Key Points
Only 16.6% of patients aged <60 years and 2.4% aged ≥60 years treated with chemotherapy are disease-free at 10 years after diagnosis.
Ten-year disease-free survivors were mostly diagnosed with core-binding factor AML with t(8;21) or inv(16), or had a normal karyotype.
Abstract
The probability that adult patients with de novo acute myeloid leukemia (AML) receiving intensive chemotherapy in the absence of allogeneic hematopoietic stem cell transplantation (Allo-HCT) in first complete remission (CR1) will be disease-free at 10 years after diagnosis, a long-term surrogate of cure, is unknown. To address this question, we examined 2551 AML patients (1607 aged <60 years, and 944 aged ≥60 years) enrolled in Cancer and Leukemia Group B treatment protocols and the cytogenetics companion protocol 8461 between 1983 and 2004. At 10 years, 267 (16.6%) of patients aged <60 years and 23 (2.4%) of those aged ≥60 years were alive and disease-free. This disease-free AML group consisted predominantly of patients with core-binding factor AML with t(8;21)(q22;q22) or inv(16)(p13q22)/t(16;16)(p13;q22) and those with a normal karyotype. Occurrences of AML beyond 10 years were infrequent and associated with cytogenetic findings different from those at diagnosis. These data provide evidence that the frequency of long-term cure of AML is low among younger and especially older patients in the absence of Allo-HCT in CR1. In older patients not appropriate for Allo-HCT, these data provide further justification for early use of alternative treatments outside of intensive chemotherapy.
Introduction
Acute myeloid leukemia (AML) is primarily a disease of the elderly, with a median age of 67 years at diagnosis.1 New studies have expanded our knowledge on AML heterogeneity and identified prognostic cytogenetic and molecular groups.2-5 Despite improved ability to identify prognostic groups, treatment for attaining and maintaining first complete remission (CR1) in AML has largely remained unchanged during the past 35 years.6 Allogeneic hematopoietic stem cell transplantation (Allo-HCT) as postremission therapy has been shown to improve outcomes in patients with normal and unfavorable karyotypes.7 However, long-term disease-free survival (DFS) of AML without Allo-HCT in CR1 is less known. A 2013 analysis of 3415 patients aged 16 to 49 years who attained CR1 demonstrated that among the 2596 patients not undergoing Allo-HCT in CR1, the relapse rate was 60%, with a median time to relapse of 28.5 months.8 In a study of 1892 patients (median age, 54 years) treated between 1965 and 1995, ∼5% of patients receiving only chemotherapy were in CR1 for over 10 years.9 Most studies using (non–Allo-HCT) chemotherapy-based approaches for AML report only 3- or 5-year DFS and overall survival (OS).10
Promising data on targeted therapies for selected AML subsets and near-universal donor availability for Allo-HCT underline the importance of identifying patients who are cured and disease-free for 10 years without an Allo-HCT.11-13 Using the well-annotated Cancer and Leukemia Group B (CALGB)/Alliance patient data, we describe the pretreatment clinical and cytogenetic features and causes of death beyond 10 years in these long-term disease-free survivors.
Methods
Patients
Patients with acute promyelocytic leukemia, secondary or treatment-related AML, and those who underwent Allo-HCT in CR1 were excluded. A total of 2551 patients diagnosed with de novo AML were enrolled into CALGB 8461, a cytogenetics companion protocol, between 1983 and 2004 (Figure 1). All patients provided written informed consent for participation in the studies, and all study protocols were in accordance with the Declaration of Helsinki and approved by institutional review boards at each treatment center.
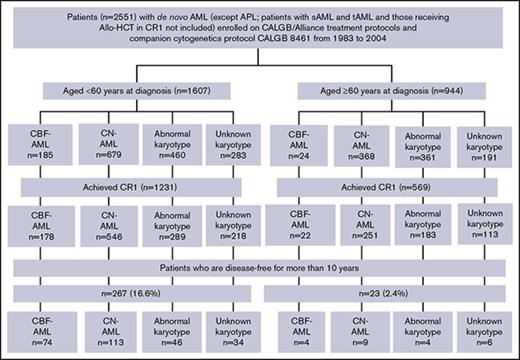
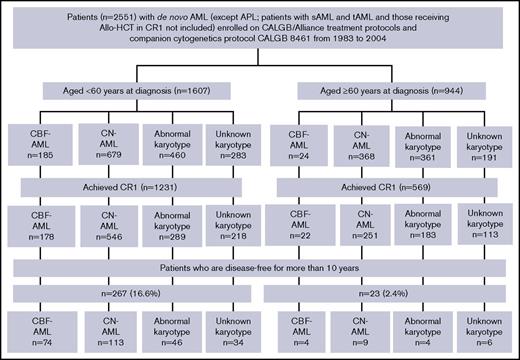
Overview of AML patients enrolled on the CALGB 8461 cytogenetic study and receiving chemotherapy-based treatment on successive CALGB trials. Abnormal karyotype indicates other abnormal karyotypes (excluding CBF-AML); unknown karyotype (due to inadequate mitoses). APL, acute promyelocytic leukemia; CALGB, Cancer and Leukemia Group B; CBF, core-binding factor; CN, cytogenetically normal; sAML, secondary AML; tAML, therapy-related AML.
Overview of AML patients enrolled on the CALGB 8461 cytogenetic study and receiving chemotherapy-based treatment on successive CALGB trials. Abnormal karyotype indicates other abnormal karyotypes (excluding CBF-AML); unknown karyotype (due to inadequate mitoses). APL, acute promyelocytic leukemia; CALGB, Cancer and Leukemia Group B; CBF, core-binding factor; CN, cytogenetically normal; sAML, secondary AML; tAML, therapy-related AML.
Cytogenetic studies
Pretreatment cytogenetic analyses of bone marrow and/or blood were performed in institutional, CALGB-approved laboratories. Karyotypes were reported according to the International System for Human Cytogenetic Nomenclature14 and karyotypes underwent central review.15 Patients were divided into 4 groups: (1) CBF-AML [inv(16)(p13.1q22)/t(16;16)(p13.1;q22) and t(8;21)(q22;q22)]; (2) normal cytogenetics (CN-AML); (3) other abnormal karyotypes (excluding CBF-AML); and (4) unknown karyotype (due to inadequate mitoses).
Induction and consolidation regimens in 10-year disease-free survivors
Induction treatment was usually with cytarabine and an anthracycline, whereas postremission therapy varied by study. Patients aged <60 years (hereafter referred to as younger) were treated on CALGB/Alliance protocols 8221 (n = 3), 8525 (n = 61), 8721 (n = 1), 8821 (n = 2), 9022 (n = 17), 9222 (n = 47), 9621 (n = 51), and 19 808 (n = 51). Patients aged ≥60 years (hereafter referred to as older) were treated on CALGB 8525 (n = 7), 8821 (n = 1), 8923 (n = 7), 9420 (n = 1), and 9720 (n = 1).
Younger patients enrolled onto the CALGB 8525 protocol were treated with cytarabine and daunorubicin induction chemotherapy and were randomly assigned to 4 cycles of consolidation with low, intermediate or high doses of cytarabine followed by maintenance treatment with four cycles of low dose cytarabine combined with daunorubicin.16 Patients enrolled onto CALGB 9022 received induction chemotherapy similar to that on CALGB 8525 followed by consolidation with 1 cycle of high-dose cytarabine (HiDAC), a cycle of cyclophosphamide and etoposide, and 1 cycle of mitoxantrone and diaziquone.17 Treatment of patients on protocol CALGB 9222 was similar, except that different doses of mitoxantrone were explored and the consolidation treatment was randomized to 3 cycles of monotherapy with HiDAC.18
Patients on CALGB 9621 were randomly assigned to receive induction chemotherapy with cytarabine, daunorubicin, and etoposide with or without a multidrug resistance protein inhibitor PSC-833 (valspodar).19 Postremission therapy depended on whether the patient had CBF-AML or not, with CBF-AML patients receiving 3 courses of HiDAC, and non-CBF-AML patients assigned to postremission therapy with HiDAC and etoposide for stem cell mobilization and myeloablative treatment with busulfan and etoposide for autologous hematopoietic stem cell transplantation (HCT). Patients not eligible for autologous HCT were administered 2 cycles of monotherapy with HiDAC. Maintenance immunotherapy consisted of a sequence of alternating low- and high-dose recombinant interleukin-2 (rIL2) or no therapy. Patients on protocols CALGB 1980820 were treated similarly to those on CALGB 9621. Patients on CALGB 8721 were treated with cytarabine and l-asparaginase. Patients on CALGB 8821 were treated with cytoxan/etoposide and diaziquone/mitoxantrone.
Older patients enrolled onto CALGB 8923 received cytarabine and daunorubicin induction treatment similar to CALGB 8525 and were then randomized to receive postremission therapy of 4 cycles of low-dose cytarabine alone or 2 cycles of intermediate dose cytarabine in combination with mitoxantrone.21 CALGB 9420 (phase 1) and CALGB 9720 (phase 3) evaluated multidrug resistance modulation by PSC-833 during induction and consolidation therapy with cytarabine, daunorubicin, and etoposide, but the PSC-833 arm was closed after random assignment of 120 patients because of a high number of early deaths.22,23 Enrollment continued for patients on the chemotherapy-only control arm. Postremission therapy consisted of a single cytarabine, daunorubicin, and etoposide consolidation course also with PSC-833 until arm closure and the patients were subsequently randomly assigned to receive or not rIL2 maintenance therapy similar to CALGB 19808.22,23
Statistical analyses and definition of clinical end points
Baseline characteristics were compared among cytogenetic groups using the Fisher exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables.24 Clinical endpoints were defined according to generally accepted criteria.25 CR required a bone marrow aspirate with cellularity >20% with maturation of all cell lines, <5% blasts and undetectable Auer rods; in blood, an absolute neutrophil count of ≥1.5 × 109/L, platelet count of >100 × 109/L, and leukemic blasts absent; and no evidence of extramedullary leukemia, all of which had to persist for ≥4 weeks.25
DFS was measured from date of achievement of CR1 until date of relapse or death from any cause.26 Median follow-up was 12.1 years. Data collection and statistical analyses were performed by the Alliance Statistics and Data Center.
Results
Of 1607 younger patients, 1231 (76.6%) achieved CR1, and 267 patients (16.6%) were disease-free for 10 years. Among 267 patients disease-free at 10 years, 74 had CBF-AML (27.7%), 113 had CN-AML (42.3%), and 46 other abnormal karyotypes (17.2%). Of the younger CBF-AML patients who achieved CR1, 41.6% were disease-free for 10 years, as were 20.7% of patients with CN-AML and 15.9% of patients with other abnormal karyotypes (Figure 1). Clinical characteristics of younger patients who survived disease-free ≥10 years and of those who did not are shown in Table 1 for patients diagnosed with CBF-AML and in Table 2 for CN-AML patients. Whereas there were no significant differences in any pretreatment features between CBF-AML patients with DFS ≥10 years and those whose DFS was shorter, long-term disease-free survivors with CN-AML had significantly lower white blood cell counts (P =.04; median, 14.8 × 109/L vs 20.7 × 109/L) and percentages of bone marrow blasts (P =.04; 63% vs 68%) than CN-AML patients with DFS <10 years.
Pretreatment features of CBF-AML patients
| Characteristic . | DFS ≥ 10 y, n = 74 . | DFS < 10 y, n = 104 . | P* . |
|---|---|---|---|
| Age, y | .52 | ||
| Median | 38 | 37 | |
| Range | 17-59 | 17-59 | |
| Male sex, n (%) | 43 (58) | 53 (51) | .36 |
| Race, n (%) | 1.00 | ||
| White | 57 (78) | 81 (78) | |
| Nonwhite | 16 (22) | 23 (22) | |
| Hemoglobin, g/dL | .64 | ||
| Median | 8.8 | 9.1 | |
| Range | 3.1-13.4 | 3.7-14.0 | |
| Platelet count, ×109/L | .38 | ||
| Median | 41 | 43 | |
| Range | 11-179 | 5-311 | |
| WBC count, ×109/L | .39 | ||
| Median | 22.8 | 18.8 | |
| Range | 1.5-206.4 | 0.9-500.0 | |
| Percentage of blood blasts | .43 | ||
| Median | 49 | 50 | |
| Range | 1-90 | 0-93 | |
| Percentage of bone marrow blasts | .88 | ||
| Median | 58 | 57 | |
| Range | 14-93 | 16-91 | |
| Extramedullary involvement, n (%) | 23 (32) | 34 (33) | 1.00 |
| CNS | 1 (1) | 4 (4) | .40 |
| Hepatomegaly | 6 (8) | 6 (6) | .56 |
| Splenomegaly | 6 (8) | 10 (10) | .80 |
| Lymphadenopathy | 11 (15) | 15 (15) | 1.00 |
| Skin infiltrates | 7 (10) | 12 (12) | .81 |
| Gum hypertrophy | 7 (10) | 7 (7) | .58 |
| Mediastinal mass | 0 (0) | 1 (1) | 1.00 |
| Characteristic . | DFS ≥ 10 y, n = 74 . | DFS < 10 y, n = 104 . | P* . |
|---|---|---|---|
| Age, y | .52 | ||
| Median | 38 | 37 | |
| Range | 17-59 | 17-59 | |
| Male sex, n (%) | 43 (58) | 53 (51) | .36 |
| Race, n (%) | 1.00 | ||
| White | 57 (78) | 81 (78) | |
| Nonwhite | 16 (22) | 23 (22) | |
| Hemoglobin, g/dL | .64 | ||
| Median | 8.8 | 9.1 | |
| Range | 3.1-13.4 | 3.7-14.0 | |
| Platelet count, ×109/L | .38 | ||
| Median | 41 | 43 | |
| Range | 11-179 | 5-311 | |
| WBC count, ×109/L | .39 | ||
| Median | 22.8 | 18.8 | |
| Range | 1.5-206.4 | 0.9-500.0 | |
| Percentage of blood blasts | .43 | ||
| Median | 49 | 50 | |
| Range | 1-90 | 0-93 | |
| Percentage of bone marrow blasts | .88 | ||
| Median | 58 | 57 | |
| Range | 14-93 | 16-91 | |
| Extramedullary involvement, n (%) | 23 (32) | 34 (33) | 1.00 |
| CNS | 1 (1) | 4 (4) | .40 |
| Hepatomegaly | 6 (8) | 6 (6) | .56 |
| Splenomegaly | 6 (8) | 10 (10) | .80 |
| Lymphadenopathy | 11 (15) | 15 (15) | 1.00 |
| Skin infiltrates | 7 (10) | 12 (12) | .81 |
| Gum hypertrophy | 7 (10) | 7 (7) | .58 |
| Mediastinal mass | 0 (0) | 1 (1) | 1.00 |
Pretreatment features of CBF-AML patients aged <60 y with DFS of ≥10 y and of those with DFS <10 y.
CNS, central nervous system; WBC, white blood cell.
P values for categorical variables are from Fisher's exact test. P values for continuous variables are from the Wilcoxon rank sum test.
Pretreatment features of CN-AML patients
| Characteristic . | DFS ≥ 10 y, n = 113 . | DFS < 10 y, n = 433 . | P* . |
|---|---|---|---|
| Age, y | .29 | ||
| Median | 43 | 45 | |
| Range | 18-59 | 18-59 | |
| Male sex, n (%) | 53 (47) | 217 (50) | .60 |
| Race, n (%) | .19 | ||
| White | 94 (84) | 384 (89) | |
| Nonwhite | 18 (16) | 48 (11) | |
| Hemoglobin, g/dL | .15 | ||
| Median | 9.2 | 9.1 | |
| Range | 4.6-14.2 | 4.3-16.0 | |
| Platelet count, ×109/L | .79 | ||
| Median | 54 | 60 | |
| Range | 3-502 | 7-569 | |
| WBC count, ×109/L | .04 | ||
| Median | 14.8 | 20.7 | |
| Range | 0.8-165.1 | 0.5-318.4 | |
| Percentage of blood blasts | .68 | ||
| Median | 48 | 50 | |
| Range | 1-94 | 0-97 | |
| Percentage of bone marrow blasts | .04 | ||
| Median | 63 | 68 | |
| Range | 10-97 | 4-97 | |
| Extramedullary involvement, n (%) | 34 (31) | 132 (31) | 1.00 |
| CNS | 2 (2) | 1 (0) | .11 |
| Hepatomegaly | 5 (5) | 23 (5) | 1.00 |
| Splenomegaly | 2 (2) | 25 (6) | .09 |
| Lymphadenopathy | 13 (12) | 61 (14) | .64 |
| Skin infiltrates | 7 (6) | 36 (8) | .56 |
| Gum hypertrophy | 16 (14) | 56 (13) | .75 |
| Mediastinal mass | 0 (0) | 1 (0) | 1.00 |
| Characteristic . | DFS ≥ 10 y, n = 113 . | DFS < 10 y, n = 433 . | P* . |
|---|---|---|---|
| Age, y | .29 | ||
| Median | 43 | 45 | |
| Range | 18-59 | 18-59 | |
| Male sex, n (%) | 53 (47) | 217 (50) | .60 |
| Race, n (%) | .19 | ||
| White | 94 (84) | 384 (89) | |
| Nonwhite | 18 (16) | 48 (11) | |
| Hemoglobin, g/dL | .15 | ||
| Median | 9.2 | 9.1 | |
| Range | 4.6-14.2 | 4.3-16.0 | |
| Platelet count, ×109/L | .79 | ||
| Median | 54 | 60 | |
| Range | 3-502 | 7-569 | |
| WBC count, ×109/L | .04 | ||
| Median | 14.8 | 20.7 | |
| Range | 0.8-165.1 | 0.5-318.4 | |
| Percentage of blood blasts | .68 | ||
| Median | 48 | 50 | |
| Range | 1-94 | 0-97 | |
| Percentage of bone marrow blasts | .04 | ||
| Median | 63 | 68 | |
| Range | 10-97 | 4-97 | |
| Extramedullary involvement, n (%) | 34 (31) | 132 (31) | 1.00 |
| CNS | 2 (2) | 1 (0) | .11 |
| Hepatomegaly | 5 (5) | 23 (5) | 1.00 |
| Splenomegaly | 2 (2) | 25 (6) | .09 |
| Lymphadenopathy | 13 (12) | 61 (14) | .64 |
| Skin infiltrates | 7 (6) | 36 (8) | .56 |
| Gum hypertrophy | 16 (14) | 56 (13) | .75 |
| Mediastinal mass | 0 (0) | 1 (0) | 1.00 |
Pretreatment features of CN-AML patients aged <60 y with DFS of ≥10 y and of those with DFS <10 y.
P values for categorical variables are from Fisher's exact test. P values for continuous variables are from the Wilcoxon rank sum test.
Our comparison of outcomes of younger CBF-AML patients who achieved CR1 and had inv(16) (n = 94) vs those with t(8;21) (n = 84) revealed a better outcome of the former (DFS: median, 6.2 vs 1.3 years, P =.008; OS: median, not reached vs 3.8 years; P < .001). Likewise, the percentage of patients who had DFS of ≥10 years tended to be greater for patients with inv(16) than for those with t(8;21): 48% vs 35% (P=.07).
Among 944 older patients, 569 (60.3%) achieved CR1, and 23 (2.4%) were disease-free for 10 years. Four of these patients had CBF-AML [17.3%, including 1 with inv(16) and 3 with t(8;21)], 9 CN-AML (39.1%) and 4 other abnormal karyotypes (17.3%) (Figure 1). Of the older patients who achieved CR1, 18.2% of CBF-AML patients, 3.6% of CN-AML patients and 2.2% of patients with other abnormal karyotypes were disease-free for 10 years. Because there were too few CBF-AML patients who were disease-free for ≥10 years, we were able to make comparisons of pretreatment features between patients who did and those who did not have DFS ≥10 years only for older patients diagnosed with CN-AML (supplemental Table 1). In contrast to younger CN-AML patients, we found no significant differences between the groups.
We examined the causes of death after 10 years to determine risk of late leukemia relapse. Nineteen deaths were observed in younger and 8 deaths in older patients. Among the former, 2 were AML-related deaths and 17 deaths occurred in CR1. Deaths in CR1 included 9 from unknown causes, 2 from secondary malignancies, 2 from Parkinson disease, 1 from myelofibrosis, 1 from congestive heart failure, 1 from myocardial infarction, and 1 from respiratory causes. Among older patients, 7 deaths occurred in CR1 and 1 was related to a second AML.
Cytogenetic findings were available in three patients with a second AML diagnosed after 10 years. In each case, karyotypes were different from those observed at original diagnosis (supplemental Table 2).
Discussion
To our knowledge, our study represents the largest US cohort of adults with de novo AML reported for 10-year DFS without Allo-HCT in CR1, and demonstrates that only 16.6% of younger and 2.4% of older patients were disease-free at 10 years. With the exception of younger CBF-AML patients, the percentage of patients achieving 10-year DFS was <25% for all other cytogenetic groups. AML-related deaths beyond 10 years were rare. Differences in cytogenetics between the initial diagnosis and diagnosis of AML after 10 years in 3 cases with available data suggest that the second disease may be a new AML different from the initial AML.
Although our study shows modest-to-poor DFS in all patients, it does not provide direct data to suggest that Allo-HCT should be considered for all AML patients in CR1. Ten-year DFS following Allo-HCT, stratified by karyotype would be helpful to evaluate long-term benefit of Allo-HCT. The largest dataset of long-term survivors in adult de novo AML is derived from a Swedish population-based registry, where among 649 patients aged 16 to 60 years, diagnosed between 1997 and 2006, 27% received Allo-HCT in CR1.27,28 The Swedish group reported that 5-year OS was superior in patients who received Allo-HCT in CR1 (61% vs 48%, P = .0005). Our study demonstrates that conventional chemotherapy approaches (still widely used) result in poor outcome for the great majority of patients when Allo-HCT is not applied during CR1.
Limitations of our retrospective analysis include the absence of prognostic molecular markers due to lack of sample availability. Although essentially all patients received anthracycline and cytarabine-based induction therapy, multiple postremission therapies were administered.16-23 Additionally, supportive care for AML patients undergoing intensive therapy has improved in the last 2 decades.
The 10-year DFS in younger CBF-AML patients in our dataset is influenced by a proportion of patients who received at least 3 courses of high-dose cytarabine consolidation shown to be important in this cytogenetic group. Specifically, among patients whose DFS was ≥10 years 74% received 3 or more courses of high-dose cytarabine postremission compared with only 54% of patients with DFS <10 years (P = .01). We also analyzed other factors reported to be prognostic in CBF-AML in the literature,29,30 such as age and platelet counts for both patients with inv(16) and those with t(8;21), trisomy of chromosome 22 in patients with inv(16), and white blood cell counts and loss of the Y chromosome in patients with t(8;21) (the latter only in male patients) but none of them was found to significantly affect disease-free survival in our cohort of patients younger than 60 years (there were too few patients with CBF-AML aged ≥60 years for a meaningful analysis). For non-CBF-AML patients, our data suggest traditional chemotherapy modalities alone are insufficient. This is particularly true for older patients with high rates of recurrent AML, where only 2.4% of patients are disease-free at 10 years even with application of intensive chemotherapy. Therefore, application of novel targeted agents, Allo-HCT, or immune-based therapies will be necessary to improve treatment results in AML.
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported in part by the National Cancer Institute of the National Institutes of Health under award numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), U10CA003927, U10CA077658, U10CA035279, U10CA180850, U10CA180857, U10CA180861, and U10CA180867.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: S.V., K.M., J.C.B., and C.D.B. designed the study; S.V., K.M., A.-K.E., L.J.S., H.B., K.H.M., D.P., J.C.B., and C.D.B. contributed to the data interpretation; S.V., K.M., J.K., and C.D.B. wrote the manuscript; J.K. and D.N. performed the statistical analyses; B.L.P., J.E.K., J.O.M., M.R.B., G.J.R., R.M.S., K.M., A.J.C., and C.D.B. were involved directly or indirectly in the care of patients and/or sample procurement; and all authors read and agreed on the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Clara D. Bloomfield, The Ohio State University Comprehensive Cancer Center, C933 James Cancer Hospital, 460 West 10th Ave, Columbus, OH 43210; e-mail: clara.bloomfield@osumc.edu.