Key Points
Pembrolizumab treatment of melanoma and concomitant sAML resulted in a significant platelet response and clearance of IDH1 mutation.
Pembrolizumab therapy and response was associated with an increased PD-L1 expression on acute myeloid leukemia blasts and T cells.
Introduction
Cancer cells use the interaction between immune-checkpoint receptor programmed cell death protein 1 (PD-1) on activated T cells and programmed cell death ligand 1 (PD-L1) on tumor cells and various cell types of the tumor microenvironment to evade immune surveillance.1,2 Blocking the interaction between PD-1 and PD-L1 with checkpoint inhibitors can improve T-cell reactions and mediate efficient antitumor activity in a variety of solid tumors including melanoma.3,4 However, clinical data from patients with myeloid diseases that are treated with these agents are limited to clinical trials in advanced disease.5 Pembrolizumab (PEM) is a humanized monoclonal antibody of the immunoglobulin G4/κ isotype designed to block PD-1/PD-L1 interactions and is approved for various solid tumors including advanced melanoma.6
Case description
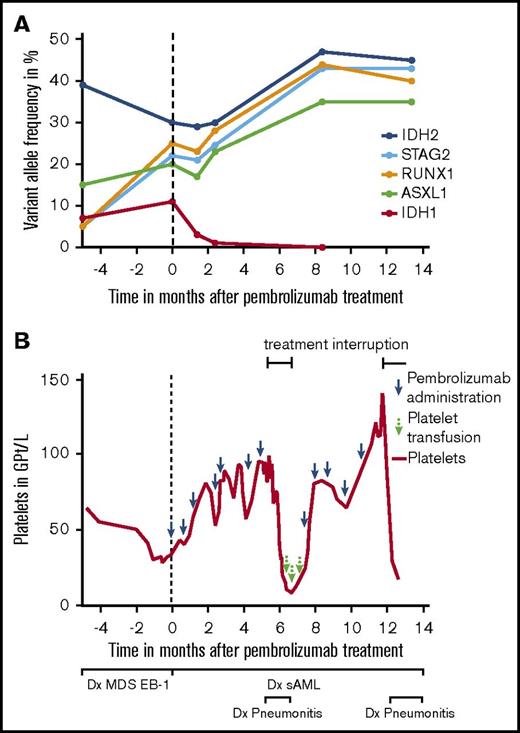
Here, we describe a patient with advanced mucosal melanoma and concomitant acute myeloid leukemia (AML), who was treated with single-agent PEM as first-line therapy. The 80-year-old patient presented initially with myelodysplastic syndrome (MDS) including transfusion-dependent anemia, neutropenia, and thrombocytopenia. MDS was type EB-1 (World Health Organization 2016) and displayed normal cytogenetics. Next-generation sequencing detected IDH1, IDH2, ASXL1, RUNX1, and STAG2 mutations (Figure 1A). Less than 3 months later, the patient was diagnosed with PD-L1-negative anorectal melanoma with satellite metastases. Because of progressive thrombocytopenia and neutropenia, an abdominoperineal resection was not performed. Positron emission tomography–based imaging verified no evidence of further metastases, and a subsequent bone marrow (BM) biopsy showed 55% blasts in line with a concomitant progression of MDS to secondary AML (FAB M1) and aggravation of thrombocytopenia (Figure 1B). Furthermore, IDH1, IDH2, ASXL1, RUNX1, and STAG2 mutations were still present (Figure 1A). The patient accepted the recommended application of PEM, but he declined locoregional radiotherapy. According to the patient’s preference, secondary AML was considered to be treated with supportive care only.
Variant allele frequency of mutations and course of platelet count prior to and during PEM therapy. (A) Variant allele frequency of single-nucleotide variants detected in IDH1, IDH2, ASXL1, RUNX1, and STAG2 in the patient before and during treatment with PEM. Analyses were done using published methods (panel next-generation sequencing analysis for 54 mutations; Trusight Myeloid Panel) including targeted ultradeep analysis for IDH1 and IDH2 with a sensitivity down to 0.1%.19 (B) After diagnosis of MDS EB-1, the patient presented with persistent thrombocytopenia. Two months after PEM treatment initiation, platelet counts increased from 34 Gpt/L to 81 Gpt/L. The course was complicated by a PEM-associated pneumonitis at ∼5 months after the start of therapy. During this period, PEM was stopped for 8 weeks, and the patient lost his platelet response subsequently. Response was regained after reintroduction of therapy. sAML, secondary AML.
Variant allele frequency of mutations and course of platelet count prior to and during PEM therapy. (A) Variant allele frequency of single-nucleotide variants detected in IDH1, IDH2, ASXL1, RUNX1, and STAG2 in the patient before and during treatment with PEM. Analyses were done using published methods (panel next-generation sequencing analysis for 54 mutations; Trusight Myeloid Panel) including targeted ultradeep analysis for IDH1 and IDH2 with a sensitivity down to 0.1%.19 (B) After diagnosis of MDS EB-1, the patient presented with persistent thrombocytopenia. Two months after PEM treatment initiation, platelet counts increased from 34 Gpt/L to 81 Gpt/L. The course was complicated by a PEM-associated pneumonitis at ∼5 months after the start of therapy. During this period, PEM was stopped for 8 weeks, and the patient lost his platelet response subsequently. Response was regained after reintroduction of therapy. sAML, secondary AML.
Methods
Clinical and molecular response assessment
The patient was seen in the outpatient department for blood checks every week while BM diagnostics including metaphase cytogenetics and molecular analysis were carried out every 2 to 3 months according to standard methods.7,8 PEM was administered according to guidelines for patients with unresectable melanoma at a dose of 2 mg/kg IV over 30 minutes every 3 weeks.
Immune monitoring
Immune profiling was performed in the blood (every 4 weeks) and BM (every 2-3 months) of the patient during therapy. Peripheral blood (PB) and BM were prepared by Ficoll-Hypaque density centrifugation and used for immunofluorescence staining. The phenotype of T cells and AML cells, as well as the frequency of regulatory T cells and myeloid-derived suppressor cells, was determined by using fluorochrome-labeled antibodies (supplemental Table 1) and analyzed by flow cytometry. Furthermore, the presence of T cells was explored in BM (immunohistochemistry, cytomorphology) and melanoma (immunohistochemistry).
Results and discussion
After 2 months of single-agent PEM treatment, platelet count increased from 34 Gpt/L to 81 Gpt/L in line with a response according to International Working Group criteria9 (Figure 1B). During the course of PEM treatment, neutropenia persisted ranging from 0.68 to 0.96 Gpt/L, and blasts in the BM remained between 60% and 80%. Because of chronic anemia, the patient required red blood cell (RBC) transfusions every 4 weeks during the first months of therapy. The clinical course was complicated by a PEM-induced pneumonitis at ∼5 months after start of therapy, necessitating corticosteroid treatment and an 8-week rest period of PEM. During drug holiday, the patient lost his platelet response, and RBC transfusion frequency increased up to every 2 weeks. After restarting therapy, platelet response was regained (Figure 1B), and RBC transfusion frequency could be extended again to every 4 weeks. Regarding the melanoma, the patient achieved stable disease throughout the observation period.
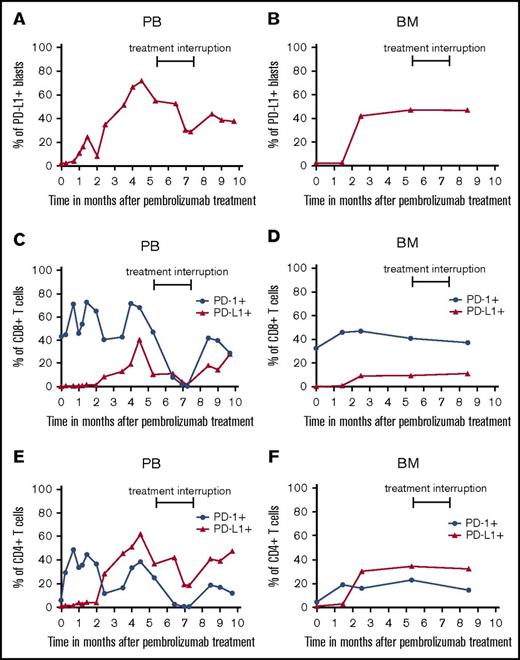
When investigating the PD-L1 expression or mutational load of tumor tissues and the frequency and phenotype of immune cells in tumor patients prior to PD-1 inhibitor therapy, several potential biomarkers have been described.10 Such studies help to select treatment responders, to discover mechanisms underlying anti-PD-1 therapy, and to identify modes of treatment resistance. Thus, it has been shown that PD-L1 expression in tumor tissues is associated with clinical responses in anti-PD-1 antibody-treated patients.11 However, other clinical trials yielded contradictory results.11 Here, we found that PD-L1 is not detectable on AML blasts in PB and BM (Figure 2A-B; supplemental Figure 1) before PEM treatment. However, a significant proportion of AML blasts increased PD-L1 expression thereafter, which was accompanied by a significant improvement of platelet count. PD-L1 expression on blasts in PB reached a maximum of 72%, which was reversible during treatment interruption. After restarting therapy, the percentage of PD-L1 expression on AML blasts in PB increased again and remained stably high with continuous therapy. This pattern is a hint for the direct linkage between PEM treatment and upregulation of PD-L1 expression on target cells induced by effective immunotherapy.12
Expression of PD-L1 and PD-1 on AML blasts and T cells prior to and during PEM therapy. PB- and BM-derived AML blasts (A-B) and T cells (C-F) were stained with fluorochrome-conjugated antibodies and analyzed by flow cytometry prior to and at various time points during PEM treatment. Blasts were defined as CD45low cells and stained for PD-L1 expression. Furthermore, T cells were characterized as CD45+, CD3+, and CD4+ or CD8+ populations. CD8+ T cells (C-D) and CD4+ T cells (E-F) were analyzed for their expression of PD-1 and PD-L1. Values in the graphs represent the percentage of cells staining positive for each molecule at indicated different time points.
Expression of PD-L1 and PD-1 on AML blasts and T cells prior to and during PEM therapy. PB- and BM-derived AML blasts (A-B) and T cells (C-F) were stained with fluorochrome-conjugated antibodies and analyzed by flow cytometry prior to and at various time points during PEM treatment. Blasts were defined as CD45low cells and stained for PD-L1 expression. Furthermore, T cells were characterized as CD45+, CD3+, and CD4+ or CD8+ populations. CD8+ T cells (C-D) and CD4+ T cells (E-F) were analyzed for their expression of PD-1 and PD-L1. Values in the graphs represent the percentage of cells staining positive for each molecule at indicated different time points.
So far, only limited clinical data are available on the pattern of PD-L1 or PD-1 expression on T cells in patients with myeloid disease.13 We found that PD-L1 and PD-1 expression also increased on PB- and BM-derived T cells during PEM therapy (Figure 2C-F; supplemental Figure 1). The direct relationship of these findings to PEM therapy is supported by the observation that T cells also showed a marked reduction of PD-L1 and PD-1 expression in PB during treatment interruption. The percentage of PD-L1- or PD-1-expressing CD4+ and CD8+ T cells in PB increased again after restarting PEM treatment, indicating that these effects are a direct result of PD-1 blockade. When exploring the frequency of regulatory T cells and myeloid-derived suppressor cells in BM and PB before and during PEM therapy, we did not observe marked changes (supplemental Figure 2A-D).
A recent study revealed that preexisting intratumoral CD8+ T cells may predict clinical response to anti-PD-1 therapy.14 In our case, frequency of BM-infiltrating T cells was within the normal range (7% of BM nucleated cells)15 (supplemental Figure 3A). Furthermore, only a few scattered T cells were detectable in melanoma tissues prior to therapy (supplemental Figure 3B).
Moreover, a higher mutation burden resulting in a higher number of tumor-derived neoantigens has been linked to better response to immune therapy.16 The number of mutations in our patient is in line with previous data, indicating that AML secondary to MDS displays a median of 4 mutations compared with 2 in de novo cases.17 During the 14 months of PEM treatment, AML staging showed no signs of progressive disease including stable blast cell number in the BM. Notably, preexisting IDH1 mutation completely disappeared, whereas IDH2, ASXL1, RUNX1, and STAG2 mutations persisted (Figure 1A).
Median survival of elderly AML patients is <1 year with first-line hypomethylating agents.18 To our knowledge, this is the first case report describing the clinical course of a patient with AML during single-agent first-line therapy with PEM. PD-1 inhibition was accompanied by a significant platelet response and upregulation of PD-1 and PD-L1, whereas IDH1 mutation completely disappeared. Our findings, therefore, indicate that single-agent therapy with PEM might display disease-modifying activity in a subset of AML patients.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Cathleen Rüger for technical assistance and the patient and his family for their cooperation.
Authorship
Contribution: A.S.K. wrote the manuscript; R.W., S.S., J.M., G.B., and C.T. designed and performed experiments; S.B., M.G., and F.M. were responsible for patient care and provided clinical data; M.S. and U.P. designed the study, interpreted data, and wrote the manuscript; and all authors reviewed, edited, and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare competing financial interests.
Correspondence: Uwe Platzbecker, Medical Clinic and Policlinic I, University Hospital Carl Gustav Carus, TU Dresden, Fetscherstr 74, 01307 Dresden, Germany; e-mail: uwe.platzbecker@uniklinikum-dresden.de.
References
Author notes
A.S.K. and R.W. contributed equally to this study as joint first authors.
M.S. and U.P. contributed equally to this study as joint senior authors.