TO THE EDITOR:
Despite recent therapeutic advances, the outcomes of patients with relapsed/refractory acute lymphoblastic leukemia (ALL) remain poor, and novel therapies are needed.1,2 Bcl-2 and Bcl-xL are highly expressed in ALL, and pharmacologic inhibition of these proteins has antileukemic activity in preclinical studies.3-9 Venetoclax-based regimens have been studied in ALL in small prospective trials, with promising results.10-12 Because upregulation of Bcl-xL is an established resistance mechanism to venetoclax, we hypothesized that adding the dual Bcl-2/Bcl-xL inhibitor navitoclax to this regimen may further improve outcomes.
Adult patients with relapsed/refractory Philadelphia chromosome–negative ALL who had not previously received venetoclax were eligible for this phase 1/2 study of mini-hyper-cyclophosphamide, vincristine, and dexamethasone alternating with methotrexate and cytarabine (CVD) in combination with venetoclax and navitoclax. The study protocol, including the complete inclusion/exclusion criteria, are detailed in the supplemental Protocol. Patients received the mini-hyper-CVD regimen at doses that have been previous reported.13 In cycle 1, patients received venetoclax with ramp-up to a maximum dose of 200 to 400 mg on days 1 to 21. For cycles 2 to 8, patients received venetoclax at the assigned dose on days 1 to 7. Navitoclax 50 mg daily was given on days 1 to 21 of cycle 1 and on days 1 to 7 of cycles 2 to 8. This dose was selected based on previously published data.14 A bone marrow assessment was performed on cycle 1, day 14, and venetoclax and navitoclax were held for patients who achieved a marrow remission. Cycles were administered every 28 days. Patients with T-cell ALL also received additional cycles of nelarabine and PEGylated (PEG)-asparaginase, given in cycles 4N and 5N (intercalated after cycles 4 and 5, respectively), as previously reported.10 The full treatment schema is detailed in the protocol in the supplemental Protocol. This study was registered at ClinicalTrials.gov as NCT03808610.
The phase 1 portion of the study was conducted using a standard “3+3” design and evaluated venetoclax at a dose of 200 mg (dose level 0) and 400 mg (dose level +1). This was followed by phase 2 using the recommended phase 2 dose of venetoclax. The primary objective of the phase 1 portion of the study was to define the maximum tolerated dose of venetoclax in the combination regimen. The primary objective of the phase 2 portion of the study was to determine the combined rate of complete remission (CR) and CR with incomplete hematologic recovery (CRi).
Between October 2021 and November 2023, a total of 22 patients with relapsed/refractory ALL were treated (Table 1). Twelve patients (55%) had B-cell ALL, and 10 patients (45%) had T-cell ALL, 3 of whom had early T-cell precursor (ETP) ALL. Eleven patients (50%) were in salvage 3 or beyond (B cell, n = 8; T cell, n = 3). Among the 12 patients with B-cell ALL, all had received prior blinatumomab, 9 (75%) had received prior inotuzumab ozogamicin (INO), and 10 (83%) had received CD19-directed chimeric antigen receptor T cells. Among the 10 patients with T-cell ALL, 5 (50%) had received prior nelarabine.
Baseline characteristics of the study population
| Characteristic . | N (%)/median (range) . |
|---|---|
| Age, y | |
| Median age | 36 (18-70) |
| ≥ 60 | 2 (9) |
| ALL subtype | |
| T-cell ALL | 10 (45) |
| B-cell ALL | 12 (55) |
| Ph-like | 6/12 (50) |
| ECOG performance status | |
| 0 | 4 (18) |
| 1 | 15 (68) |
| 2 | 3 (14) |
| Isolated extramedullary disease | 3 (14) |
| Baseline blood parameters | |
| White blood cells, ×109/L | 6.9 (0.1-143.3) |
| Hemoglobin, g/dL | 10.4 (7.1-13.9) |
| Platelets, ×109/L | 35 (6-205) |
| Bone marrow blasts, % | 83 (0-97) |
| Cytogenetics | |
| Diploid | 8 (36) |
| Complex | 5 (23) |
| Miscellaneous | 7 (32) |
| Insufficient metaphases/not done | 2 (9) |
| Prior venetoclax | 1 (5) |
| Prior alloSCT | 6 (27) |
| Prior therapies (B-cell ALL) | |
| Median prior lines of therapy | 3 (1-6) |
| Prior INO | 9/12 (75) |
| Prior Blina | 12/12 (100) |
| Prior INO and Blina | 9/12 (75) |
| Prior CD19 CAR T cells | 9/12 (75) |
| Prior therapies (T-cell ALL) | |
| Median prior lines of therapies | 2 (1-4) |
| Prior nelarabine | 5/10 (50) |
| Characteristic . | N (%)/median (range) . |
|---|---|
| Age, y | |
| Median age | 36 (18-70) |
| ≥ 60 | 2 (9) |
| ALL subtype | |
| T-cell ALL | 10 (45) |
| B-cell ALL | 12 (55) |
| Ph-like | 6/12 (50) |
| ECOG performance status | |
| 0 | 4 (18) |
| 1 | 15 (68) |
| 2 | 3 (14) |
| Isolated extramedullary disease | 3 (14) |
| Baseline blood parameters | |
| White blood cells, ×109/L | 6.9 (0.1-143.3) |
| Hemoglobin, g/dL | 10.4 (7.1-13.9) |
| Platelets, ×109/L | 35 (6-205) |
| Bone marrow blasts, % | 83 (0-97) |
| Cytogenetics | |
| Diploid | 8 (36) |
| Complex | 5 (23) |
| Miscellaneous | 7 (32) |
| Insufficient metaphases/not done | 2 (9) |
| Prior venetoclax | 1 (5) |
| Prior alloSCT | 6 (27) |
| Prior therapies (B-cell ALL) | |
| Median prior lines of therapy | 3 (1-6) |
| Prior INO | 9/12 (75) |
| Prior Blina | 12/12 (100) |
| Prior INO and Blina | 9/12 (75) |
| Prior CD19 CAR T cells | 9/12 (75) |
| Prior therapies (T-cell ALL) | |
| Median prior lines of therapies | 2 (1-4) |
| Prior nelarabine | 5/10 (50) |
alloSCT, allogeneic stem cell transplantation; Blina, blinatumomab; CAR T-cells, chimeric antigen receptor T cells; ECOG, Eastern Cooperative Oncology Group; Ph-like, Philadelphia-like.
Ten patients were enrolled into the phase 1 portion of the study (4 patients with venetoclax 200 mg and 6 patients with venetoclax 400 mg). Seven patients had T-cell ALL, and 3 had B-cell ALL. No dose-limiting toxicities were observed, and venetoclax at a dose of 400 mg was chosen as the recommended phase 2 dose for further study in this combination with mini-hyper-CVD and navitoclax.
The composite CR/CRi rate was 50%. Ten patients (45%) achieved CR, and 1 patient (5%) achieved CRi. Five of the 11 responding patients (45%) achieved measurable residual disease negativity by multiparameter flow cytometry, with sensitivity of 0.01%. The CR/CRi rate in patients with B-cell ALL was 33% (4/12) and for T-cell ALL was 70% (7/10; P = .09), and the rates of multiparameter flow cytometry measurable residual disease–negative remission rates were 8% (1/12) and 40% (4/10), respectively (P = .07). All 3 patients with ETP ALL achieved CR. The CR/CRi rate was 50% for both patients treated in first salvage (4/8) and those in second or later salvage (7/14). Among patients with 1 to 2 prior therapies, the CR/CRi rates for B-cell and T-cell ALL were 25% (1/4) and 71% (5/7), respectively (P = .14). The median number of cycles of the mini-hyper-CVD, venetoclax, and navitoclax regimen was 2 (range, 1-8). Among 10 patients with T-cell ALL, 2 patients received at least 1 cycle of nelarabine plus PEG-asparaginase.
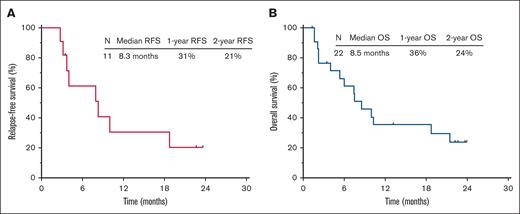
The median follow-up is 17.9 months (range, 1.3-22.6). Patient disposition is shown in supplemental Figure 1. Among the 11 responders, 5 patients (23% overall and 45% of responders) proceeded to allogeneic stem cell transplant. The median relapse-free survival was 8.3 months, and the 1-year relapse-free survival rate was 31% (Figure 1A). The median overall survival (OS) was 8.5 months, and the 1-year OS rate was 36% (Figure 1B). Outcomes were superior in patients treated in first salvage (median OS, 17.1 months; 1-year OS rate, 50%) vs those treated in second salvage or beyond (median OS, 7.5 months; 1-year OS rate, 26%; P = .08; supplemental Figure 2). The OS for patients with B-cell and T-cell ALL were similar (median OS, 7.5 months vs 10.0 months; P = .83; supplemental Figure 3).
Outcomes of the study cohort. (A-B) Relapse-free survival (A) and OS (B). RFS, relapse-free survival.
Outcomes of the study cohort. (A-B) Relapse-free survival (A) and OS (B). RFS, relapse-free survival.
Nonhematologic adverse events are shown in supplemental Table 1. The most common grade ≥3 nonhematologic adverse events were infection in 13 patients (59%) and febrile neutropenia in 5 patients (23%). Five patients (23%) had a bleeding event of any grade. There were 3 on-study deaths (2 due to infection in the setting of refractory leukemia and 1 due to unknown causes). The 30- and 60-day mortality rates were 0% and 9%, respectively. Among responding patients, the median time in cycle 1 to absolute neutrophil count >1 × 109/L was 25 days (range, 0-45) and to platelet count >100 × 109/L was 26 days (range, 11-41). In cycle 2, the time to neutrophil and platelet recovery was 18 days (range, 14-28) and 24 days (range, 18-28), respectively. Ten patients (45%) had a dose reduction or interruption of venetoclax or navitoclax (supplemental Figure 4). No patient discontinued venetoclax due to related adverse events. One patient discontinued navitoclax in cycle 4 due to persistent thrombocytopenia.
In this prospective study of mini-hyper-CVD, venetoclax, and navitoclax, the overall CR/CRi rate was 50%, with the highest response rate in patients with T-cell ALL (70%). We observed particularly encouraging responses in ETP ALL (3/3 responders), a high-risk T-cell ALL subtype.15 Given the high response rate and limited other therapeutic options for T-cell ALL, this regimen could be an effective bridge to allogeneic stem cell transplant in this population. However, given discontinuation of the clinical development of navitoclax, this strategy would need to be investigated using alternative Bcl-xL inhibitors. These subgroup analyses should also be interpreted with caution given the small sample size, although it is notable that the high response rate in T-cell ALL is consistent with preclinical data suggesting that T-cell ALL (particularly ETP ALL) may be particularly susceptible to Bcl-2 and/or Bcl-xL inhibition.7
Several other retrospective and prospective studies have suggested benefit of Bcl-2 or Bcl-xL inhibition in ALL.10,14,16,17 In a phase 1 study of venetoclax, navitoclax, and minimal chemotherapy (PEG-asparaginase, vincristine, and corticosteroids) in children and adults with relapsed/refractory ALL, the CR/CRi rate was 60%, and the median OS was 7.8 months.12 However, only a minority of patients in this study had received novel immunotherapies such as blinatumomab or INO. In contrast, in our study, 75% of patients with B-cell ALL had received both blinatumomab and INO, and 50% of patients with T-cell ALL had received nelarabine, representing a very heavily pretreated population with few therapeutic options.18 Similarly, we previously published results from a similar clinical trial of mini-hyper-CVD plus venetoclax (without navitoclax) in patients with relapsed/refractory ALL.10 The CR/CRi rates and median OS in these 2 studies appear similar, and therefore, the added benefit of Bcl-xL inhibition with navitoclax remains uncertain. However, it is important to note that the population in this study was more heavily pretreated, which confounds our ability to compare results from these 2 studies.
In conclusion, the combination of low-intensity chemotherapy (mini-hyper-CVD), venetoclax, and navitoclax was effective in heavily treated relapsed/refractory ALL. Future studies evaluating combination strategies with Bcl-2 and/or Bcl-xL inhibitors are warranted, particularly for T-cell ALL, in which the benefit of this approach may be greatest.
This was part of an institutional review board–approved clinical trial.
Acknowledgments: This research is supported in part by MD Anderson Cancer Center Leukemia SPORE grant CA100632 and National Institutes of Health/National Cancer Institute Cancer Center support grant P30 CA016672. AbbVie provided funding for the clinical trial as well as free venetoclax and navitoclax.
Contribution: N.J.S. and E.J. designed the study, enrolled patients, collected and analyzed data, and wrote the manuscript; H.K., N.J., T.M.K., J.S., F.G.H., K.S., R.A., M.Z., M.K., and F.R. enrolled patients; O.K., M.M.M., and R.G. collected and analyzed data and performed statistical analyses; L.X. and X.H. performed statistical analyses; and all authors reviewed and edited the manuscript and approved of the final version.
Conflict-of-interest disclosure: N.J.S. reports consulting fees from Amgen, Pfizer Inc, GlaxoSmithKline, Adaptive Biotechnologies, Autolus, and Sanofi; research funding from Takeda Oncology, Astellas Pharma Inc, Xencor, GlaxoSmithKline, NextCure, Ascentage, Novartis, Hemogenyx, and Vironexis; and honoraria from Adaptive Biotechnologies, Novartis, Amgen, Takeda Oncology, Pfizer Inc, Astellas Pharma Inc, and Sanofi. M.K. is a member of the advisory boards of AbbVie, Auxenion, Dark Blue Therapeutics, Legend, MEI Pharma, and Menarini/Stemline Therapeutics; reports consulting fees from AbbVie, Adaptive, Curis, Intellisphere, Janssen, Menarini/Stemline Therapeutics, Novartis, Sanofi Aventis, Servier, Syndax, and Vincerx; and research funding from AbbVie, Janssen, and Klondike Biopharma. E.J. reports research funding and honoraria from AbbVie. The remaining authors declare no competing financial interests.
Correspondence: Nicholas J. Short, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; email: nshort@mdanderson.org; and Elias Jabbour, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; email: ejabbour@mdanderson.org.
References
Author notes
The data sets used and/or analyzed during the current study are available on reasonable request from the corresponding authors, Nicholas J. Short (nshort@mdanderson.org) and Elias Jabbour (ejabbour@mdanderson.org).
The full-text version of this article contains a data supplement.