Key Points
The preferred donor for patients with poor-risk AML in CR1 proceeding to alloHSCT include MRD or 10/10 MUD.
Alternative donors are 9/10 MUD, UCB grafts, and especially haplo, but sufficient numbers and follow-up to define a hierarchy are lacking.
Abstract
Allogeneic hematopoietic stem cell transplantation (alloHSCT) remains the treatment of choice to consolidate remission in patients with poor-risk acute myeloid leukemia (AML). With increasing alternative donors available, the preferred donor or stem cell source is debated. We set out to study outcome in recipients of alloHSCT with poor-risk AML in first complete remission (CR1) by donor type. A total of 6545 adult patients with poor-risk AML in CR1 receiving an alloHSCT using matched related donor (MRD, n = 3511) or alternative donors, including 10/10 (n = 1959) or 9/10 matched unrelated donors (MUDs, n = 549), umbilical cord blood (UCB) grafts (n = 333), or haplo-identical (haplo) donors (n = 193) were compared. Overall survival (OS) at 2 years following MRD alloHSCT was an estimated 59 ± 1%, which did not differ from 10/10 MUD (57 ± 1%) and haplo alloHSCT (57 ± 4%). OS, however, was significantly lower for 9/10 MUD alloHSCT (49 ± 2%) and UCB grafts (44 ± 3%), respectively (P < .001). Nonrelapse mortality (NRM) depended on donor type and was estimated at 26 ± 3% and 29 ± 3% after haplo alloHSCT and UCB grafts at 2 years vs 15 ± 1% following MRD alloHSCT. Multivariable analysis confirmed the impact of donor type with OS following MRD, 10/10 MUD, and haplo alloHSCT not being statistically significantly different. NRM was significantly higher for alternative donors as compared with MRD alloHSCT. Collectively, these results suggest that alloHSCT with MRDs and 10/10 MUDs may still be preferred in patients with poor-risk AML in CR1. If an MRD or 10/10 MUD is not available, then the repertoire of alternative donors includes 9/10 MUD, UCB grafts, and haplo-identical donors. The latter type of donor is increasingly applied and now approximates results with matched donors.
Introduction
Allogeneic hematopoietic stem cell transplantation (alloHSCT) is the most effective postremission treatment for prevention of relapse in poor-risk acute myeloid leukemia (AML) in first complete remission (CR1).1,2 Although most patients lack an HLA-matched related donor (MRD), an alternative donor is available for almost every patient in need of an alloHSCT.3 Although the probability of identifying an adult matched unrelated donor (MUD) can be as high as 60% to 80% for Caucasian patients, finding a suitable MUD for patients from ethnic minorities is less successful.4-6 One allele mismatched unrelated donor (MMUD) may serve as a good alternative, but outcome has been associated with approximately 10% loss in overall survival (OS), which has predominantly been ascribed to increased nonrelapse mortality (NRM).7 Following the favorable results in pediatric patients, alloHSCT with umbilical cord blood (UCB) grafts was also developed in adult patients. Results of UCB alloHSCT in retrospective registry studies approximated those of MUD alloHSCT, although hematopoietic recovery is delayed compared with MUD alloHSCT, and graft failure was more frequently observed.8-10 More recently, a revived interest in the use of haplo-identical (haplo) donors has become apparent because of improved transplantation techniques and pharmacological manipulation of host-versus-graft and graft-versus-host reactions.11 Although each type of donor and/or stem cell source has its own advantages and drawbacks, comparative studies evaluating survival estimates in well-defined groups of patients are scarce. Here, we set out to compare outcome in patients with poor-risk AML in CR1 receiving alloHSCT between 2000 and 2014, using either MRD, 10/10 or 9/10 MUDs, haplo, or UCB grafts.
Methods
Patients
A total of 6545 adult patients with poor-risk AML in CR1 receiving an alloHSCT between 2000 and 2014 and reported to the European Society for Blood and Marrow Transplantation (EBMT) Acute Leukemia Working Party and Eurocord were eligible for the analysis. Patients were transplanted with an MRD (n = 3511) or alternative donors, including 10/10 (n = 1959) or 9/10 MUDs (n = 549), UCB grafts (n = 333), or haplo (n = 193). Poor-risk AML was defined as described previously,12 with either white blood cell count (WBC) >100 × 109/L at diagnosis, secondary AML, cytogenetic abnormalities associated with adverse risk according to European LeukemiaNET classification,13 presence of an fms-like tyrosine kinase 3 internal tandem duplication (FLT3-ITD), or no CR after 1 cycle of induction chemotherapy. Patients received conditioning therapy followed by infusion of donor cells with either MRD or alternative donors. In vivo T-cell depletion was defined as the use of either antithymocyte globulin (ATG) or alemtuzumab. No ex vivo T-cell depletion was performed on any of the patient grafts. The degree of HLA matching was 9 or 10 of a 10 allele match for the MUDs, with mismatches allowed at HLA-A, HLA-B, HLA-C, HLA-DR, or HLA-DQ levels for the 9/10 MUDs (supplemental Table 1). AlloHSCT with UCB grafts was performed with either single or double cords. HLA matching for UCB was done according to the standard criteria on antigen level for A and B and allele level for DRB1. Haplo was defined as a ≥4/8 HLA match.
This study was a retrospective multicenter analysis and was performed in accordance with the principles of the Declaration of Helsinki and approved by the Acute Leukemia Working Party of the EBMT group. The EBMT is a nonprofit, scientific society representing more than 600 transplant centers, mainly in Europe. The EBMT promotes all activity aiming to improve stem cell transplantation or cellular therapy, which includes registering all the activity relating to stem cell transplants. Data are entered, managed, and maintained in a central database with Internet access; each EBMT center is represented in this database. There are no restrictions on centers for reporting data, except for those required by the law on patient consent, data confidentiality, and accuracy. Quality control measures included several independent systems: confirmation of validity of the entered data by the reporting team, selective comparison of the survey data with minimum essential data A data sets in the EBMT registry database, cross-checking with the national registries, and regular in-house and external data audits. Since 1990, patients have provided informed consent authorizing the use of their personal information for research purposes.
End points
The primary end point of the study was OS at 2 years. Secondary end points included relapse-free survival (RFS), relapse, NRM, and acute and chronic graft-versus-host disease (GVHD) at 2 years. All outcome parameters were measured from the date of transplantation. The event for OS was death, whatever the cause, and patients were censored at the date of last contact if alive. The events for RFS were death in CR1, designated as NRM, or hematological relapse of the leukemia. Cumulative incidences of chronic GVHD were estimated in patients without graft failure with death without chronic GVHD as competing risk.
Statistical methods
Patient, disease, and transplant characteristics were compared by using the χ2 test for categorical variables and the Kruskal-Wallis test for continuous variables. Probabilities of OS and RFS were calculated using the Kaplan-Meier estimate.14 Cumulative incidence curves were used to estimate NRM and relapse because NRM and relapse were competing events.15 Outcome estimates are at 2 years unless explicitly stated otherwise. Multivariable Cox regression analysis for OS, RFS, relapse, and NRM was applied with adjustment for covariates, which were selected based on a P value <.05 by univariate analysis. All analyses were done with Stata Statistical Software, release 13.1 (Stata Corporation; 2013, College Station, TX).
Results
Patient characteristics
A total of 6545 patients with poor-risk AML in CR1 were included. Patients received an alloHSCT from either an MRD (n = 3511), 10/10 MUD (n = 1959), 9/10 MUD (n = 549), UCB graft (n = 333), or haplo (n = 193). Patient characteristics are shown in Table 1, including the different poor-risk features of the AML, which were differentially distributed among the donor types. Recipients of UCB grafts were significantly younger compared with the other donor types (median, 48 vs 52 years; P < .001). Poor-risk cytogenetics and a FLT3-ITD were more frequently present in recipients of UCB grafts and haplo alloHSCT (P = .016 and P < .001). The median time from diagnosis to alloHSCT was 4.7 months, which was significantly shorter for recipients of an MRD compared with the alternative donors (median, 5.8 months; P < .001). In addition, alternative donor transplantation has been performed more frequently in recent years as compared with MRD alloHSCT (P < .001). The median follow-up of patients still alive differed between the patient groups, with a follow-up time of 43 months (range, 1-188) for recipients of MRD alloHSCT, whereas follow-up was shorter for 10/10 MUD (24 months; range, 1-159), 9/10 MUD (26 months; range, 1-139), UCB (24 months; range, 2-124), and haplo (22 months; range, 1-120).
Patient characteristics
| . | Donor source . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | MRD (N = 3511) . | MUD 10/10 (N = 1959) . | MUD 9/10 (N = 549) . | UCB (N = 333) . | Haplo (N = 193) . | |||||
| Sex | P = .019 | |||||||||
| Male | 1809 | 52% | 1034 | 53% | 275 | 50% | 146 | 44% | 110 | 57% |
| Female | 1701 | 48% | 925 | 47% | 273 | 50% | 187 | 56% | 83 | 43% |
| Age, y | P < .001 | |||||||||
| Median | 50 | 54 | 52 | 48 | 51 | |||||
| Range | 18-74 | 18-80 | 18-74 | 18-72 | 18-75 | |||||
| WBC at diagnosis | P = .009 | |||||||||
| >100 | 1716 | 49% | 897 | 46% | 264 | 48% | 177 | 53% | 69 | 36% |
| ≤100 | 435 | 12% | 176 | 9% | 47 | 9% | 36 | 11% | 8 | 4% |
| Missing | 1360 | 39% | 886 | 45% | 238 | 43% | 120 | 36% | 116 | 60% |
| Secondary AML | P < .001 | |||||||||
| No | 2207 | 63% | 1068 | 55% | 318 | 58% | 208 | 62% | 116 | 60% |
| Yes | 1304 | 37% | 891 | 45% | 231 | 42% | 125 | 38% | 77 | 40% |
| Poor-risk cytogenetics (−7/−5/complex/11q23) | P = .016 | |||||||||
| No | 2028 | 58% | 1109 | 57% | 300 | 55% | 163 | 49% | 101 | 52% |
| Yes | 1483 | 42% | 850 | 43% | 249 | 45% | 170 | 51% | 92 | 48% |
| FLT3-ITD | P < .001 | |||||||||
| No | 366 | 10% | 271 | 14% | 83 | 15% | 53 | 16% | 50 | 26% |
| Yes | 605 | 17% | 353 | 18% | 101 | 18% | 72 | 22% | 42 | 22% |
| Missing | 2540 | 72% | 1335 | 68% | 365 | 66% | 208 | 62% | 101 | 52% |
| CR reached after | P < .001 | |||||||||
| Cycle 1 (early CR) | 2536 | 72% | 1529 | 78% | 404 | 74% | 253 | 76% | 144 | 75% |
| Cycle 2 (late CR) | 975 | 28% | 430 | 22% | 145 | 26% | 80 | 24% | 49 | 25% |
| Time from diagnosis to PRT, mo | P < .001 | |||||||||
| Median | 4.7 | 5.6 | 6.1 | 5.9 | 6.2 | |||||
| IQR | 3.7-6.1 | 4.3-7.2 | 4.7-8.1 | 4.8-7.8 | 4.5-8.7 | |||||
| Year of PRT | P < .001 | |||||||||
| Median | 2008 | 2011 | 2011 | 2010 | 2012 | |||||
| Range | 2000-2014 | 2000-2014 | 2000-2014 | 2000-2014 | 2000-2014 | |||||
| . | Donor source . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | MRD (N = 3511) . | MUD 10/10 (N = 1959) . | MUD 9/10 (N = 549) . | UCB (N = 333) . | Haplo (N = 193) . | |||||
| Sex | P = .019 | |||||||||
| Male | 1809 | 52% | 1034 | 53% | 275 | 50% | 146 | 44% | 110 | 57% |
| Female | 1701 | 48% | 925 | 47% | 273 | 50% | 187 | 56% | 83 | 43% |
| Age, y | P < .001 | |||||||||
| Median | 50 | 54 | 52 | 48 | 51 | |||||
| Range | 18-74 | 18-80 | 18-74 | 18-72 | 18-75 | |||||
| WBC at diagnosis | P = .009 | |||||||||
| >100 | 1716 | 49% | 897 | 46% | 264 | 48% | 177 | 53% | 69 | 36% |
| ≤100 | 435 | 12% | 176 | 9% | 47 | 9% | 36 | 11% | 8 | 4% |
| Missing | 1360 | 39% | 886 | 45% | 238 | 43% | 120 | 36% | 116 | 60% |
| Secondary AML | P < .001 | |||||||||
| No | 2207 | 63% | 1068 | 55% | 318 | 58% | 208 | 62% | 116 | 60% |
| Yes | 1304 | 37% | 891 | 45% | 231 | 42% | 125 | 38% | 77 | 40% |
| Poor-risk cytogenetics (−7/−5/complex/11q23) | P = .016 | |||||||||
| No | 2028 | 58% | 1109 | 57% | 300 | 55% | 163 | 49% | 101 | 52% |
| Yes | 1483 | 42% | 850 | 43% | 249 | 45% | 170 | 51% | 92 | 48% |
| FLT3-ITD | P < .001 | |||||||||
| No | 366 | 10% | 271 | 14% | 83 | 15% | 53 | 16% | 50 | 26% |
| Yes | 605 | 17% | 353 | 18% | 101 | 18% | 72 | 22% | 42 | 22% |
| Missing | 2540 | 72% | 1335 | 68% | 365 | 66% | 208 | 62% | 101 | 52% |
| CR reached after | P < .001 | |||||||||
| Cycle 1 (early CR) | 2536 | 72% | 1529 | 78% | 404 | 74% | 253 | 76% | 144 | 75% |
| Cycle 2 (late CR) | 975 | 28% | 430 | 22% | 145 | 26% | 80 | 24% | 49 | 25% |
| Time from diagnosis to PRT, mo | P < .001 | |||||||||
| Median | 4.7 | 5.6 | 6.1 | 5.9 | 6.2 | |||||
| IQR | 3.7-6.1 | 4.3-7.2 | 4.7-8.1 | 4.8-7.8 | 4.5-8.7 | |||||
| Year of PRT | P < .001 | |||||||||
| Median | 2008 | 2011 | 2011 | 2010 | 2012 | |||||
| Range | 2000-2014 | 2000-2014 | 2000-2014 | 2000-2014 | 2000-2014 | |||||
IQR, interquartile range; PRT, postremission treatment.
Transplant characteristics
Myeloablative conditioning (MAC) was applied in a higher proportion in recipients of haplo (54%) and MRD (56%) compared with patients receiving MUD (46%) or UCB grafts (45%) (Table 2). The vast majority of MRD and MUD received peripheral blood stem cells, whereas 52% of the haplo recipients received bone marrow cells. Both family donors had a cytomegalovirus (CMV) donor match in more than two-thirds of the transplants, whereas a CMV mismatch was present in MUDs and UCB grafts in about 40% of the transplants. Conditioning with total body irradiation was performed mostly in recipients of UCB grafts (69%), particularly in patients who received a UCB graft following reduced intensity conditioning (RIC) (80%). In vivo T-cell depletion with either ATG or alemtuzumab was used in the majority of MUDs (75%). In the haplo group, 90 (47%) haplo recipients received posttransplant cyclophosphamide without ATG, whereas 62 (32%) patients received an ATG-based regimen as GVHD prophylaxis. Fourteen (7%) patients received both ATG and posttransplant cyclophosphamide following a haplo donor.
Transplant characteristics
| . | Donor source . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | MRD (N = 3511) . | MUD 10/10 (N = 1959) . | MUD 9/10 (N = 549) . | UCB (N = 333) . | Haplo (N = 193) . | |||||
| Conditioning | P < .001 | |||||||||
| MAC | 1951 | 56% | 888 | 45% | 256 | 47% | 145 | 44% | 104 | 54% |
| RIC | 1518 | 43% | 1056 | 54% | 291 | 53% | 183 | 55% | 89 | 46% |
| Missing | 42 | 1% | 15 | 1% | 2 | 0% | 5 | 2% | 0 | |
| Peripheral stem cells | P < .001 | |||||||||
| No | 712 | 20% | 315 | 16% | 76 | 14% | 333 | 100% | 100 | 52% |
| Yes | 2799 | 80% | 1644 | 84% | 473 | 86% | 0 | 93 | 48% | |
| CMV patient/donor | P < .001 | |||||||||
| neg/neg | 717 | 20% | 585 | 30% | 129 | 23% | 64 | 19% | 32 | 17% |
| pos/neg | 522 | 15% | 582 | 30% | 166 | 30% | 93 | 28% | 35 | 18% |
| neg/pos | 324 | 9% | 166 | 8% | 74 | 13% | 42 | 13% | 12 | 6% |
| pos/pos | 1587 | 45% | 582 | 30% | 166 | 30% | 78 | 23% | 111 | 58% |
| Missing | 361 | 10% | 44 | 2% | 14 | 3% | 56 | 17% | 3 | 2% |
| TBI given | P < .001 | |||||||||
| No | 2480 | 71% | 1422 | 73% | 424 | 77% | 130 | 39% | 141 | 73% |
| Yes | 1028 | 29% | 535 | 27% | 124 | 23% | 203 | 69% | 52 | 27% |
| Female donor to male recipient | P < .001 | |||||||||
| No | 2710 | 77% | 1739 | 89% | 472 | 86% | 273 | 82% | 147 | 76% |
| Yes | 801 | 23% | 220 | 11% | 77 | 14% | 60 | 18% | 46 | 24% |
| In vivo T-cell depletion | P < .001 | |||||||||
| No | 2354 | 67% | 535 | 27% | 73 | 13% | 198 | 59% | 111 | 58% |
| ATG | 707 | 20% | 1201 | 61% | 393 | 72% | 123 | 37% | 78 | 40% |
| Alemtuzumab | 237 | 7% | 216 | 11% | 80 | 15% | 0 | 3 | 2% | |
| Missing | 213 | 6% | 7 | 0% | 3 | 1% | 12 | 4% | 1 | 1% |
| . | Donor source . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | MRD (N = 3511) . | MUD 10/10 (N = 1959) . | MUD 9/10 (N = 549) . | UCB (N = 333) . | Haplo (N = 193) . | |||||
| Conditioning | P < .001 | |||||||||
| MAC | 1951 | 56% | 888 | 45% | 256 | 47% | 145 | 44% | 104 | 54% |
| RIC | 1518 | 43% | 1056 | 54% | 291 | 53% | 183 | 55% | 89 | 46% |
| Missing | 42 | 1% | 15 | 1% | 2 | 0% | 5 | 2% | 0 | |
| Peripheral stem cells | P < .001 | |||||||||
| No | 712 | 20% | 315 | 16% | 76 | 14% | 333 | 100% | 100 | 52% |
| Yes | 2799 | 80% | 1644 | 84% | 473 | 86% | 0 | 93 | 48% | |
| CMV patient/donor | P < .001 | |||||||||
| neg/neg | 717 | 20% | 585 | 30% | 129 | 23% | 64 | 19% | 32 | 17% |
| pos/neg | 522 | 15% | 582 | 30% | 166 | 30% | 93 | 28% | 35 | 18% |
| neg/pos | 324 | 9% | 166 | 8% | 74 | 13% | 42 | 13% | 12 | 6% |
| pos/pos | 1587 | 45% | 582 | 30% | 166 | 30% | 78 | 23% | 111 | 58% |
| Missing | 361 | 10% | 44 | 2% | 14 | 3% | 56 | 17% | 3 | 2% |
| TBI given | P < .001 | |||||||||
| No | 2480 | 71% | 1422 | 73% | 424 | 77% | 130 | 39% | 141 | 73% |
| Yes | 1028 | 29% | 535 | 27% | 124 | 23% | 203 | 69% | 52 | 27% |
| Female donor to male recipient | P < .001 | |||||||||
| No | 2710 | 77% | 1739 | 89% | 472 | 86% | 273 | 82% | 147 | 76% |
| Yes | 801 | 23% | 220 | 11% | 77 | 14% | 60 | 18% | 46 | 24% |
| In vivo T-cell depletion | P < .001 | |||||||||
| No | 2354 | 67% | 535 | 27% | 73 | 13% | 198 | 59% | 111 | 58% |
| ATG | 707 | 20% | 1201 | 61% | 393 | 72% | 123 | 37% | 78 | 40% |
| Alemtuzumab | 237 | 7% | 216 | 11% | 80 | 15% | 0 | 3 | 2% | |
| Missing | 213 | 6% | 7 | 0% | 3 | 1% | 12 | 4% | 1 | 1% |
TBI, total body irradiation.
Transplant outcome
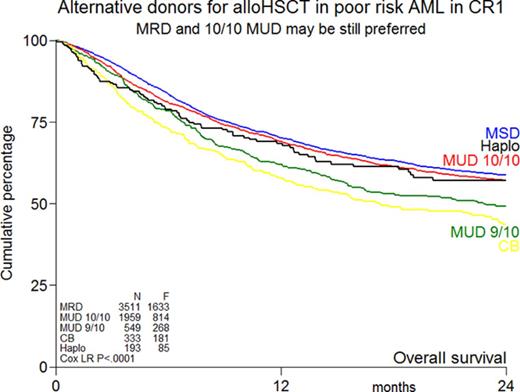
The rate of graft failure at 100 days after transplant was significantly higher in recipients of UCB grafts and haplo compared with MRD and 10/10 MUD alloHSCT (9% and 6% vs 2% and 1%, P < .001, respectively; Table 3). Maximum grade of acute GVHD was slightly increased in recipients of 9/10 MUDs and UCB grafts (P < .001; Table 3; supplemental Table 2). Limited chronic GVHD was less frequently present in recipients of UCB grafts, and the highest incidence of chronic extensive GVHD was observed in MRD recipients (Table 3). The cumulative incidence of chronic GVHD by MRD, 10/10 MUD, 9/10 MUD, UCB, and haplo at 2 years is an estimated 38 ± 1%, 36 ± 1%, 33 ± 2%, 24 ± 2%, and 37 ± 4%, respectively. With a median follow-up of 32 months, OS at 2 years was not significantly different between MRD alloHSCT, 10/10 MUD alloHSCT, and haplo alloHSCT (59 ± 1%, 57 ± 1%, and 57 ± 4%, respectively, P = .19; Figure 1A). However, OS was significantly lower in recipients of 9/10 MUD alloHSCT and UCB grafts compared with MRD alloHSCT (49 ± 2%, 44 ± 3%, and 59 ± 1%, respectively, P < .001; Figure 1A). These results were similar in subgroups of poor-risk cytogenetics and secondary AML (supplemental Figure 1). RFS was 53 ± 1%, 53 ± 1%, and 52 ± 4% at 2 years following MRD, 10/10 MUD, and haplo alloHSCT, respectively, which was significantly (P < .001) better than following UCB grafts (41 ± 3%) or 9/10 MUD alloHSCT (44 ± 2%) (Figure 1B). The cumulative incidence of relapse at 2 years is an estimated 22 ± 3% for haplo alloHSCT, whereas other types of donor transplantation were associated with a relapse incidence of about 30% (Figure 1C). NRM depended on donor type and is an estimated 26 ± 3% and 29 ± 3% after haplo and UCB alloHSCT at 2 years, respectively, vs 15 ± 1% following MRD alloHSCT (Figure 1D). Causes of death by donor type are shown in supplemental Table 3. Infections and GVHD were the most common causes of nonrelapse death, which were increased in the alternative donor transplants.
Transplant outcome
| . | Donor source . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | MRD (N = 3511 . | MUD 10/10 (N = 1959) . | MUD 9/10 (N = 549) . | UCB (N = 333) . | Haplo (N = 193) . | |||||
| Graft failure | P < .001 | |||||||||
| No. of patients | 53 | 2% | 29 | 1% | 20 | 4% | 30 | 9% | 11 | 6% |
| Time to engraftment, d | P < .001 | |||||||||
| Median | 16 | 17 | 17 | 23 | 18 | |||||
| IQ range | 13-20 | 14-20 | 14-20 | 17-30 | 15-23 | |||||
| Acute GVHD (maximum grade) | P < .001 | |||||||||
| Grade 0-1 | 2623 | 75% | 1369 | 70% | 374 | 68% | 220 | 66% | 140 | 73% |
| Grade 2-4 | 769 | 22% | 518 | 26% | 156 | 28% | 99 | 30% | 49 | 25% |
| Unknown | 119 | 3% | 72 | 4% | 19 | 3% | 14 | 4% | 4 | 2% |
| Time transplant to acute GVHD, d | P < .11 | |||||||||
| Median | 28 | 27 | 25 | 28 | 29 | |||||
| IQR | 18-47 | 17-43 | 16-42 | 19-38 | 18-48 | |||||
| Chronic GVHD | P < .001 | |||||||||
| Mild | 533 | 15% | 283 | 14% | 81 | 15% | 28 | 8% | 39 | 20% |
| Extensive | 712 | 20% | 310 | 16% | 73 | 13% | 37 | 11% | 18 | 9% |
| Time transplant to chronic GVHD, mo | P < .001 | |||||||||
| Median | 6.0 | 5.4 | 5.6 | 4.9 | 6.4 | |||||
| IQ range | 4.0-9.6 | 3.7-8.3 | 3.7-8.3 | 4.1-6.9 | 4.1-9.6 | |||||
| Outcome at 2 y | ||||||||||
| OS | 59 ± 1% | 57 ± 1% | 49 ± 2% | 44 ± 3% | 57 ± 4% | |||||
| RFS | 53 ± 1% | 53 ± 1% | 44 ± 2% | 41 ± 3% | 52 ± 4% | |||||
| Relapse | 32 ± 1% | 27 ± 1% | 31 ± 2% | 30 ± 3% | 22 ± 3% | |||||
| NRM | 15 ± 1% | 20 ± 1% | 24 ± 2% | 29 ± 3% | 26 ± 3% | |||||
| . | Donor source . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | MRD (N = 3511 . | MUD 10/10 (N = 1959) . | MUD 9/10 (N = 549) . | UCB (N = 333) . | Haplo (N = 193) . | |||||
| Graft failure | P < .001 | |||||||||
| No. of patients | 53 | 2% | 29 | 1% | 20 | 4% | 30 | 9% | 11 | 6% |
| Time to engraftment, d | P < .001 | |||||||||
| Median | 16 | 17 | 17 | 23 | 18 | |||||
| IQ range | 13-20 | 14-20 | 14-20 | 17-30 | 15-23 | |||||
| Acute GVHD (maximum grade) | P < .001 | |||||||||
| Grade 0-1 | 2623 | 75% | 1369 | 70% | 374 | 68% | 220 | 66% | 140 | 73% |
| Grade 2-4 | 769 | 22% | 518 | 26% | 156 | 28% | 99 | 30% | 49 | 25% |
| Unknown | 119 | 3% | 72 | 4% | 19 | 3% | 14 | 4% | 4 | 2% |
| Time transplant to acute GVHD, d | P < .11 | |||||||||
| Median | 28 | 27 | 25 | 28 | 29 | |||||
| IQR | 18-47 | 17-43 | 16-42 | 19-38 | 18-48 | |||||
| Chronic GVHD | P < .001 | |||||||||
| Mild | 533 | 15% | 283 | 14% | 81 | 15% | 28 | 8% | 39 | 20% |
| Extensive | 712 | 20% | 310 | 16% | 73 | 13% | 37 | 11% | 18 | 9% |
| Time transplant to chronic GVHD, mo | P < .001 | |||||||||
| Median | 6.0 | 5.4 | 5.6 | 4.9 | 6.4 | |||||
| IQ range | 4.0-9.6 | 3.7-8.3 | 3.7-8.3 | 4.1-6.9 | 4.1-9.6 | |||||
| Outcome at 2 y | ||||||||||
| OS | 59 ± 1% | 57 ± 1% | 49 ± 2% | 44 ± 3% | 57 ± 4% | |||||
| RFS | 53 ± 1% | 53 ± 1% | 44 ± 2% | 41 ± 3% | 52 ± 4% | |||||
| Relapse | 32 ± 1% | 27 ± 1% | 31 ± 2% | 30 ± 3% | 22 ± 3% | |||||
| NRM | 15 ± 1% | 20 ± 1% | 24 ± 2% | 29 ± 3% | 26 ± 3% | |||||
Outcome by different donor types. Kaplan-Meier estimates of OS (A), RFS (B), relapse (C), and NRM (D) by donor type of patients with poor-risk AML in CR1. F, number of failures (ie, death whatever the cause); Cox LR, Cox likelihood ratio.
Outcome by different donor types. Kaplan-Meier estimates of OS (A), RFS (B), relapse (C), and NRM (D) by donor type of patients with poor-risk AML in CR1. F, number of failures (ie, death whatever the cause); Cox LR, Cox likelihood ratio.
Multivariable analysis
The multivariable analysis is shown in Table 4 and was performed with adjustment for donor type, age, cytogenetics, secondary AML, time interval from diagnosis to transplant, year of transplant, in vivo T-cell depletion, and conditioning type. OS was not significantly different comparing alloHSCT following MRD with 10/10 MUD, and haplo alloHSCT (hazard ratio [HR], 0.99 and 1.12, respectively). OS following both 9/10 MUD and UCB grafts was significantly worse compared with MRD (HR, 1.23; P = .005; and HR, 1.54; P < .001, respectively). A similar pattern was found for RFS with nonsignificant differences for MRD, 10/10 MUD, and haplo alloHSCT, whereas both 9/10 MUD alloHSCT and UCB grafts were associated with worse RFS. Relapse was decreased for 10/10 MUD (HR, 0.74; P < .001) and haplo (HR, 0.60; P = .001) compared with MRD alloHSCT. NRM was significantly higher for all alternative donors compared with MRD alloHSCT. Older age was associated with increased risk for all outcome parameters. Both poor-risk cytogenetics and secondary AML had an increased HR for OS, RFS, and relapse, whereas a shorter time from diagnosis to transplant predicted for better OS, RFS, and relapse. A higher HR for relapse was found for RIC compared with MAC (HR, 1.23; P < .001), which was counterbalanced by a lower HR for NRM (0.78, P < .001), resulting in similar OS and RFS comparing RIC and MAC.
Multivariable analysis
| . | OS . | RFS . | Relapse . | NRM . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | HR* . | 95% CI . | P value . | HR* . | 95% CI . | P value . | HR* . | 95% CI . | P value . | HR* . | 95% CI . | P value . |
| Donor source | ||||||||||||
| MUD 10/10 vs MRD | 0.99 | 0.90-1.09 | .89 | 0.93 | 0.85-1.02 | .11 | 0.74 | 0.66-0.83 | <.001 | 1.39 | 1.19-1.62 | <.001 |
| MUD 9/10 vs MRD | 1.23 | 1.07-1.42 | .005 | 1.17 | 1.02-1.34 | .023 | 0.93 | 0.78-1.11 | .44 | 1.77 | 1.43-2.20 | <.001 |
| UCB vs MRD | 1.54 | 1.31-1.81 | <.001 | 1.37 | 1.17-1.60 | <.001 | 0.96 | 0.77-1.19 | .69 | 2.41 | 1.92-3.04 | <.001 |
| Haplo vs MRD | 1.12 | 0.89-1.40 | .34 | 1.00 | 0.81-1.25 | .97 | 0.60 | 0.44-0.84 | .001 | 1.98 | 1.47-2.68 | <.001 |
| Age† | 1.17 | 1.13-1.21 | <.001 | 1.13 | 1.09-1.16 | <.001 | 1.07 | 1.02-1.12 | .002 | 1.22 | 1.16-1.29 | <.001 |
| Poor-risk cytogenetics (yes vs no) | 1.31 | 1.20-1.44 | <.001 | 1.36 | 1.25-1.48 | <.001 | 1.66 | 1.49-1.85 | <.001 | 0.95 | 0.82-1.09 | .47 |
| Secondary AML (yes vs no) | 1.35 | 1.23-1.48 | <.001 | 1.33 | 1.22-1.46 | <.001 | 1.28 | 1.14-1.44 | <.001 | 1.40 | 1.21-1.61 | <.001 |
| Time from diagnosis to transplant‡ | 0.99 | 0.98-1.00 | .095 | 0.99 | 0.98-1.00 | .039 | 0.97 | 0.96-0.99 | .002 | 1.00 | 0.99-1.02 | .69 |
| Year of transplant§ | 0.96 | 0.85-1.09 | .50 | 0.95 | 0.84-1.06 | .36 | 1.03 | 0.89-1.19 | .68 | 0.80 | 0.66-0.98 | .029 |
| Conditioning (RIC vs MAC) | 0.98 | 0.90-1.06 | .59 | 1.03 | 0.95-1.12 | .42 | 1.23 | 1.11-1.37 | <.001 | 0.78 | 0.68-0.89 | <.001 |
| In vivo T-cell depletion (yes vs no) | 1.04 | 0.95-1.13 | .37 | 1.06 | 0.98-1.15 | .15 | 1.13 | 1.02-1.25 | .023 | 0.94 | 0.82-1.07 | .35 |
| . | OS . | RFS . | Relapse . | NRM . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | HR* . | 95% CI . | P value . | HR* . | 95% CI . | P value . | HR* . | 95% CI . | P value . | HR* . | 95% CI . | P value . |
| Donor source | ||||||||||||
| MUD 10/10 vs MRD | 0.99 | 0.90-1.09 | .89 | 0.93 | 0.85-1.02 | .11 | 0.74 | 0.66-0.83 | <.001 | 1.39 | 1.19-1.62 | <.001 |
| MUD 9/10 vs MRD | 1.23 | 1.07-1.42 | .005 | 1.17 | 1.02-1.34 | .023 | 0.93 | 0.78-1.11 | .44 | 1.77 | 1.43-2.20 | <.001 |
| UCB vs MRD | 1.54 | 1.31-1.81 | <.001 | 1.37 | 1.17-1.60 | <.001 | 0.96 | 0.77-1.19 | .69 | 2.41 | 1.92-3.04 | <.001 |
| Haplo vs MRD | 1.12 | 0.89-1.40 | .34 | 1.00 | 0.81-1.25 | .97 | 0.60 | 0.44-0.84 | .001 | 1.98 | 1.47-2.68 | <.001 |
| Age† | 1.17 | 1.13-1.21 | <.001 | 1.13 | 1.09-1.16 | <.001 | 1.07 | 1.02-1.12 | .002 | 1.22 | 1.16-1.29 | <.001 |
| Poor-risk cytogenetics (yes vs no) | 1.31 | 1.20-1.44 | <.001 | 1.36 | 1.25-1.48 | <.001 | 1.66 | 1.49-1.85 | <.001 | 0.95 | 0.82-1.09 | .47 |
| Secondary AML (yes vs no) | 1.35 | 1.23-1.48 | <.001 | 1.33 | 1.22-1.46 | <.001 | 1.28 | 1.14-1.44 | <.001 | 1.40 | 1.21-1.61 | <.001 |
| Time from diagnosis to transplant‡ | 0.99 | 0.98-1.00 | .095 | 0.99 | 0.98-1.00 | .039 | 0.97 | 0.96-0.99 | .002 | 1.00 | 0.99-1.02 | .69 |
| Year of transplant§ | 0.96 | 0.85-1.09 | .50 | 0.95 | 0.84-1.06 | .36 | 1.03 | 0.89-1.19 | .68 | 0.80 | 0.66-0.98 | .029 |
| Conditioning (RIC vs MAC) | 0.98 | 0.90-1.06 | .59 | 1.03 | 0.95-1.12 | .42 | 1.23 | 1.11-1.37 | <.001 | 0.78 | 0.68-0.89 | <.001 |
| In vivo T-cell depletion (yes vs no) | 1.04 | 0.95-1.13 | .37 | 1.06 | 0.98-1.15 | .15 | 1.13 | 1.02-1.25 | .023 | 0.94 | 0.82-1.07 | .35 |
CI, confidence interval; NRM, time as RFS with event death in first CR and censored at relapse; relapse, time as RFS and with event relapse and censored at death in first CR.
The HRs are the estimates of the effect of covariates for each outcome parameter, adjusted for donor, age, poor-risk cytogenetics, secondary AML, time from diagnosis to transplant, year of transplant, conditioning type, and in vivo T-cell depletion.
Linear with estimates of 10-y difference.
Linear with estimates of 1-mo difference.
Linear with estimates of 10-y difference.
A detailed analysis of the different alternative donors following either a RIC or MAC preparative regimen is presented in supplemental Table 4. Higher NRM associated with 9/10 MUD, UCB, and following haplo donors was observed after both RIC and MAC, but appeared most pronounced after MAC. OS again showed similar survival following MRD and 10/10 MUD.
Discussion
Postremission therapy by alloHSCT remains the treatment of choice in poor-risk AML patients upon achieving CR1 and qualifying for intensive therapy.1,2 Possible donor sources currently include MRDs or alternative donors such as MUDs with either a 10/10 or 9/10 HLA match, UCB grafts, or haplo. The present retrospective study from the EBMT Acute Leukemia Working Party demonstrates similar OS for patients with poor-risk AML in CR1 following alloHSCT with either MRD or 10/10 MUD. In contrast, recipients of 9/10 MUD and UCB grafts experienced worse outcome compared with MRD or 10/10 MUD, which was mainly the result of increased NRM. Recipients of T-cell replete haplo alloHSCT showed encouraging outcomes, which appeared not statistically different from MRD and 10/10 MUD, although a larger cohort and longer follow-up may be needed.
Historically, alloHSCT with an MRD has been the preferred type of donor for patients with hematological diseases. However, 70% of the patients lack a suitable MRD and the use of older MRD in elderly AML patients has recently been questioned.16,17 The present study confirms that an MRD should still be considered the preferred donor. AlloHSCT with a 10/10 MUD yielded similar survival in the present study, confirming 10/10 MUD as the preferred alternative if an MRD is not available. Several study groups have compared outcome of transplantation using either MRD or MUD in patients with AML and reported similar survival rates.18-22 Some studies reported slightly higher NRM following MUD, whereas counterbalancing lower relapse resulted in similar outcome compared with MRD, which was also found in the present study.
Inferior survival was found for recipients of MMUDs (9/10 HLA match) compared with MRD, which was primarily caused by increased incidence of (severe) GVHD and subsequent NRM. A recent meta-analysis of 7 retrospective studies comparing 10/10 MUD and 9/10 MUD alloHSCT showed a 27% increased risk of mortality for recipients of a 9/10 MUD.23 Here, a similarly increased risk (25%) was found when comparing 9/10 MUDs with 10/10 MUDs in patients with poor-risk AML in CR1. These results suggest that transplants using 9/10 MUD may be followed by more stringent prevention of GVHD to limit NRM. Studies addressing the value of intensified GVHD prophylaxis, such as being applied in a haplo alloHSCT setting, may possibly direct how to improve transplants with MMUDs.11,24
Following the initial favorable results in pediatric patients, alloHSCT with UCB grafts was also developed in adults with acute leukemia. Although a higher incidence of graft failure and delayed hematopoietic recovery are associated with UCB grafts, largely similar outcomes compared with MRD, MUD, or MMUD alloHSCT were reported.9,10,25,26 However, these studies included different groups of patients, which hampered a precise comparison. The present study in a homogenous group of patients shows that alloHSCT with UCB grafts is still associated with higher NRM compared with MRD, which resulted in significantly lower OS. The incidence of graft failure following alloHSCT with UCB grafts in our study is an estimated 9% and the majority of causes of death were infections, to which graft failure and delayed recovery contributed. No significant differences in outcome were found between single vs double UCB grafts, and no difference between UCB with low vs high total nucleated cells at infusion of the UCB grafts were found, although information was not available for all UCB grafts (data not shown). These results suggest that improving hematopoietic engraftment and hematopoietic recovery remains a major challenge in UCB graft alloHSCT in adult patients, which is currently addressed and studied by several groups exploring expansion of UCB hematopoietic stem cells.27,28
Allogeneic transplantation with a haplo-identical family donor was extensively studied by the Perugia group.29 Although that approach consisting of transplantation with high numbers of CD34+ cells, intensified conditioning and stringent GVHD prophylaxis appeared to result in favorable engraftment in the majority of patients, a relatively high NRM precluded application on a broader scale. More recently, both the approach by the Baltimore group based on posttransplant cyclophosphamide and the Chinese approach based on in vivo T-cell depletion were demonstrated to result in favorable engraftment, limited GVHD, and limited NRM.30,31 A recent biologically randomized study from China suggested similar outcomes using matched related or haplo-identical family donors.30 Updated results from the Baltimore group also suggested similar survival following haplo alloHSCT and matched donor alloHSCT.31 A recent retrospective study from the Acute Leukemia Working Party of the EBMT showed a similar relapse incidence in patients receiving T cell–replete and T cell–depleted haplo alloHSCT compared with MRD, which suggested a similar graft-versus-leukemia effect.32 The present study included T cell–replete haplo alloHSCT, which appeared associated with a stronger graft-versus-leukemia effect compared with MRD (HR, 0.60), whereas severe grades of acute and chronic GVHD were relatively low compared with the other donor types. A large retrospective study of the Center for International Blood and Marrow Transplant Research comparing MRD with haplo alloHSCT using posttransplant cyclophosphamide also found less GVHD following haplo alloHSCT and an overall similar outcome.33 Our study showed a relatively low incidence of relapse following haplo alloHSCT, but a higher incidence of NRM, resulting in a similar outcome compared with MRD alloHSCT. Both the relatively short follow-up and unknown patient selection preclude more definite conclusions as regards the comparison with sibling donors. However, haplo alloHSCT was also suggested to result in better outcome compared with UCB alloHSCT. In a less homogenous group of AML patients, UCB and haplo alloHSCT were previously suggested to result in similar overall outcome.34 The latter results are in line with the 2 parallel phase 2 trials of Brunstein et al, which addressed UCB grafts and unmanipulated haplo alloHSCT including posttransplant cyclophosphamide. Although haplo was associated with less NRM, a higher relapse rate counterbalanced that favorable effect, resulting in similar RFS. Currently, a prospective randomized phase 3 trial is being conducted comparing UCB and haplo alloHSCT (BMT CTN 1101), which will address the question how UCB and haplo alloHSCT compare in patients with hematological malignancies.
Our study may have several limitations. First, a center’s preference regarding preferred alternative donors may result in selection bias.35 Reasons for the choice of an alternative donor transplant are not registered in the EBMT database and therefore not known. We focused on a homogenous group of poor-risk AML patients with no important differences in baseline characteristics, but time intervals from diagnosis to transplant did differ, which may be associated with selection resulting from exclusion of early relapses in the group of alloHSCT recipients with the longest timeframe. Second, an increasingly important parameter is the presence or absence of minimal residual disease, which was unknown in the present study, but has recently been shown to strongly predict for subsequent relapse and overall outcome.36-39 Although not recorded, residual disease is not routinely assessed in most centers and also not uniformly used for risk-adapted treatment; as a result, it is unlikely to have resulted in a strong selection bias. Last, the retrospective multicenter nature of our study implies that the physician/centers intention and/or preference is not taken into account, which can only be addressed in a prospective randomized study. However, prospective studies with more than 2 or 3 donor types will be extremely difficult, necessitating larger registry studies. To our knowledge, our study is the largest comparative study of MRD and alternative donors in the homogenous subgroup of patients with poor-risk ALM in CR1 in urgent need of an alloHSCT. Our results compare well with a recent study by Raiola et al, who performed a single-center retrospective study of 459 patients that received alloHSCT using different donors, including unmanipulated haplo, MRD, MUD, or UCB grafts. Although the recipients suffered from various hematological malignancies and haplo has been performed more frequently in recent years, their results also suggested higher NRM in MMUD alloHSCT and following alloHSCT with UCB grafts.40
In conclusion, our study suggests that well-matched donors including MRD and 10/10 MUD are preferred over UCB and MMUD patients with poor-risk AML in CR1. Nine of 10 MUD, UCB grafts, and haplo-identical donors could be used as alternatives in case a fully matched donor is not available or an urgent transplant is required. Haplo-identical donors are increasingly used, and results are encouraging. However, comparative prospective studies of haplo alloHSCT with other donor types are warranted and longer follow-up after haplo alloHSCT may be needed to definitely establish its place in the hierarchy of alternative donors.
The full-text version of this article contains a data supplement.
Acknowledgments
Following European Society for Blood and Marrow Transplantation (EBMT) publication rules, coauthorship was offered to centers contributing the highest number of patients. Nevertheless, the authors highly appreciate the contribution by many physicians and data managers throughout the EBMT, who made this analysis possible.
Authorship
Contribution: J.V., M.L., J.J.C., and A.N. contributed to the study design; all authors provided study materials or patients; all authors were involved in collection and assembly of clinical data; J.V., M.L., J.J.C., and A.N. were involved in analyzing and interpreting the data and writing this report; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation appears in the online appendix.
Correspondence: Jan J. Cornelissen, Department of Hematology, Erasmus University Medical Center Cancer Institute, Groene Hilledijk 301, 3075 EA, Rotterdam, The Netherlands; e-mail: j.cornelissen@erasmusmc.nl.
References
Author notes
J.J.C. and A.N. contributed equally to this study.
Competing Interests
Presented by J.V. as an oral presentation at the 56th annual meeting of the American Society of Hematology, San Francisco, CA, 6-9 December 2014, and at the 42nd annual meeting of the European Society for Blood and Marrow Transplantation, Valencia, Spain, 3-6 April 2016.