Key Points
Early progression of disease within 2 years of initial therapy independently predicts inferior survival in CLL.
Early progression of disease is a robust clinical end point and a useful posttreatment risk stratification tool.
Abstract
Chemoimmunotherapy for chronic lymphocytic leukemia (CLL) promotes clonal evolution of aggressive clones, which in some patients may lead to early progression of disease (POD). We studied the prognostic value of early POD in a cohort of patients with CLL enrolled between 2010 and 2014 in the Connect CLL Registry. Overall, 829 eligible patients receiving first-line therapy were categorized into 3 groups: early POD (progression <2 years after treatment initiation), late POD (progression ≥2 years after treatment initiation), and no POD as of 1 May 2017. Baseline demographics, treatment characteristics, and overall survival (OS) were analyzed. Logistic regression models identified independent predictors of early POD; Cox regression models were used to evaluate the risk of early POD. With a median follow-up of 48.8 months, 209 (25.2%), 162 (19.5%), and 458 (55.3%) patients had early, late, and no POD, respectively. Patients with early POD were older and had inferior response to similar first-line treatment regimens vs late and no POD groups (overall response rate: 53% vs 80% vs 84%). Patients with early POD were more likely to have unfavorable-risk cytogenetics (del[11q]/del[17p]) than patients with no POD (34% vs 20%; P = .04). Early POD was associated with an inferior OS across all patients (hazard ratio, 3.6; 95% confidence interval, 2.6-5.1; P < .01) and in patients treated with fludarabine, cyclophosphamide plus rituximab, and bendamustine plus rituximab (P < .05). Early POD within 2 years of first-line therapy is a robust clinical prognostic factor for inferior OS in patients with CLL. The Connect CLL Registry was registered at www.clinicaltrials.gov as #NCT01081015.
Introduction
Chronic lymphocytic leukemia (CLL) is a biologically heterogeneous cancer. In a study by Landau et al, more than 20 driver mutations were found by whole exome sequencing in 149 patients with CLL.1 The majority of patients has more than 1 mutant clonal subpopulation detectable by deep sequencing.2,3 As a result of this biological diversity and the heterogeneity of clinical outcomes of CLL, many tools exist to help stratify patients into different prognostic groups. Tools commonly used in clinical practice include Rai and Binet staging,4-6 detection of genetic aberrations by cytogenetics and fluorescence in situ hybridization (FISH),6-8 and immunoglobulin heavy-chain variable region gene (IgHV) mutation testing.6,8,9 The widely used CLL-International Prognostic Index includes cytogenetics and IgHV mutation testing alongside clinical prognostic factors to stratify patients into 1 of 4 risk groups.6
A common feature of established prognostic tools is the use of pretreatment factors; little is known about risk stratification after treatment. It is now well-established that the clonal composition of CLL changes during treatment,10 and chemoimmunotherapy selectively enriches for certain mutations. Subclones with TP53 mutations, for instance, may be resistant to chemoimmunotherapy and later drive disease progression or transformation.11,12 The presence of certain driver mutations before treatment initiation was associated with a limited duration of response and shorter survival in a prospective study.13
Because the onset of disease progression after initial treatment may reflect differences in disease biology, early progression may be a strong clinical prognostic factor for survival. Previous clinical trials have shown poor survival in patients with CLL who relapse within 2 to 3 years of first-line therapy.14,15 Based on these trial data, a cutoff point of 2 to 3 years has been widely adopted in the assessment of frontline chemoimmunotherapy for CLL.16 However, the validity of this approach in the real-world setting has not been previously explored. Here, we characterize the prognostic significance of early progression of disease (POD) in patients enrolled in the Connect CLL Registry and treated mainly outside of the clinical trial setting. The Connect CLL Registry is, to our knowledge, the largest prospective study of patients with CLL starting first-line treatment in practices across the United States. Understanding the significance of early POD in chemoimmunotherapy-treated patients with CLL represents a benchmark against which novel and targeted therapies can be compared.
Patients and methods
Patients and study design
The Connect CLL Registry is a large US-based, multicenter, prospective observational cohort study. A total of 1494 patients with CLL were enrolled between 2010 and 2014 at 199 academic, community, and government sites in the United States. The study was noninterventional, with all medical care and follow-up performed at the discretion of the treating physician. The registry protocol was approved by a central institutional review board (IRB) (Quorum Review IRB, Seattle, WA) or by the IRB at each participating site, and conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent. Eligible patients had a diagnosis of CLL based on International Workshop on CLL guidelines17 and were enrolled within 2 months of initiating treatment of CLL, with either first-line or subsequent line of therapy. Demographic and clinical data were collected at baseline; clinical data included baseline comorbidities, genetic characteristics, and treatment regimens. Posttreatment clinical data, including response data, were collected every 3 months for ≤60 months after enrollment. To minimize loss to follow-up, patients were recontacted at regular intervals to obtain overall survival (OS) data. Baseline medical history and preexisting condition data were used to generate a Charlson comorbidity index (CCI) for use as a prognostic indicator of overall risk of death from comorbidities and to measure the burden of comorbid disease at registry enrollment.18,19 Baseline genetic aberrations were detected by interphase FISH, metaphase karyotype, and/or IgHV mutational status. Full details on the design and conduct of the registry have been described previously.20 This manuscript was developed in line with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines for reporting of observational studies.21
Definition of treatment groups
First-line regimens used in the study cohort were categorized into combination chemoimmunotherapy, monotherapy, and others. Combination chemoimmunotherapy indicated regimens pairing a monoclonal antibody with 1 or more cytotoxic agents. Monotherapy was defined by single-agent use of a monoclonal antibody, a cytotoxic agent, or an immunomodulatory agent.
Definition of patient subgroups
Eligible patients receiving first-line therapy were stratified into 3 groups on the basis of onset of first event: early POD (disease progression <2 years from enrollment); late POD (disease progression ≥2 years from enrollment); and no POD (patients with no disease progression) as of the data cutoff of 1 May 2017. An event was defined as posttreatment disease progression including both CLL and Richter’s histologic transformation, as per the International Workshop on CLL guidelines.17 No POD was defined as (1) the patient was still alive at the data cutoff date with no documented POD or (2) the patient’s cause of death was attributed to causes other than CLL disease progression. Because this is a purely observational study, disease progression was defined by the treating physician without the need to follow formal response criteria and was not centrally assessed. The 2-year cutoff was selected because it closely approximated the median time to progression (TTP) observed in 829 evaluable patients (21 months) and was also used in previous studies.14,16,22 Sensitivity analyses were conducted to assess the biases in the association of the late POD group with OS.
Statistical analysis
OS was defined as time from enrollment until death, study discontinuation, or the data cutoff of 1 May 2017, whichever was earliest. Probabilities of OS were estimated by the Kaplan-Meier method and compared among subgroups using the log-rank test. Cox regression models including disease progression as a time-varying covariate were used to measure the risk of death associated with early POD. Logistic regression with an outcome variable for early POD (yes vs no) was used to identify additional risk factors associated with onset of early POD. Variables significant at the P < .10 level, as identified in the univariate analysis, were included in the multivariable logistic regression and Cox regression analyses. A best model was selected using the score-based variable selection process. Sensitivity analyses were conducted to test the robustness of the results in the presence of potentially biasing factors. Patients who were lost to follow-up were censored. As per registry protocol, missing data were not imputed. In the statistical analyses, missing data were assumed to be missing at random. All tests were 2-sided. P < .05 was considered statistically significant. Statistical analyses were performed using SAS software, version 9.2 (SAS Institute, Cary, NC).
Results
Patient characteristics
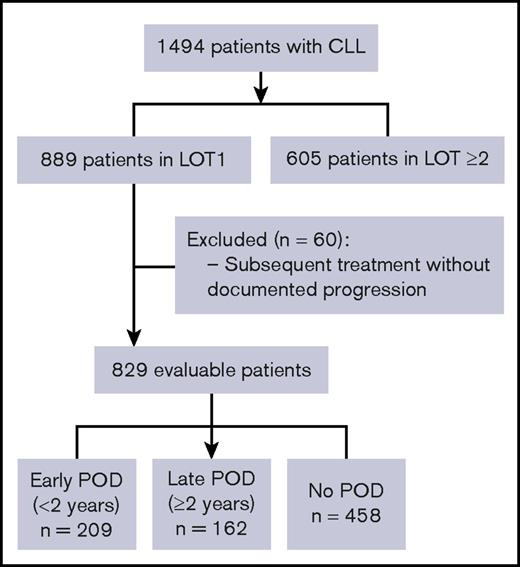
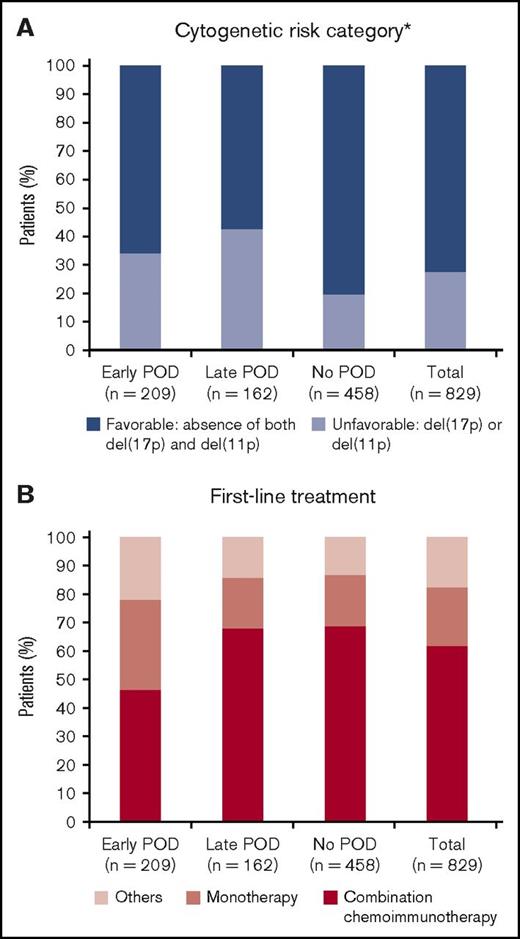
Of 889 patients who received first-line therapy for CLL, 829 patients were considered evaluable (Figure 1). The remaining 60 patients were excluded from the analyses because of subsequent treatment without documented disease progression. Approximately 25% of patients enrolled to the registry were lost to follow-up, which is as expected for a registry. With a median follow-up of 48.8 months, 371 (44.8%) patients experienced POD. The median TTP in all evaluable patients was 21 months. Based on the cutoff of 2 years from treatment initiation, 3 subgroups were defined: early POD (209 patients, 25.2%); late POD (162 patients, 19.5%); and no POD (458 patients, 55.3%). Baseline characteristics are summarized in Table 1. The median age of all patients was 68 years, and 63.6% were male. Rai stage was available for 76.2% of patients; 54.0% of these patients were Rai stage 0-I. Of the 476 patients (57.4%) whose cytogenetic risk category was known, 27.7% had an unfavorable cytogenetic risk profile, defined as the presence of del(11q) or del(17p). Patients in the early POD group were older (≥75 years) (P < .01) and more likely to have either del(11q) or del(17p) (P = .04) vs the no POD group (Figure 2A). The distribution of other baseline characteristics, including sex, race, CCI, and Rai stage, was similar among the 3 subgroups (all P > .05). IgHV mutation status was available in only 8.0% of the cohort (supplemental Table 1) and was therefore excluded from further analyses.
Consolidated Standards of Reporting Trials diagram for participant selection. Progression includes relapsed/refractory CLL and histologic transformation. Patients who died or received subsequent treatment without documented progression were excluded. LOT1, first-line therapy; LOT ≥2, second or further lines of therapy.
Consolidated Standards of Reporting Trials diagram for participant selection. Progression includes relapsed/refractory CLL and histologic transformation. Patients who died or received subsequent treatment without documented progression were excluded. LOT1, first-line therapy; LOT ≥2, second or further lines of therapy.
Baseline characteristics of patients receiving first-line therapy for CLL at enrollment in the Connect CLL Registry
| Characteristic . | Early POD . | Late POD . | No POD . | All patients . |
|---|---|---|---|---|
| N (% of total) | 209 (25.2) | 162 (19.5) | 458 (55.3) | 829 (100.0) |
| Age | ||||
| Median (range), y | 71.0 (41-91) | 67.5 (22-94) | 67.0 (27-99) | 68.0 (22-99) |
| ≥65 y, n (%) | 140 (67.0) | 93 (57.4) | 278 (60.7) | 511 (61.6) |
| ≥75 y, n (%) | 81 (38.8) | 35 (21.6) | 123 (26.9) | 239 (28.8) |
| Sex, n (%) | ||||
| Male | 137 (65.6) | 110 (67.9) | 280 (61.1) | 527 (63.6) |
| Female | 72 (34.4) | 52 (32.1) | 178 (38.9) | 302 (36.4) |
| Race | ||||
| Available, n | 204 | 157 | 443 | 804 |
| White, n (% of available) | 185 (90.7) | 143 (91.1) | 415 (93.7) | 743 (92.4) |
| African American, n (% of available) | 15 (7.4) | 13 (8.3) | 25 (5.6) | 53 (6.6) |
| Other, n (% of available) | 4 (2.0) | 1 (0.6) | 3 (0.7) | 8 (1.0) |
| Geographic region | ||||
| Available, n | 204 | 161 | 456 | 821 |
| West, n (% of available) | 42 (20.5) | 26 (16.1) | 67 (14.7) | 135 (16.4) |
| Midwest, n (% of available) | 65 (31.9) | 54 (33.5) | 137 (30.0) | 256 (31.2) |
| Northeast, n (% of available) | 24 (11.8) | 22 (13.7) | 56 (12.3) | 102 (12.4) |
| South, n (% of available) | 73 (35.8) | 59 (36.6) | 196 (43.0) | 328 (40.0) |
| Charlson comorbidity index, n (% of available) | ||||
| Low (≤2) | 115 (55.0) | 95 (58.6) | 264 (57.6) | 474 (57.2) |
| High (≥3) | 94 (45.0) | 67 (41.4) | 194 (42.4) | 355 (42.8) |
| Rai stage | ||||
| Available, n | 151 | 135 | 346 | 632 |
| 0-I, n (% of available) | 80 (53.0) | 82 (60.7) | 179 (51.7) | 341 (54.0) |
| II-IV, n (% of available) | 71 (47.0) | 53 (39.3) | 167 (48.3) | 291 (46.0) |
| Cytogenetic risk category* | ||||
| Available, n | 117 | 94 | 265 | 476 |
| Unfavorable, n (% of available) | 40 (34.2) | 40 (42.6) | 52 (19.6) | 132 (27.7) |
| Favorable, n (% of available) | 77 (65.8) | 54 (57.4) | 213 (80.4) | 344 (72.3) |
| Median TTP in all patients, mo | 12 | 37 | — | 21 |
| Median TTP after first-line FCR, mo | 16 | 38 | — | 26 |
| Median TTP after first-line BR, ms | 13 | 41 | — | 25 |
| Patients who received subsequent therapy and had available treatment data, n | 160 | 130 | — | 290 |
| TTNT in all patients, ms | 12 | 38 | — | 22 |
| Characteristic . | Early POD . | Late POD . | No POD . | All patients . |
|---|---|---|---|---|
| N (% of total) | 209 (25.2) | 162 (19.5) | 458 (55.3) | 829 (100.0) |
| Age | ||||
| Median (range), y | 71.0 (41-91) | 67.5 (22-94) | 67.0 (27-99) | 68.0 (22-99) |
| ≥65 y, n (%) | 140 (67.0) | 93 (57.4) | 278 (60.7) | 511 (61.6) |
| ≥75 y, n (%) | 81 (38.8) | 35 (21.6) | 123 (26.9) | 239 (28.8) |
| Sex, n (%) | ||||
| Male | 137 (65.6) | 110 (67.9) | 280 (61.1) | 527 (63.6) |
| Female | 72 (34.4) | 52 (32.1) | 178 (38.9) | 302 (36.4) |
| Race | ||||
| Available, n | 204 | 157 | 443 | 804 |
| White, n (% of available) | 185 (90.7) | 143 (91.1) | 415 (93.7) | 743 (92.4) |
| African American, n (% of available) | 15 (7.4) | 13 (8.3) | 25 (5.6) | 53 (6.6) |
| Other, n (% of available) | 4 (2.0) | 1 (0.6) | 3 (0.7) | 8 (1.0) |
| Geographic region | ||||
| Available, n | 204 | 161 | 456 | 821 |
| West, n (% of available) | 42 (20.5) | 26 (16.1) | 67 (14.7) | 135 (16.4) |
| Midwest, n (% of available) | 65 (31.9) | 54 (33.5) | 137 (30.0) | 256 (31.2) |
| Northeast, n (% of available) | 24 (11.8) | 22 (13.7) | 56 (12.3) | 102 (12.4) |
| South, n (% of available) | 73 (35.8) | 59 (36.6) | 196 (43.0) | 328 (40.0) |
| Charlson comorbidity index, n (% of available) | ||||
| Low (≤2) | 115 (55.0) | 95 (58.6) | 264 (57.6) | 474 (57.2) |
| High (≥3) | 94 (45.0) | 67 (41.4) | 194 (42.4) | 355 (42.8) |
| Rai stage | ||||
| Available, n | 151 | 135 | 346 | 632 |
| 0-I, n (% of available) | 80 (53.0) | 82 (60.7) | 179 (51.7) | 341 (54.0) |
| II-IV, n (% of available) | 71 (47.0) | 53 (39.3) | 167 (48.3) | 291 (46.0) |
| Cytogenetic risk category* | ||||
| Available, n | 117 | 94 | 265 | 476 |
| Unfavorable, n (% of available) | 40 (34.2) | 40 (42.6) | 52 (19.6) | 132 (27.7) |
| Favorable, n (% of available) | 77 (65.8) | 54 (57.4) | 213 (80.4) | 344 (72.3) |
| Median TTP in all patients, mo | 12 | 37 | — | 21 |
| Median TTP after first-line FCR, mo | 16 | 38 | — | 26 |
| Median TTP after first-line BR, ms | 13 | 41 | — | 25 |
| Patients who received subsequent therapy and had available treatment data, n | 160 | 130 | — | 290 |
| TTNT in all patients, ms | 12 | 38 | — | 22 |
TTNT, time to next therapy.
Cytogenetic risk category was defined by the hierarchical model. Unfavorable cytogenetic risk category was defined by del(11q) or del(17p) detected by interphase FISH or metaphase karyotype. Favorable-risk category was defined by the absence of both del(11q) and del(17p).
Comparison of baseline and treatment characteristics in early, late, and no POD groups. (A) Cytogenetic risk category defined by the hierarchical model. (B) Treatment patterns divided by combination chemoimmunotherapy, monotherapy, and others. *Percentage of patients undergoing genetic testing.
Comparison of baseline and treatment characteristics in early, late, and no POD groups. (A) Cytogenetic risk category defined by the hierarchical model. (B) Treatment patterns divided by combination chemoimmunotherapy, monotherapy, and others. *Percentage of patients undergoing genetic testing.
Treatment characteristics
Patients in the early POD group more frequently received monotherapy (31.6% vs 17.9% vs 17.9%) and less frequently had combination chemoimmunotherapy (46.4% vs 67.9% vs 68.8%) than patients with late or no POD (Figure 2B; supplemental Table 1). Fludarabine, cyclophosphamide, and rituximab (FCR) was the most commonly used combination chemoimmunotherapy (26.4% of 829 evaluable patients), followed by bendamustine and rituximab (BR) (21.5%), with a similar distribution of usage among all POD groups. Rituximab and chlorambucil were the 2 most commonly used monotherapies: in 11.1% and 4.5% of patients, respectively. First-line treatment with immunomodulatory agents, such as lenalidomide, was rare (<1.0%), and no patients received ibrutinib. Use of second-line therapies was comparable between early and late POD groups with regard to use of combination chemoimmunotherapy and monotherapy. Second-line ibrutinib was received significantly more often by patients in the late POD group compared with the early POD group (33.1% vs 12.5%, respectively; P < .10) (supplemental Table 3).
OS
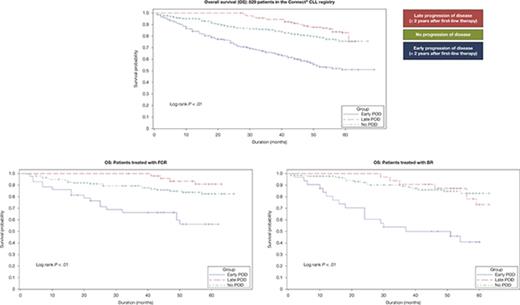
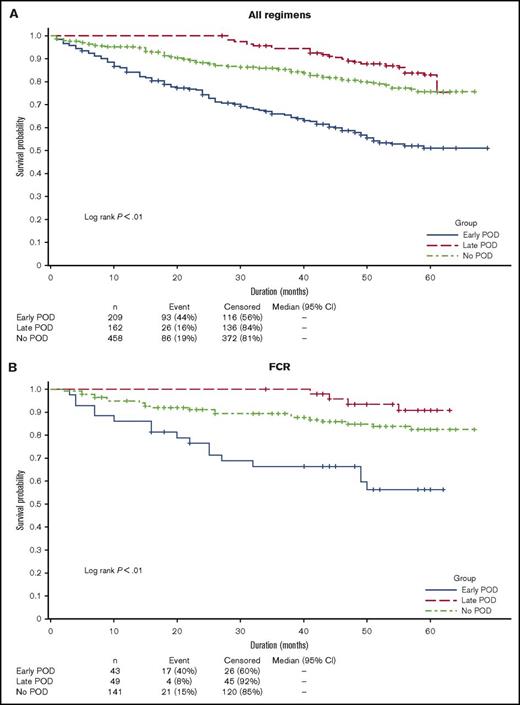
As of May 1, 2017, 93 (44%) patients in the early POD group had died compared with 26 (16%) in the late POD group and 86 (19%) in the no POD group. Early POD was associated with significantly inferior OS compared with late and no POD in a univariate analysis (hazard ratio [HR], 4.8; 95% confidence interval [CI], 3.6-6.4; P < .01). Three-year OS in the early, late, and no POD groups was 65.9% (95% CI, 58.9-72.0), 94.4% (95% CI, 89.5-97.0), and 85.5% (95% CI, 81.6-88.6), respectively (Figure 3A). To assess the impact of treatment regimens as potential confounding factors for survival, we selected the 3 most commonly used first-line regimens and performed survival analyses among patients who received homogeneous therapy. Among 219 patients treated with first-line FCR, those with early POD (n = 38 [17.4%]) had significantly inferior survival compared with patients with late (n = 48 [21.9%]) or no POD (n = 133 [60.7%]) in a univariate analysis (HR, 7.0; 95% CI, 3.6-13.5; P < .01). Similar results were observed in patients treated with first-line BR (n = 178: early POD, 31 [17.4%]; late POD, 29 [16.3%]; no POD, 118 [66.3%]) (HR, 7.4; 95% CI, 3.9-14.1; P < .01; Figure 3C). There was no significant survival difference between POD groups in patients treated with rituximab monotherapy (n = 92: early POD, 29 [31.5%]; late POD, 16 [17.4%]; no POD, 47 [51.1%]) (HR, 1.2; 95% CI 0.5-3.0; P < .66; Figure 3D).
Overall survival in patients with early, late, or no POD. (A) All evaluable patients. (B) Patients treated with first-line FCR. (C) Patients treated with first-line BR. (D) Patients treated with first-line rituximab monotherapy. Includes death after registry discontinuation.
Overall survival in patients with early, late, or no POD. (A) All evaluable patients. (B) Patients treated with first-line FCR. (C) Patients treated with first-line BR. (D) Patients treated with first-line rituximab monotherapy. Includes death after registry discontinuation.
Early POD is an independent predictor of survival
To determine the prognostic significance of early POD, Cox regression analyses were performed using more than 20 pre-, intra-, and posttreatment factors; 14 were associated with OS in univariate analysis (supplemental Table 4). Five independent predictors of inferior OS were identified in a multivariable analysis: early POD, high comorbidity index (CCI ≥ 3), age ≥75 years, Rai stage ≥II, and treatment duration ≤4 months (Table 2). Early POD was strongly and significantly associated with a higher risk of death (HR, 3.6; 95% CI, 2.6-5.1; P < .01). Because treatment duration ≤4 months was a significant predictor of survival, we looked at the most frequent reasons for short treatment duration (≤4 months). These were: completed course of therapy (46%), toxicity (21%), remission (9%), resistance (5%), and other reasons (15%). Of patients with a treatment duration ≤4 months, 25% received FCR, 18% received BR, and 16% received rituximab monotherapy. We observed more frequent use of rituximab monotherapy (24%) and less frequent use of combination chemoimmunotherapy regimens such as FCR (19%) and BR (17%) in patients who were considered to have completed a course of therapy of ≤4 months.
Multivariable Cox regression analysis of the association between early POD and risk of death
| Variable . | Adverse factor* . | Adjusted HR (95% CI) . | P . |
|---|---|---|---|
| Progression as a time varying covariate | Early POD (<2 y) | 3.6 (2.6-5.1) | <.01 |
| Age | Age ≥75 y | 2.2 (1.6-3.0) | <.01 |
| Comorbidity | Charlson comorbidity index ≥3 | 2.1 (1.5-2.8) | <.01 |
| Treatment duration | Treatment duration ≤4 mo | 1.8 (1.3-2.5) | <.01 |
| Disease stage | Rai stage ≥II | 1.4 (1.1-2.0) | .01 |
| Variable . | Adverse factor* . | Adjusted HR (95% CI) . | P . |
|---|---|---|---|
| Progression as a time varying covariate | Early POD (<2 y) | 3.6 (2.6-5.1) | <.01 |
| Age | Age ≥75 y | 2.2 (1.6-3.0) | <.01 |
| Comorbidity | Charlson comorbidity index ≥3 | 2.1 (1.5-2.8) | <.01 |
| Treatment duration | Treatment duration ≤4 mo | 1.8 (1.3-2.5) | <.01 |
| Disease stage | Rai stage ≥II | 1.4 (1.1-2.0) | .01 |
Factors that reached statistical significance in the univariate but not the multivariable model were: insurance other than private, first-line regimens other than FCR and BR, first-line regimens other than FCR, Eastern Cooperative Oncology Group performance status 0 vs ≥1, anemia, splenomegaly as a reason for treatment initiation, and creatinine clearance at baseline.
Additionally, we assessed the prognostic value of early POD in the setting of subsequent therapy (early POD2, defined as progression <2 years after second-line therapy). We divided 340 patients who were followed up after progressing on first-line therapy into 3 subgroups: early, late, and no POD (based on time from the onset of first progression to the onset of second progression). Multivariate Cox analysis demonstrated that early POD2 was a statistically significant predictor of inferior survival (supplemental Table 5).
Predictors of early POD
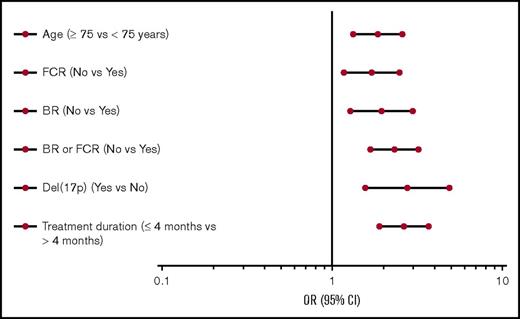
Factors associated with early POD in a univariate as well as multivariate analysis were age ≥75 years, presence of del(17p), first-line therapy other than FCR or BR, and treatment duration ≤4 months (P < .01 for all tests; Figure 4; supplemental Table 6). Cytogenetic changes other than del(17p), insurance status (private vs other), and reason for treatment initiation were not significantly associated with early POD.
Subgroup analyses of the risk of early POD. ORs and 95% CIs for selected factors associated with the risk of early POD (all P < .01). OR, odds ratio.
Subgroup analyses of the risk of early POD. ORs and 95% CIs for selected factors associated with the risk of early POD (all P < .01). OR, odds ratio.
Sensitivity analyses
To control for immortal time bias, which can be introduced when the exposure of interest is defined by a future event (in this case, that the late POD group was restricted to those patients who survived ≥24 months), the primary analysis treated POD as a time-varying covariate. To evaluate whether the time-varying approach appropriately removed this bias, a series of sensitivity analyses were conducted. Landmark analyses of OS were performed using landmarks at 12 and 24 months. The results confirmed the initial findings; early POD was a significant predictor of inferior OS for 717 patients followed for ≥12 months (HR, 2.24; 95% CI, 1.52-3.31; P < .01) and for 626 patients followed for ≥24 months (HR, 2.01; 95% CI, 1.29-3.23; P < .01) (supplemental Figure 1).
An additional analysis was performed to control the effect of the introduction of targeted agents on patient outcomes. This analysis excluded patients who experienced a POD event before the initial approval of ibrutinib by the US Food and Drug Administration (FDA) (ie, before 13 February 2014). The results confirmed that early POD is a significant predictor of inferior survival even in the era of targeted agents. In total, 63 (21.7%) of the 371 patients who had disease progression after first-line therapy received ibrutinib as a single agent or in combination with an anti-CD20 monoclonal antibody (supplemental Table 3). Patients who were not subsequently treated with ibrutinib monotherapy or ibrutinib-based combination therapy at any time point after the first progression had significantly inferior OS compared with those who received ibrutinib (HR, 3.1; 95% CI, 1.9-5.1, P < .0001). The favorable survival associated with subsequent therapy with ibrutinib was consistently observed when we limited the survival analysis to patients who had disease progression after 2014, when ibrutinib was first approved for treatment of relapsed/refractory CLL.
Furthermore, a landmark sensitivity analysis of patients followed for >4 months confirmed that treatment duration ≤4 months was an independent predictor of inferior OS and of early POD. A sensitivity analysis using a more stringent categorization of treatment regimens FCR, BR, and rituximab monotherapy, which excluded patients who received combination FCR and an additional antitumor agent (for example, FCR plus lenalidomide), also confirmed that patients who had early POD continued to have significantly shorter survival. The results of a final sensitivity analysis that excluded patients with del(17p) confirmed again that early POD is a predictor of inferior OS (data not shown).
Discussion
In this study, we found that early POD (ie, within 2 years of registry enrollment) was an independent predictor of inferior OS in the Connect CLL registry in patients who were treated with chemoimmunotherapy combinations in community-based practices. The adverse impact of early POD on survival was consistently observed after adjusting for first-line treatment regimens, such as FCR and BR. Older age, del(17p), treatment with regimens other than FCR and BR, and treatment duration ≤4 months were associated with a higher risk of early POD.
TTP has biological implications because it may represent clonal fitness and growth trajectory after initial therapy. Inferior outcomes may be driven by clonal expansion of driver mutations. For instance, del(17p) or TP53 mutation consistently predicted poor outcome after treatment with chemoimmunotherapy10 or kinase inhibitors,23 and a higher risk of relapse after allogeneic stem cell transplantation.24 Our study also demonstrated a strong association between del(17p) and the risk of early POD. Treatment, in this context, can be viewed as a selective pressure that confers a fitness advantage to clones with existing somatic mutations, such as TP53.13 Furthermore, treatment itself may facilitate new genomic changes and increase clonal complexity. In sequencing studies using matched pre- and posttreatment samples, clonal evolution occurred in almost all CLL patients receiving chemoimmunotherapy.10 In another study, copy number aberrations significantly increased after treatment (from 1 per pretreatment sample to 8 per posttreatment sample) and increasing copy number aberrations were associated with a shorter time to next treatment or death.25 Although evidence suggests that early treatment failure is related to biologically aggressive molecular changes and should be addressed separately from late relapses,14,16 there is a paucity of studies designed to recognize and prospectively intervene to prevent early progression in patients with unfavorable-risk CLL.
Time to POD may potentially serve as a posttreatment risk stratification tool that offers a simple readout of clonal fitness in the context of first-line treatment without the need for additional sophisticated or costly genomic analyses. Current risk stratification of CLL heavily uses pretreatment factors. For example, the CLL-International Prognostic Index is a tool developed for prognostication of previously untreated patients using 5 baseline clinical and genetic parameters.6 Minimal residual disease negativity remains the only established prognostic factor applicable after treatment.26,27 If we use TTP as a prognostic factor, patients who progress within 2 years of starting first-line therapy with conventional chemotherapy regimens can be prioritized for clinical trials of highly active targeted agents, in combination or in sequence, rather than being treated with conventional approaches using chemoimmunotherapy or a single-agent kinase inhibitor.
Early POD within 2 years may also be used as a surrogate end point for OS. Previous studies have used a similar cutoff point of 24 to 36 months based on median OS outcomes.14,16,22 Identifying surrogate end points has become increasingly important because the introduction of monoclonal antibodies and targeted agents has markedly extended the survival of patients with CLL. Ibrutinib treatment led to a 30-month OS of 65% and 60-month OS of 57% for this high-risk population of patients with relapsed/refractory CLL with del(17p).23,28 If validated, the application of early POD as a surrogate end point for survival can allow identification of target populations for trials and facilitate drug development.
In the Connect CLL registry, the selection of first-line therapy appeared to be paramount to long-term outcome in CLL. Patients in the early POD group were more likely to receive monotherapy (32%) than those in the late and no POD groups (18% in each group). Combination chemoimmunotherapy was more frequently prescribed in the late (68%) and no (69%) POD groups than in the early POD group (46%). The increased use of monotherapy in the early POD group could not be fully explained by factors such as age, comorbidities, geographic region, or insurance status because these factors were relatively similar among the 3 subgroups. Furthermore, univariate analyses confirmed that age, comorbidities, and insurance status were not associated with early POD; however, treatment with regimens other than first-line FCR or BR was associated with early POD. The Connect CLL cohort had a large number of patients predominantly treated in community practices, where rituximab was the most commonly used monotherapy; this was given to 92 patients (54%) of those who received monotherapy. Single-agent rituximab is an inappropriate treatment of CLL in the modern era, unless it is given to treat autoimmune cytopenia. Monotherapy with conventional cytotoxic agents or rituximab is associated with inferior initial response and long-term outcomes compared with combination chemoimmunotherapy.29,30
More recently, 4 randomized trials demonstrated superior efficacy of targeted agents over monoclonal antibody monotherapy.31-34 Although we do not have direct evidence describing the rationale for treatment selections, we doubt that all 92 patients in our cohort received single-agent rituximab to control autoimmune cytopenias; this means that this approach may represent suboptimal treatment of some patients. It could be that early POD was not a predictor of inferior survival in the setting of rituximab monotherapy because the regimen itself was suboptimal for all patients in the treatment subgroup. Patients who experience early POD after rituximab monotherapy should not automatically be considered to have high-risk disease, unlike those presenting with early POD after FCR or BR; the next treatment of these patients should be an appropriate first-line regimen.
There are several limitations to this study worth noting. First, because of the choice of a 2-year progression cutoff, all patients in the late POD group in this analysis were, by definition, alive at 2 years, introducing immortal time bias. Such problems have been encountered in similar analyses of registry data, and several approaches are possible.35 To overcome this limitation, we selected a time-dependent analysis to control for immortal time bias. Sensitivity analyses, including landmark analyses and a Cox model using progression as a time-varying covariate, confirmed these findings. Second, baseline cytogenetics and IgHV mutation status were assessed in limited subsets of patients (58% and 8%, respectively), and their prognostic impact on survival could not be directly compared with that of early POD. However, it has previously been shown that patients in the Connect CLL registry with missing cytogenetics data do not differ from the rest of the cohort with regard to baseline characteristics and treatment outcomes.20 Despite the small number of patients undergoing cytogenetic testing, del(17p) was strongly associated with early POD, supporting the notion that early POD is driven by the growth of genetically aggressive clones. Third, the Connect CLL registry was an observational study without a standardized schedule for imaging and laboratory follow-ups. The lack of scheduled follow-up studies affects progression-free survival and hampers comparison of these observational data with clinical trial results. Furthermore, patients were enrolled to the registry between 2010 and 2014, before the FDA approval of novel agents for frontline therapy of CLL. None of the 829 evaluable patients received ibrutinib as first-line therapy; 21.7% received it as second-line therapy. The FDA initially approved ibrutinib in 2014, and 2 additional targeted agents were approved in the following 2 years, which has dramatically changed the treatment landscape of this disease. Future studies should explore the prognostic value of early POD in the context of targeted agents and if subsequent treatment with new agents can improve inferior survival among patients with early POD. Last, because this was a registry-based study, assessment of response and progression was performed at the discretion of the treating clinician. Evaluation intervals may have varied between patients, particularly between those with and without POD, because there were no required scheduled clinic visits.
In summary, this study of patients in the Connect CLL Registry shows that POD within 2 years of initial chemoimmunotherapy is an independent predictor of inferior OS in CLL. In view of the clonal heterogeneity and evolution of CLL, early POD indicates the presence of CLL clones capable of surviving treatment and leading to disease progression. Early POD may function as a posttreatment risk stratification tool and a robust clinical end point, which should be further explored in the context of targeted agents. This analysis from the Connect CLL registry provides important, real-world insights into the experiences of patients and has enabled us to answer clinically meaningful questions about treatment patterns and outcomes of patients with CLL treated in clinical practices.
The full-text version of this article contains a data supplement.
Acknowledgments
All authors were involved in the maintenance of the Connect CLL Registry. The Connect CLL Scientific Steering Committee acknowledges the contributions of all past and current members of the committee for their guidance in the design of the registry and participation in the analysis of the data, including Matthew Davids, Charles Farber, Ian Flinn, Christopher R. Flowers, David L. Grinblatt, Neil E. Kay, Michael Keating, Thomas J. Kipps, Mark F. Kozloff, Nicole Lamanna, Susan Lerner, Anthony Mato, Chadi Nabhan, Chris L. Pashos, Jeff P. Sharman, and Mark Weiss.
The Connect CLL Registry is sponsored and funded by Celgene Corporation. Celgene Corporation supported the authors in collecting and analyzing the data reported in this registry. The authors received medical writing services provided by Victoria Edwards and Nicky Dekker of Excerpta Medica BV in the preparation of this manuscript, funded by Celgene Corporation.
Authorship
Contribution: All authors directed the development, reviewed, and approved this manuscript and are fully responsible for all content and editorial decisions.
Conflict-of interest disclosure: C.M.F. received research funding from Genentech and Gilead; received consulting and lecturing fees from Celgene Corporation, Genentech, Gilead, Janssen, Pharmacyclics, and Seattle Genetics; served on the advisory committee for Celgene Corporation; and received honoraria from Janssen. M.S.D. received research funding and served at the scientific advisory board for Genentech, Infinity, Pharmacyclics, and TG Therapeutics, and received consulting fees from AbbVie, Celgene Corporation, Gilead, Infinity, Janssen, Merck, and AstraZeneca. D.L.G. received consulting and lecturing fees from Celgene Corporation. N.E.K. received research funding from Gilead, Morphosys, Celgene Corporation, and Pharmacyclics. N.L. received research funding from AbbVie, Genentech, Gilead, Infinity, and Pronai; received consulting fees from AbbVie, Genentech, Gilead, Pharmacyclics, and Pronai; and served on the advisory committee for Celgene Corporation. A.M. received research funding from AbbVie, Gilead, Pronai, and TG Therapeutics; received consulting fees from AbbVie; and received lecturing fees from Celgene Corporation. C.N. is an employee of Cardinal Health. P.K., A.S.S., E.D.F., and K.S. are employees of Celgene Corporation and have equity ownership in Celgene Corporation. J.P.S. received consulting fees from Celgene Corporation, Genentech, Gilead, Pharmacyclics, and TG Therapeutics, and received lecturing fees from Gilead. C.R.F. received research funding from AbbVie, Acerta, Gilead, Millennium, Infinity, Janssen, Pharmacyclics, Spectrum, and TG Therapeutics, and received consulting fees from Bayer, Celgene Corporation, Genentech/Roche, Gilead, Millennium, Optum Rx, and Seattle Genetics. I.E.A. declares no competing financial interests.
Correspondence: Christopher R. Flowers, Emory Lymphoma Program, Winship Cancer Institute, Emory University, 1365 Clifton Rd NE, B4300, Atlanta, GA 30322; e-mail: crflowe@emory.edu.