) . | P . | P (differential effect) . | |
| OS | |||||
| Age, per year | 1.00 (0.99-1.02) | .72 | 1.00 (.98-1.03) | .82 | .97 |
| RAEB-1 or −2 | 2.78 (1.11-6.96) | .028 | .81 (.33-2.00) | .65 | .060 |
| Transformation to AML | 3.69 (1.49-9.16) | .005 | 1.08 (.43-2.67) | .88 | .060 |
| Non-CR at transplant | 2.47 (1.44-4.25) | .001 | 1.32 (.70-2.49) | .39 | .14 |
| Bone marrow vs peripheral blood* | 1.17 (0.58-2.36) | .51 | .72 (.39-1.32) | .29 | .31 |
| Female donor to male recipient | 1.34 (0.78-2.28) | .29 | 2.02 (.97-4.21) | .059 | .37 |
| CM-positive recipient | 1.89 (1.04-3.45) | .038 | 1.19 (.49-2.86) | .70 | .39 |
| ≥2 HLA loci mismatch | 1.26 (0.68-2.31) | .46 | — | — | — |
| MAC | 1.52 (0.79-2.92) | .21 | 3.12 (1.21-8.04) | .018 | .22 |
| TBI | 0.90 (0.48-1.66) | .73 | 2.66 (.95-7.47) | .062 | .075 |
| In vivo TCD | 0.86 (0.45-1.65) | .60 | — | — | — |
| Ex-vivo TCD | 0.78 (0.31-1.96) | .60 | — | — | — |
| PFS | |||||
| Age, per year | 1.00 (0.99-1.02) | .76 | 1.01 (.99-1.04) | .29 | .51 |
| RAEB-1 or −2 | 2.58 (1.13-5.88) | .025 | .66 (.27-1.60) | .36 | .028 |
| Transformation to AML | 3.14 (1.38-7.15) | .007 | .87 (.36-2.14) | .77 | .040 |
| Non-CR at transplant | 2.31 (1.38-3.88) | .002 | 1.55 (.84-2.87) | .16 | .33 |
| Bone marrow vs peripheral blood* | 1.43 (0.73-2.78) | .30 | .91 (.50-1.63) | .74 | .32 |
| Female donor to male recipient | 1.22 (0.73-2.04) | .45 | 2.02 (1.00-4.08) | .050 | .26 |
| CMV-positive recipient | 1.62 (0.92-2.87) | .095 | 1.20 (.53-2.74) | .66 | .56 |
| ≥2 HLA loci mismatch | 1.28 (0.72-2.25) | .40 | — | — | — |
| MAC | 1.46 (0.76-2.80) | .25 | 2.71 (1.13-6.51) | .026 | .27 |
| TBI | 0.81 (0.45-1.44) | .47 | 2.58 (.99-6.69) | .052 | .041 |
| In vivo TCD | 0.87 (0.45-1.68) | .68 | — | — | — |
| Ex vivo TCD | 0.89 (0.37-2.15) | .79 | — | — | — |
| NRM | |||||
| Age, per year | 1.01 (0.99-1.03) | .47 | 1.02 (.99-1.05) | .31 | .68 |
| RAEB-1 or −2 | 1.72 (0.67-4.43) | .26 | .60 (.20-1.82) | .37 | .16 |
| Transformation to AML | 2.50 (0.98-6.35) | .055 | 1.00 (.33-2.98) | >.99 | .21 |
| Non-CR at transplant | 2.36 (1.28-4.35) | .006 | 1.16 (.54-2.50) | .70 | .16 |
| Bone marrow vs peripheral blood* | 0.86 (0.38-1.96) | .73 | .72 (.33-1.57) | .42 | .76 |
| Female donor to male recipient | 1.39 (0.77-2.51) | .28 | 2.27 (.95-5.39) | .064 | .36 |
| CMV-positive recipient | 1.96 (0.99-3.89) | .053 | 1.42 (.45-4.50) | .56 | .63 |
| ≥2 HLA loci mismatch | 1.24 (0.62-2.49) | .54 | — | — | — |
| MAC | 2.03 (0.95-4.36) | .068 | 2.01 (.75-5.39) | .16 | .99 |
| TBI | 0.99 (0.38-1.97) | .98 | 1.53 (.49-4.81) | .46 | .52 |
| In vivo TCD | 0.84 (0.41-1.75) | .74 | — | — | — |
| Ex vivo TCD | 0.58 (0.21-1.64) | .31 | — | — | — |
Results presented are pooled over imputed data sets.
—, number of patients were too few to perform statistical analysis.
*Comparison of bone marrow or peripheral blood as the source of stem cells, the reference is peripheral blood.
Regarding no PT-CY, MDS transformed into AML, non-CR at the time of transplant, MDS with blast excess, and positive recipient CMV serostatus were deleterious for OS, PFS, and NRM. The use of MAC only impacted NRM with borderline significance (P = .068) and had no impact on OS and PFS (Table 4).
Regarding PT-CY, the use of MAC resulted in significantly worse OS and PFS. A female donor for a male recipient was the other variable impacting OS, PFS, and NRM, but the significance was only borderline. A total body irradiation regimen was deleterious for OS and PFS. It was striking to see that no factor related to the disease impacted the outcome: MDS with blast excess, non-CR at the time of transplant, and transformation into AML at the time of transplant were not predictive of a poor outcome in PT-CY patients. The source of the stem cells (bone marrow or peripheral stem cell) had no impact on outcomes.
Discussion
On behalf of the Chronic Malignancy Working Party of the EBMT, in this article, we report the activity of HLA-mismatched related transplant in patients with a primary diagnosis of MDS between 2007 and 2014. Ex vivo TCD was performed uniformly over time for ∼5 patients per year from 2007 to 2014. Regarding T-cell replete HLA-mismatched related transplant, there was a progressive increase in the use of PT-CY over time and the procedure without PT-CY represented 18% of the total haplo in MDS in 2014. The results of HLA-mismatched related transplantation were generally similar to the results expected for other HLA-mismatched sources of stem cells, with approximately one-third of patients alive without active disease after transplant.3,9
Haploidentical transplant procedures have changed significantly over time. In the 1990s, haploidentical transplantation using CD34-positive cell selection provided encouraging results, especially in patients with AML, but these patients are at higher risk of infection and relapse due to profound immune defects. More recently, several methods using T-cell replete grafts have been reported, including the use of PT-CY,5 or the use of granulocyte colony-stimulating factor–primed bone marrow combined with intensive GVHD prophylaxis, as described by Chinese and European teams.6,7 In the present study, it appears that EBMT centers are currently using T-cell replete transplantation, with a majority of centers choosing PT-CY in more recent years, but not exclusively.
In this study, the GVHD rate was relatively low, and GVHD was the cause of death for a minority of patients, especially after PT-CY, where it was the cause of death for <5% of patients. Nevertheless, NRM remained high at >40%, regardless of the type of GVHD prophylaxis used, and chronic GVHD was still quite frequent, involving ∼30% of patients, which is in the higher range according to a recent review.17,18 Of note, the main cause of NRM was infection, which occurred in patients without active GVHD at the time of death. This issue has been rarely highlighted, but immune defects after haplo transplantation might be profound, and immune recovery is usually delayed as compared with HLA-matched transplantation. In a series of 57 children receiving either haplo (n = 24) or UCB (n = 33) transplantation, Clave et al19 analyzed immune recovery, including thymic function, and found that the source of stem cells did not influence immune recovery, with a prolonged defect in both haplo and UCB transplantation. Our results from the EBMT registry may represent “real life” and include patients who may be at high risk with advanced disease and/or comorbidities and who are not eligible for a prospective study, since frequently results from registry studies are worse than from prospective studies. Although the recent enthusiasm for T-cell replete haplo transplantation with intensive GVHD prophylaxis is mainly related to the very low rate of NRM, we failed to observe this low rate, and one could be relatively disappointed by our results.8,20 However, the NRM rate has progressively decreased and was lower in PT-CY patients. As was observed for all other kind of transplants, the NRM rate was higher when MAC was used, reaching 59% at 3 years, and this deleterious effect also translates to worse OS and PFS rates. The higher NRM after MAC does not confirm a CIBMTR study, which reported a 3-year NRM of only 14%.10 Of note, the CIBMTR study did not include MDS patients, but included patients with AML, who may have different outcomes after transplant. Generally, the posttransplant outcomes of standard AML patients are better than those of MDS patients, who often have active disease and comorbidities and are older.3,21-23 Other risk factors for outcomes differed between no PT-CY and PT-CY patients; for instance, more advanced disease was especially deleterious in no PT-CY patients, whereas it did not impact outcomes for PT-CY patients. It has been suspected that haplo transplantation using PT-CY may be less efficient in patients with active disease. Di Stasi et al24 reported a 3-year PFS rate of only 10% for AML/MDS haplo transplant recipients not in remission, but it was not confirmed in this study. Haplo transplantation may be also an option for patients with advanced disease or disease that is not in remission.
Recently, the largest series of MDS patients transplanted from T-cell replete haplos has been reported by Wang et al,25 who compared 226 haplos with 228 matched sibling donors using intensive immunosuppressive therapy with antithymocyte globulins, cyclosporine, methotrexate, and mycophenolate mofetil. The outcomes of haplo transplant recipients with only 1 or 2 HLA mismatches were not significantly different from those of HLA-matched sibling transplant recipients, with OS and DFS rates of 63% and 73%, respectively, and 63% and 71%, respectively. The patients who had more than 2 HLA mismatches had worse results with respect to OS and DFS rates, which were both at 58%, but had much better results than those of the present EBMT registry, including a patient who received a T-cell replete transplant. Furthermore, patients from the Chinese Bone Marrow Transplantation registry were very young, >25 years younger than patients from the EBMT registry, and age (cut off, 50 years) was an independent risk factor for survivals and NRM in the Chinese study. Di Bartolomeo et al7 have reported results of haplo transplantation from G-CSF–primed bone marrow with intensive immunosuppressive therapy. In standard- and high-risk patients, the DFS was 54% and 33%, respectively, and the 1-year NRM rate was 35%, which is close to our results.7 We hypothesize that EBMT patients had high-risk MDS because many of them had MDS transformed into AML at the time of transplant, <20% had MDS without excess bone marrow blasts, and almost all received treatment before transplant. Unfortunately, and this is a major limitation in this study, cytogenetic data were missing for most of patients, preventing their inclusion in statistical analysis. Nevertheless, presuming that these patients had high-risk MDS, the results were close to those reported by Di Bartolomeo et al.7
In conclusion, data from MDS patients reported in the EBMT registry shows that T-cell replete transplant using PT-CY has become the most common transplant procedure, but NRM remains relatively high, leaving room to improve management of these patients. Haplo transplantation using PT-CY and a reduced-intensity conditioning regimen seems to be an acceptable option for MDS patients lacking an HLA-matched donor, including patients with advanced disease. More studies are needed to prospectively compare HLA-mismatched grafts in MDS patients of a specific population, patients who are older, and who have uncontrolled disease at the time of transplant.
Authorship
Contribution: M.R. and N.K. designed the study; L.K. provided data management; R.P. performed statistical analysis; and M.R., F.C., M.T.v.L., S.S., G.E., D.B., Z.G., S.G.M., M.M., A.V., L.K., J.M., J.S., D.S., A.R., J.L.D.-M., C.H.A., D.N., H.H., A.M., Y.K., T.d.W., and N.K. provided patients and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marie Robin, Hôpital Saint-Louis, Assistance Publique Hôpitaux de Paris, Paris, France, INSERM 1131, Université Paris 7, 1 Ave Claude Vellefaux, 75010 Paris, France; e-mail: marie.robin@aphp.fr.