Key Points
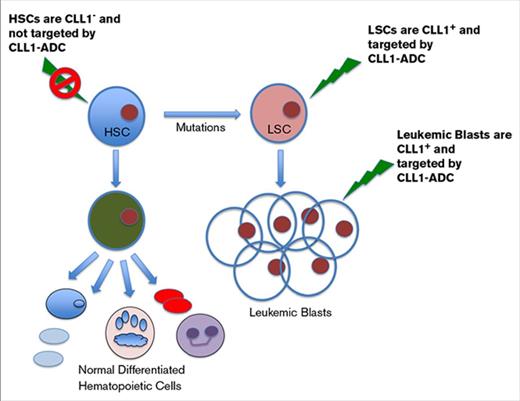
CLL1-ADC targets both AML blasts and LSCs.
Unlike CD33-ADC, CLL1-ADC does not affect normal HSCs.
Abstract
The current standard of care for acute myeloid leukemia (AML) is largely ineffective with very high relapse rates and low survival rates, mostly due to the inability to eliminate a rare population of leukemic stem cells (LSCs) that initiate tumor growth and are resistant to standard chemotherapy. RNA-sequencing analysis on isolated LSCs confirmed C-type lectin domain family 12 member A (CLL1, also known as CLEC12A) to be highly expressed on LSCs but not on normal hematopoietic stem cells (HSCs) or other healthy organ tissues. Expression of CLL1 was consistent across different types of AML. We developed CLT030 (CLL1-ADC), an antibody-drug conjugate (ADC) based on a humanized anti-CLL1 antibody with 2 engineered cysteine residues linked covalently via a cleavable linker to a highly potent DNA-binding payload, thus resulting in a site-specific and homogenous ADC product. The ADC is designed to be stable in the bloodstream and to release its DNA-binding payload only after the ADC binds to CLL1-expressing tumor cells, is internalized, and the linker is cleaved in the lysosomal compartment. CLL1-ADC inhibits in vitro LSC colony formation and demonstrates robust in vivo efficacy in AML cell tumor models and tumor growth inhibition in the AML patient-derived xenograft model. CLL1-ADC demonstrated a reduced effect on differentiation of healthy normal human CD34+ cells to various lineages as observed in an in vitro colony formation assay and in an in vivo xenotransplantation model as compared with CD33-ADC. These results demonstrate that CLL1-ADC could be an effective ADC therapeutic for the treatment of AML.
Introduction
Acute myeloid leukemia (AML) remains a major therapeutic challenge and an unmet need in hematologic oncology with estimated new cases of 19 950 and 10 430 deaths in 2016 in the United States.1 AML is a disease resulting in uncontrollable accumulation of immature myeloid blasts in the bone marrow and peripheral blood, and the disease has multiple subtypes that contribute to the challenge in developing an encompassing targeted therapy. Although there is an increased understanding in the molecular genetics of the disease, there have been relatively few novel therapies approved for AML in the past 40 years.2
Antibody-drug conjugates (ADCs) take advantage of the specificity of antibody to deliver a potent toxin to the targeted cells. Impressive clinical data generated by ADCs against CD30, Her2, and CD22 have led to successful approval of therapies by the US Food and Drug Administration (FDA).3-5 For AML, an ADC targeting CD33, gemtuzumab ozogamicin (Mylotarg), was approved by the FDA in 2000, but was later removed voluntarily from the market due to toxicity and no added benefit over the conventional standard of care. Recently, gemtuzumab ozogamicin was reapproved upon demonstrating benefit in patients by implementing a fractionated dosing regimen in the clinic.6 Another ADC targeting CD33 was withdrawn from phase 3 clinical development due to increased fatalities.7
The current standard of care for AML is largely ineffective, yielding a 5-year overall survival of only 27%.8 This is largely due to inability to remove a relatively rare population of leukemic stem cells (LSCs), which is likely to contribute to disease relapse in AML patients following chemotherapy induction treatments.9 Thus, development of a targeted therapy that can eliminate LSCs should yield a more durable response for AML patients. Although current efforts in targeting CD33 and CD123 with an ADC approach using different linkers and toxin payloads has generated promising results in the clinic and preclinical settings,10-12 the expression levels of these molecules on normal hematopoietic stem cells (HSCs) could present unwanted toxicities.13 The C-type lectin domain family 12 member A (CLL1 or also known as CLEC12A and MICL) is highly expressed on LSC and AML blast cells, but not on normal HSCs.14,15 In this article, we describe CLL1 as an attractive ADC target; anti-CLL1 antibodies were developed, characterized, and validated for use as an ADC therapeutic. The lead anti-CLL1 antibody was humanized; lead ADC (CLT030, CLL1-ADC) was selected and characterized in vitro and in vivo using several AML cell line models and AML patient samples. The CLL1-ADC demonstrated superior safety in eliminating normal HSCs compared with an ADC targeting CD33.
Materials and methods
Human AML cell lines and patient samples
AML cell lines were obtained from American Type Culture Collection (ATCC; Manassas, VA) or Deutche Sammlung von Mikrooganismen und Zelkulturen (DMSZ; Braunschweig, Germany), and cells were maintained in growth media according to supplier instructions using heat-inactivated fetal bovine sera. Patient AML samples were obtained under an approved institutional review board protocol at Cleveland Clinic and in accordance with the Declaration of Helsinki or purchased from All Cells Inc and Conversant Biologics Inc.
Fluorescent-activated cell sorting/analysis and LSC and normal HSC isolation
LSCs from patients or HSCs from healthy bone marrow donors were enriched by fluorescent-activated cell sorting (FACS) using a BD Aria II cell sorter, and samples were stained with antibodies against CD34, CD38, CD90, and lineage depletion markers including CD2, CD3, CD11b, CD14, CD15, CD16, CD19, CD56, CD235a antibodies (Biolegend, BD Biosciences, or R&D Systems). Analyses of CLL1 staining in LSCs were done by examining the percentage positivity and mean fluorescent intensity (MFI) of CLL1 antibody staining in the CD34+CD38− fraction relative to that of immunoglobulin G (IgG) control antibody. Similarly, MFI analysis was done for CLL1 and CD33 staining among various normal hematopoietic cell types based on lineage markers (ie, CD3+ for T cells, CD19+ for B cells, CD14+ for monocytes, CD66b+ for neutrophils, CD235a+ for erythrocytes, and CD41+ for platelets). FACS analysis was performed using FACSGallios, FACSCalibur, or FACSAriaII; MFI was calculated using FlowJo software.
AML subcutaneous or orthotopic tumor models in mice
All animal experiments were conducted in a facility accredited by the Association for Assessment of Laboratory Animal Care (AALAC) under institutional animal care and use committee (IACUC) guidelines and appropriate animal research approval. For the subcutaneous model, 10 × 106 HL60 or OCI-AML2 cells were injected at the flank of CB-17/Scid Beige mice. Once tumors reached 100 to 150 mm3, mice were predosed with 30 mg/kg human IgG 1 day prior to dosing with a specific ADC to block FcR expressed on myeloid cells. Tumor volumes were measured twice per week. For the orthotopic model, NOD/SCID mice were irradiated with a Faxitron CP-160 (Tucson, AZ) to yield a total dose of 2.5 Gy 1 day prior to tumor cell injection, and 5 × 106 HL60 or 1 × 106 OCI-AML2 cells were intravenously injected into host mice. Six days following tumor injection, ADC was given at a dosing schedule of once a week for 3 weeks (Q1W×3). After 28 days, bone, spleen, and peripheral blood were collected from treated mice and analyzed for human cells by FACS via anti-human CD45 and CD33 staining. Tumor burden is indicated as a percentage of human cells among endogenous mouse cells and the median percentage of human cells was plotted and analyzed by Prism program. For the patient-derived xenograft (PDX) tumor, NOD.Cg-PrkdcscidIL2rgtm1Wjl/SzJ (NSG) mice were irradiated with 2.5 Gy 1 day prior to cell injection, and 2 × 106 to 10 × 106 AML patient cells were injected intravenously. After 6 weeks of tumor cell injection, mice were treated Q1W×3 with ADC and after 9 to 10 weeks, bone marrow, spleen, and peripheral blood were collected and analyzed for percentage of human cells using the Prism program and P values were calculated.
In vivo CD34+ engraftment study
Nine-week-old female NSG mice were conditioned with 2.7 Gy of radiation using a Faxitron delivering 0.71 Gy per minute in a single dose. Twenty-four hours afterward, 2 × 106 CD34+ cells were administered to anesthetized mice by retro-orbital injection. Twenty-four hours following cell dosing, 0.5 mg/kg ADC was administered via intraperitoneal injection. After euthanasia, bones from both femurs and tibia were excised, crushed, filtered through nylon mesh, subjected to ACK lysis, washed with Hank's balanced salt solution containing 0.25% human serum albumin (Octapharma, Sacramento, CA) and stained with antibodies against CD45, CD14, CD3, CD15, CD2, CD19, and CD33 (ThermoFisher, Invitrogen, BD Biosciences). Stained cells were acquired on a Gallios flow cytometer (Beckman Coulter, Indianapolis, IN).
Results
Identification of CLL1 target on LSCs of AML samples
To identify genes that selectively expressed in LSCs, LSC-enriched cells (CD34+CD38−CD90−) were isolated from 8 AML patient samples and normal HSCs (CD34+CD38−CD90+) were obtained from 6 healthy donors by FACS according to markers and methods described previously.16,17 Total RNA was extracted and subjected to whole-genome expression analysis via the Illumina HiSeq analysis. Results from the sequencing analysis identified CLL1 as one of the most promising candidates for development of an antibody therapeutic for AML as the expression was high in AML blast cells and LSCs while minimal to none in normal HSCs and other healthy organs. These sequencing results were supported by the data assembled from The Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx) databases, showing the median RNA transcript levels of CLL1 in 173 AML samples was at least fourfold higher than the median of the highest normal lung tissues (Figure 1A). TaqMan analysis of RNA extracted from LSCs and AML blast cells vs normal HSCs and other critical organs from healthy donors (Figure 1B) further supports that CLL1 expression levels in LSC and AML blast samples ranged from twofold to 2500-fold above that of the highest healthy organ tissue (lung tissue being the highest among all the healthy organs). Immunohistochemistry (IHC) analysis of AML samples and normal healthy tissues indicated that the expression of CLL1 protein was abundant in AML samples and moderately common in normal bone marrow tissue (supplemental Figure 1A), and its expression appeared to be at the cell surface. However, CLL1 expression in other normal healthy tissue was weakly detectable in the kidney tissues and appeared to be in luminal ductal cells so it is unlikely to contribute to toxicity due to limited access to an antibody/ADC therapeutic (supplemental Table 1). A positive CLL1 staining was also observed with tissue-resident macrophages in lung (supplemental Figure 1B). All other normal tissues did not display any detectable CLL1+ staining by IHC analysis (supplemental Table 1). In healthy blood and bone marrow, CLL1 expression was found on renewable monocytes and granulocytes, but not on HSCs, multipotent progenitors, erythrocytes, platelets, B cells, or T cells (Figure 1C), whereas the same analysis showed that the expression of CD33 was detectable on HSCs. In 31 patient AML samples, median CLL1 expression on LSCs was more abundant than that of CD33, both in terms of percentage of positive cells and MFI (Figure 1D; supplemental Figure 2), whereas the estimated receptor copy number on AML blast patient cells was similar to that of CD33 (Figure 1E). Furthermore, expression of CLL1+ cells was more commonly found in AML LSCs with various subtypes of AML, such as French-American-British (FAB) classification or cytogenetic risk categories, compared with expression of CD33 with 27 of 31 patient samples (87%) positive for CLL1 relative to 20 of 31 (65%) positive for CD33. A larger FACS staining analysis with CLL1 antibody on 90 patients showed that 81 of 90 AML patient samples (90%) and >30% positive cells were considered CLL1+ (Table 1). Heterogeneous expression of CLL1 was observed in AML blasts for CLL1 staining (in the range of 0%-100% CLL1+ cells) with a mean value of 49.9% (supplemental Figure 3). Given these compelling expression profiles, CLL1 appears to be an optimal antibody/ADC therapeutic target for treatment of AML patients.
CLL1 expression in normal healthy tissues, AML, and LSC. (A) RNA levels of CLL1 in various tumor and normal tissues based on data from TCGA and GTEx. Red dots represent cancer samples, green dots are cancer-matched normal samples, and blue dots are normal samples from GTEx. (B) TaqMan analysis of CLL1 RNA levels from isolated LSC (blue circle) and blast population (green circle) of AML patient samples, from HSC (blue circle) and multipotent progenitor cells (green circle) of healthy bone marrow samples, and from various healthy organ tissues including brain, colon, heart, kidney, liver, lung, pancreas, skin, and stomach (red open circle). Lines connecting the 2 circles indicate samples isolated from the same patient. (C) FACS analysis of CLL1 and CD33 expression in various hematopoietic lineages from healthy donor and from AML patient samples. Relative MFI is determined by dividing the MFI of the CLL1 or CD33 antibody signal by the MFI of IgG control antibody. (D) FACS analysis of CLL1 and CD33 expression in LSC of 31 AML samples. Percent-positive cells and relative MFI was determined relative to IgG control staining of CD34+CD38− population in AML patient samples. (E) Estimated receptor copy number of CLL1 vs CD33 on AML patient samples. ACC, adrenocortical cancer; BLCA, bladder cancer; BRCA, breast cancer; CESC, cervical cancer; CHOL, cholangiocarcinoma; CMP, common myeloid progenitor; COAD, colorectal cancer; DLBC, diffused large B-cell lymphoma; GBM, glioblastoma; GMP, granulocyte-macrophage progenitor; HNSC, head and neck carcinoma; KICH/KIRC/KIRP, kidney cancers; LAML, acute myeloid leukemia (AML); LGG, brain glioma; LIHC, liver cancer; LUAD/LUSC, lung cancer; MEP, megakaryocyte-erythroid progenitor; MPP, multipotent progenitor; OV, ovarian cancer; PAAD, pancreatic cancer; PCPG, pheochromocytoma; PRAD, prostate cancer; READ, rectum cancer; SARC, sarcoma; SKCM, melanoma; TGCT, testicular tumor; THCA, thyroid cancer; UCEC, uterine/endometrial cancer; UCS, uterine cancer.
CLL1 expression in normal healthy tissues, AML, and LSC. (A) RNA levels of CLL1 in various tumor and normal tissues based on data from TCGA and GTEx. Red dots represent cancer samples, green dots are cancer-matched normal samples, and blue dots are normal samples from GTEx. (B) TaqMan analysis of CLL1 RNA levels from isolated LSC (blue circle) and blast population (green circle) of AML patient samples, from HSC (blue circle) and multipotent progenitor cells (green circle) of healthy bone marrow samples, and from various healthy organ tissues including brain, colon, heart, kidney, liver, lung, pancreas, skin, and stomach (red open circle). Lines connecting the 2 circles indicate samples isolated from the same patient. (C) FACS analysis of CLL1 and CD33 expression in various hematopoietic lineages from healthy donor and from AML patient samples. Relative MFI is determined by dividing the MFI of the CLL1 or CD33 antibody signal by the MFI of IgG control antibody. (D) FACS analysis of CLL1 and CD33 expression in LSC of 31 AML samples. Percent-positive cells and relative MFI was determined relative to IgG control staining of CD34+CD38− population in AML patient samples. (E) Estimated receptor copy number of CLL1 vs CD33 on AML patient samples. ACC, adrenocortical cancer; BLCA, bladder cancer; BRCA, breast cancer; CESC, cervical cancer; CHOL, cholangiocarcinoma; CMP, common myeloid progenitor; COAD, colorectal cancer; DLBC, diffused large B-cell lymphoma; GBM, glioblastoma; GMP, granulocyte-macrophage progenitor; HNSC, head and neck carcinoma; KICH/KIRC/KIRP, kidney cancers; LAML, acute myeloid leukemia (AML); LGG, brain glioma; LIHC, liver cancer; LUAD/LUSC, lung cancer; MEP, megakaryocyte-erythroid progenitor; MPP, multipotent progenitor; OV, ovarian cancer; PAAD, pancreatic cancer; PCPG, pheochromocytoma; PRAD, prostate cancer; READ, rectum cancer; SARC, sarcoma; SKCM, melanoma; TGCT, testicular tumor; THCA, thyroid cancer; UCEC, uterine/endometrial cancer; UCS, uterine cancer.
CLL1 expression in AML patient blasts and comparison of percent positivity in the CD33 and CLL1 expression in LSCs
| AML subtype . | Positive CLL1 expression in AML patient blast cells* . | CLL1 and CD33 expression and percent positivity in LSCs . | ||
|---|---|---|---|---|
| CD33 . | CLL1 . | |||
| FAB subtype† | M1 | 22/22 | 10/11 | 10/11 |
| M2 | 20/22 | 5/8 | 7/8 | |
| M4 | 17/18 | 2/6 | 6/6 | |
| M5 | 12/13 | 3/4 | 4/4 | |
| Others | 3/8 | |||
| N/A | 6/7 | 0/2 | 0/2 | |
| Cytogenetic risk categories‡ | Poor§ | 29/34 | 3/7 | 7/7 |
| Intermediate|| | 33/34 | 11/14 | 12/14 | |
| Favorable¶ | 15/15 | 4/7 | 6/7 | |
| N/A | 4/7 | 2/3 | 2/3 | |
| Bone marrow | 8/8 | |||
| Peripheral blood | 73/82 | |||
| Total | 81/90 (90%) | 20/31 | 27/31 | |
| AML subtype . | Positive CLL1 expression in AML patient blast cells* . | CLL1 and CD33 expression and percent positivity in LSCs . | ||
|---|---|---|---|---|
| CD33 . | CLL1 . | |||
| FAB subtype† | M1 | 22/22 | 10/11 | 10/11 |
| M2 | 20/22 | 5/8 | 7/8 | |
| M4 | 17/18 | 2/6 | 6/6 | |
| M5 | 12/13 | 3/4 | 4/4 | |
| Others | 3/8 | |||
| N/A | 6/7 | 0/2 | 0/2 | |
| Cytogenetic risk categories‡ | Poor§ | 29/34 | 3/7 | 7/7 |
| Intermediate|| | 33/34 | 11/14 | 12/14 | |
| Favorable¶ | 15/15 | 4/7 | 6/7 | |
| N/A | 4/7 | 2/3 | 2/3 | |
| Bone marrow | 8/8 | |||
| Peripheral blood | 73/82 | |||
| Total | 81/90 (90%) | 20/31 | 27/31 | |
N/A, not defined.
AML blast population with >30% positive for CLL1 staining in FACS analysis was considered to be a CLL1+. The mean value for the percentage of CLL1+ in AML blast is 49.9% ± 0.3%. Contaminating nonneoplastic cells were removed by gating the AML blast population using CD45 and side scatter properties. Blasts typically show dim CD45 expression and low side scatter properties, which allows easy separation from lymphocytes, granulocytes, and monocytes.
AML patient samples were subcategorized according to FAB subtype. “Others” category is patient samples classified as M0, M6, or CML blast crisis (N = 2). “N/A” category is patient samples that were unable to be subcategorized into FAB subtypes due to lack of pathological information.
Cytogenetic risk categories were defined following guidelines of “National Comprehensive Cancer Network” version 2.2014 Acute Myeloid Leukemia.
Poor: Complex (3 or more chromosomal abnormalities); Monosomal karyotype −5, 5q−, −7, 7q-11q23 - non t(9;11)inv(3), t(3;3) t(6;9) t(9;22).
Intermediate: Normal cytogenetics + 8 (isolated) t(9;11).
Favorable: inv(16) or t(16;16) t(8;21) t(15;17).
Characterization of anti-CLL1 antibodies
As an ideal ADC therapeutic target, it is important that the CLL1 molecule internalizes upon its antibody binding on the cell surface. The anti-CLL1 antibody was conjugated with an acidic pH-sensitive pHrodo and incubated with HL60 cells that endogenously express CLL1. Unlike a nonbinding control IgG-pHrodo conjugate, the CLL1 antibody-pHrodo conjugate emitted bright pHrodo fluorescence, which is a result of target-dependent internalization of the CLL1 antigen-antibody complex into the acidic endosomal/lysosomal compartment (supplemental Figure 4A-B). To determine internalization kinetics of anti-CLL1 antibody, anti-CLL1 antibody–Alexa 488 conjugate was incubated with HL60 cells for 0 to 5.5 hours at 37°C. As seen from supplemental Figure 4C, rapid internalization kinetics were observed for the CLL1 antigen-antibody complex demonstrating that CLL1 is an excellent ADC target.
A fully humanized anti-CLL1 antibody was generated with a comparable antigen-binding affinity to that of chimeric mouse anti-CLL1 monoclonal antibody (mAb) as shown in supplemental Figure 5. The binding affinity of the humanized antibody, as determined by ForteBio, was Kd of 7.32 nM; the chimeric antibody was Kd of 2.88 nM.
Production of CLT030 (CLL1-ADC)
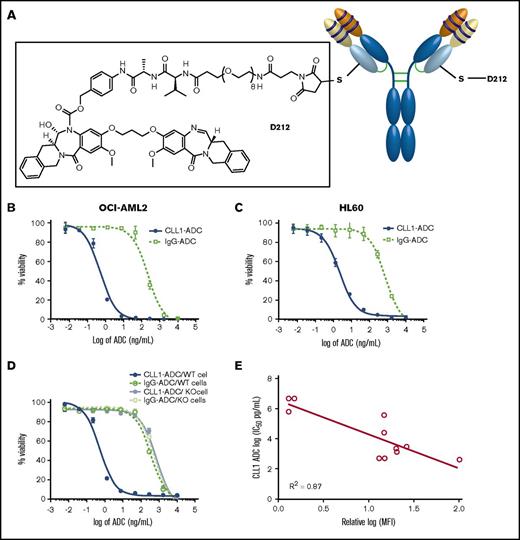
To use an ADC as a cancer therapeutic, we have screened and identified the D211 payload, isoquinolidinobenzodiazepine (IQB), which induces cell toxicity in various AML cell lines with a picomolar 50% inhibitory concentration (IC50), similar to that of a pyrrolobenzodiazepine (PBD) dimer.18 The D212 linker-payload is the D211 payload with a cleavable Val-Ala linker, PEG-8 spacer, and maleimide functional group to conjugate the linker-payload to reactive thiol groups on the antibody consisting of 2 engineered cysteine residues (Figure 2A). A nonbinding control antibody (IgG antibody) was also conjugated with D212 linker-payload and used as nonbinding ADC control in all in vitro and in vivo studies. An ADC against CD33 was generated by engineering anti-CD33 antibody as described previously11 and conjugated with a D212 linker payload. All D212 ADCs consisted of a drug-to-antibody ratio of 1.7-1.8 with >95% monomer and <0.5 EU/mg endotoxin levels in the ADC preparation (supplemental Table 2).
Functional characterization of CLL1-ADC. (A) A schematic of CLT030 (CLL1-ADC) including anti-CLL1 antibody, linker, and payload structures. Cytotoxicity activity of CLL1-ADC on (B) OCI-AML2, (C) HL60, and (D) OCI-AML5 cells or OCI-AML5 cells devoid of CLL1 expression (KO cells). CLL1-ADC in solid blue line, IgG-ADC in dotted green line on OCI-AML5 cells, CLL1-ADC on CLL1 KO cells in gray solid line, and IgG-ADC on CLL1 KO cells in dotted light green line. (E) Correlation plot of log of IC50 determine from CLL1-ADC cytotoxicity on AML cells as listed in Table 2 vs log of MFI of CLL1 antibody binding to the same listed cells.
Functional characterization of CLL1-ADC. (A) A schematic of CLT030 (CLL1-ADC) including anti-CLL1 antibody, linker, and payload structures. Cytotoxicity activity of CLL1-ADC on (B) OCI-AML2, (C) HL60, and (D) OCI-AML5 cells or OCI-AML5 cells devoid of CLL1 expression (KO cells). CLL1-ADC in solid blue line, IgG-ADC in dotted green line on OCI-AML5 cells, CLL1-ADC on CLL1 KO cells in gray solid line, and IgG-ADC on CLL1 KO cells in dotted light green line. (E) Correlation plot of log of IC50 determine from CLL1-ADC cytotoxicity on AML cells as listed in Table 2 vs log of MFI of CLL1 antibody binding to the same listed cells.
Target-specific in vitro and in vivo potency of CLL1-ADC in AML tumor models
When the D212 linker-payload was conjugated to the anti-CLL1-antibody (CLT030; CLL1-ADC) and tested on AML cells, specific killing of the target positive cell lines (ie, OCI-AML2, OCI-AML5, HL60) was observed but not of the target negative cell lines (OCI-AML5 devoid of CLL1; Figure 2B-D). The IC50 of CLL1+ cell toxicity ranged from 0.5 to 27 ng/mL (Table 2) on CLL1-expressing cells. Moreover, there was a strong inverse correlation between the expression of CLL1 based on MFI by FACS analysis vs IC50 of CLL1-ADC with a correlation coefficient of R2 = 0.87, indicating a target-specific killing (Figure 2E). A well-characterized PBD linker-payload (SGD1910)11 to the anti-CLL1 antibody and to a nonbinding control antibody (IgG antibody) was also conjugated. The resulting ADCs were tested on various AML cell lines. The results demonstrate that the ADC with PBD dimer payload and the ADC with IQB dimer payload have similar potency (Table 3). The in vitro plasma stability of CLL1-ADC was tested by incubating CLL1-ADC with human plasma and the total antibody, as well as ADC levels, were measured as described in supplemental Figure 6A. These results demonstrated that both total anti-CLL1 antibody and CLL-ADC quantities in the plasma were similar up to 5 days, indicating that conjugated linker-payload is stable in the plasma (supplemental Figure 6B).
Relationship between CLL1 target copy number and potency of CLL1-ADC in various AML cells lines
| Cell line . | Anti-CLL1 antibody/isotype control antibody MFI ratio . | Relative copy no. . | CLL1-ADC IC50, ng/mL . |
|---|---|---|---|
| EOL-1 | 10.0 | 35 740 | 1.4 |
| HEL92.1.7 | 1.6 | 5 575 | >5000 |
| HL60 | 17.7 | 66 000 | 1.7 |
| Nomo-1 | 3.7 | 13 045 | 328 |
| OCI-AML2 | 17.1 | 61 220 | 0.6 |
| OCI-AML5 | 16.6 | 63 000 | 0.7 |
| OCI-M1 | 1.2 | 4 180 | 1535 |
| PL21 | 11.2 | 39 920 | 27.0 |
| TF-1 | 1.3 | 4 570 | >5000 |
| TF-1/CLL1 | 8.6 | 30 560 | 1.8 |
| U937 | 2.8 | 9 830 | 384 |
| Cell line . | Anti-CLL1 antibody/isotype control antibody MFI ratio . | Relative copy no. . | CLL1-ADC IC50, ng/mL . |
|---|---|---|---|
| EOL-1 | 10.0 | 35 740 | 1.4 |
| HEL92.1.7 | 1.6 | 5 575 | >5000 |
| HL60 | 17.7 | 66 000 | 1.7 |
| Nomo-1 | 3.7 | 13 045 | 328 |
| OCI-AML2 | 17.1 | 61 220 | 0.6 |
| OCI-AML5 | 16.6 | 63 000 | 0.7 |
| OCI-M1 | 1.2 | 4 180 | 1535 |
| PL21 | 11.2 | 39 920 | 27.0 |
| TF-1 | 1.3 | 4 570 | >5000 |
| TF-1/CLL1 | 8.6 | 30 560 | 1.8 |
| U937 | 2.8 | 9 830 | 384 |
Relative MFI is determined by CLL1 antibody staining fluorescent intensity divided by IgG control antibody staining fluorescent intensity. CLL1 target copy number in various AML cell lines is estimated using FACS-based assay with standard markers. IC50 of toxicity on cells is determined by incubation of various concentrations of CLL1-ADC with AML cells.
Potency (IC50 in nanograms per milliliter) of CLL1-D212 and CLL1-PBD ADCs in various AML cell lines
| Cell line . | CLL1-D212 . | IgG-D212 . | CLL1-PBD . | IgG-PBD . |
|---|---|---|---|---|
| ADC, IC50, ng/mL . | ||||
| HL60 | 1.7 | 615 | 0.6 | 588 |
| OCI-AML2 | 0.6 | 230 | 0.2 | 162 |
| OCI-AML5 | 0.7 | 517 | 0.5 | 580 |
| OCI-AML5/CLL1 KO | 650 | 804 | 869 | 897 |
| OCI-M1 | 1535 | 2979 | 477 | 1173 |
| Namo-1 | 328 | 1184 | 136 | 859 |
| Cell line . | CLL1-D212 . | IgG-D212 . | CLL1-PBD . | IgG-PBD . |
|---|---|---|---|---|
| ADC, IC50, ng/mL . | ||||
| HL60 | 1.7 | 615 | 0.6 | 588 |
| OCI-AML2 | 0.6 | 230 | 0.2 | 162 |
| OCI-AML5 | 0.7 | 517 | 0.5 | 580 |
| OCI-AML5/CLL1 KO | 650 | 804 | 869 | 897 |
| OCI-M1 | 1535 | 2979 | 477 | 1173 |
| Namo-1 | 328 | 1184 | 136 | 859 |
KO, knockout.
Subcutaneous and orthotopic AML models were used to demonstrate efficacy of CLL1-ADC in vivo. Established HL60 subcutaneous tumor models were treated with a single injection of CLL1-ADC, CD33-ADC, or control IgG ADC. A dose of 1.0 mg/kg CLL1-ADC completely eliminated the HL60-derived tumors (8 of 8 animals) for the duration of the study (102 days) whereas 1 mg/kg CD33-ADC resulted in complete tumor regressions in 7 of 8 animals and minimal effect on the tumor growth in 1 of 8 animals (Figure 3A-B). At 0.5 mg/kg, both CLL1-ADC and CD33-ADC showed complete regressions in 6 of 8 animals. Similarly, a single dose of 0.3 mg/kg CLL1-ADC resulted in complete regression of the OCI-AML cell-derived tumors for 86 days whereas 0.1 mg/kg resulted in partial regression (Figure 3C-D). Consistent results were seen in HL60-derived orthotopic models where a dose of 0.1 mg/kg CLL1-ADC reduced median tumor burden in bone marrow and peripheral blood by 13- and 25-fold, respectively, relative to that of control IgG-ADC treatments. A higher dose of 0.5 mg/kg reduced the median tumor burden in bone marrow and peripheral blood to below 0.4% and 0.001%, respectively, whereas the median percentage in untreated mice was 52% and 8% (Figure 3E-F). These data indicate that CLL1-ADC is effective in eliminating AML tumor cells in vivo.
In vivo efficacy characterization of CLL1-ADC. CLL1-ADC or CD33-ADC or IgG-ADC treatment (single dose, intraperitoneal injection) of (A) HL60 tumor cell derived subcutaneous tumors and (C) OCI-AML2–derived tumors. Average tumor volume and SEM from 8 individual tumor-bearing mice is plotted against time with CLL1-ADC treatment shown in blue/gray color, CD33-ADC treatment in red/orange color and IgG-ADC control treatment in green/light green color. Survival curves (B,D) from subcutaneous tumor model were determined by number of days taken for tumor to volume to reach >1000 mm3 from the day of implantation, which then resulted in sacrificing these mice. (E-F) CLL1-ADC treatment (Q1W×3 dosing, intraperitoneal injection) of HL60 tumor cell–derived orthotopic tumor-bearing NOD/SCID mice. Bone marrow and peripheral blood were harvested following ADC treatments, processed, and analyzed for percentage of human cell based on staining with human-specific CD45 and CD33 antibodies. Data from 6 to 8 individual mice are shown, the lines represent median values of each group, and error bars represent interquartile range (ie, 25th percentile and 75th percentile). *The group in which datasets are statistically significant (P < .05) relative to control treatment based on 1-way ANOVA analysis.
In vivo efficacy characterization of CLL1-ADC. CLL1-ADC or CD33-ADC or IgG-ADC treatment (single dose, intraperitoneal injection) of (A) HL60 tumor cell derived subcutaneous tumors and (C) OCI-AML2–derived tumors. Average tumor volume and SEM from 8 individual tumor-bearing mice is plotted against time with CLL1-ADC treatment shown in blue/gray color, CD33-ADC treatment in red/orange color and IgG-ADC control treatment in green/light green color. Survival curves (B,D) from subcutaneous tumor model were determined by number of days taken for tumor to volume to reach >1000 mm3 from the day of implantation, which then resulted in sacrificing these mice. (E-F) CLL1-ADC treatment (Q1W×3 dosing, intraperitoneal injection) of HL60 tumor cell–derived orthotopic tumor-bearing NOD/SCID mice. Bone marrow and peripheral blood were harvested following ADC treatments, processed, and analyzed for percentage of human cell based on staining with human-specific CD45 and CD33 antibodies. Data from 6 to 8 individual mice are shown, the lines represent median values of each group, and error bars represent interquartile range (ie, 25th percentile and 75th percentile). *The group in which datasets are statistically significant (P < .05) relative to control treatment based on 1-way ANOVA analysis.
Effect of CLL1-ADC on AML patient tumor cells and colony formation
The ability of CLL1-ADC to inhibit the growth/survival of the in vitro colony formation and in vivo patient-derived xenograft model of the AML patient samples was assessed. The impact of CLL1-ADC on colony formation, a surrogate property of LSCs, was studied by treating AML patient samples in colony formation cultures with CLL1-ADC and scored for total colony formation units (CFUs). CLL1-ADC inhibited total AML cell-derived CFUs, whereas the inhibition by control IgG-ADC was less pronounced (Figure 4A). The in vivo effect of CLL1-ADC on patient AML cells was studied in a PDX orthotopic tumor model. The tumor with an AML patient sample was established in NSG mice and treated with CLL1-ADC. Three doses of 0.25 mg/kg CLL1-ADC reduced tumor burden in bone marrow and peripheral blood by 3.3- and threefold, respectively, relative to that of untreated mice, and higher doses of 0.5 mg/kg resulted in sevenfold and 22-fold reduction of tumor burden in the bone marrow and blood, respectively (Figure 4B). These data suggest that CLL1-ADC can effectively inhibit the growth/survival of AML patient cells in both in vitro and in vivo settings.
CLL1-ADC inhibits colony formation of AML patient samples and tumor growth in the AML PDX model. (A) CLL1-ADC impact on colony formation ability as determined by number of colonies that can be grown from 70 000 PBMCs of AML patient samples. IgG-ADC treatment is shown in green bars, CLL1-ADC in blue bars, and untreated is shown in purple bars. Error bars represent SEM calculated from 3 individually treated wells/plates. (B) AML patient samples (10 × 106 AML patient cells) were injected intravenously into NOD/SCID/IL-2Rγ−/−. After 6 weeks of tumor cell injection, mice were treated Q1W×3 dosing schedule with CLL1-ADC, and at the end of the ninth week, bone marrow and peripheral blood were collected and analyzed for percentage of human cells. *The group in which datasets are statistically significant (P < .05) relative to control treatment based on 1-way ANOVA analysis.
CLL1-ADC inhibits colony formation of AML patient samples and tumor growth in the AML PDX model. (A) CLL1-ADC impact on colony formation ability as determined by number of colonies that can be grown from 70 000 PBMCs of AML patient samples. IgG-ADC treatment is shown in green bars, CLL1-ADC in blue bars, and untreated is shown in purple bars. Error bars represent SEM calculated from 3 individually treated wells/plates. (B) AML patient samples (10 × 106 AML patient cells) were injected intravenously into NOD/SCID/IL-2Rγ−/−. After 6 weeks of tumor cell injection, mice were treated Q1W×3 dosing schedule with CLL1-ADC, and at the end of the ninth week, bone marrow and peripheral blood were collected and analyzed for percentage of human cells. *The group in which datasets are statistically significant (P < .05) relative to control treatment based on 1-way ANOVA analysis.
Effect of CLL1-ADC on normal HSCs
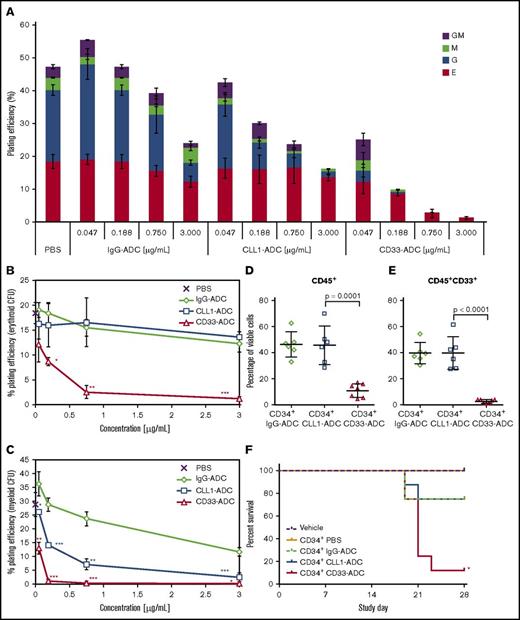
CLL1 expression is substantially lower on primitive HSCs in normal human subjects compared with AML patients whereas CD33 is expressed in HSCs and multipotent progenitor cells in healthy human subjects (Figure 1C). The effect of CLL1-ADC on hematopoietic differentiation was compared with CD33-ADC in CFU assays. CD34+ cells from healthy donors were treated with phosphate-buffered saline (PBS), IgG-ADC, CLL1-ADC, or CD33-ADC and colonies representing various hematopoietic lineages were scored. Results indicate that CLL1-ADC has a less severe impact on colony formation compared with CD33-ADC (Figure 5A-C). CLL1-ADC has little impact on erythroid colony formation (not significant compared with IgG-ADC control) whereas CD33-ADC treatment results in a significant decrease in erythroid CFU development compared with CLL1-ADC (Figure 5B). This is consistent with CD33 being expressed more broadly on the various hematopoietic subpopulations (common myeloid progenitor [CMP], granulocyte-macrophage progenitor [GMP], and megakaryocyte-erythrocyte progenitor [MEP]) whereas CLL1 has little to no expression in MEPs in healthy donor samples (Figure 1C). Although both CLL1-ADC and CD33-ADC decreased myeloid CFU formation (Figure 5C), the extent varied depending on the type of CFU formed (Figure 5A). The CLL1-ADC significantly (P < .05) decreases myeloid CFU formation compared with the IgG-ADC control (Figure 5C). The decrease in CFU formation by the IgG-ADC control may likely be due to FcR binding on the CD34+ cells. Because CLL1 is known to be expressed in myeloid cells, the continuous presence of CLL1-ADC in the CFU media may have caused a decrease in myeloid colony formation. At each concentration examined, the decrease in overall myeloid colony formation (granulocyte [G], monocyte/macrophage [M], and granulocyte-macrophage [GM]) is more pronounced when cells were treated with CD33-ADC compared with CLL1-ADC (Figure 5C).
Effects of CLL1-ADC and CD33-ADC on in vitro and in vivo differentiation of normal CD34+ stem cells. (A-C) Enriched CD34+ cells from a pool of 3 donors were treated with PBS or the indicated doses of IgG-ADC (nonbinding control), CLL1-ADC, or CD33-ADC. Treated cells were seeded in methylcellulose-based hematopoietic colony-forming media (250 cells per plate) in triplicate and scored for erythroid (E), granulocyte (G), monocyte/macrophage (M), or granulocyte macrophage (GM) colony formation (average and standard deviation of n = 3). (A) The percentage of cells plated that gave rise to each colony type is shown. The percentage of cells that gave rise to (B) erythroid or (C) combined G, M, or GM myeloid colonies is shown. *P < .05; **P < .01; ***P < .001 for CD33-ADC compared with CLL1-ADC or CLL1-ADC compared with IgG-ADC applied at the same concentration using the 2-tailed equal variance Student t test. (D-E) Sublethally irradiated NSG mice (6-7 animals per group) were dosed with 2 × 106 CD34+ cells (pooled from 3 healthy G-CSF–mobilized peripheral blood donors) and treated with 0.5 mg/kg IgG-ADC, CLL1-ADC, or CD33-ADC 24 hours after cell administration. (D) Fourteen days following cell dosing, overall bone marrow engraftment is shown as the percentage of human CD45+ cells and (E) myeloid engraftment is shown as the percentage of human CD45+CD33+ cells. (F) Kaplan-Meier survival analysis for 28 days following cell infusion is shown for a similar in vivo study for sublethally irradiated NSG mice (8 animals per group) dosed with 2 × 106 CD34+ cells from a pool of three different mobilized peripheral blood donors. One day following irradiation, mice were dosed with vehicle (PBS) only or CD34+ cells. Twenty-four hours after cell administration, mice were treated with PBS or 0.5 mg/kg IgG-ADC, CLL1-ADC, or CD33-ADC. *P = 0.0226 for CLL1-ADC vs CD33-ADC survival using the log-rank (Mantel-Cox) test.
Effects of CLL1-ADC and CD33-ADC on in vitro and in vivo differentiation of normal CD34+ stem cells. (A-C) Enriched CD34+ cells from a pool of 3 donors were treated with PBS or the indicated doses of IgG-ADC (nonbinding control), CLL1-ADC, or CD33-ADC. Treated cells were seeded in methylcellulose-based hematopoietic colony-forming media (250 cells per plate) in triplicate and scored for erythroid (E), granulocyte (G), monocyte/macrophage (M), or granulocyte macrophage (GM) colony formation (average and standard deviation of n = 3). (A) The percentage of cells plated that gave rise to each colony type is shown. The percentage of cells that gave rise to (B) erythroid or (C) combined G, M, or GM myeloid colonies is shown. *P < .05; **P < .01; ***P < .001 for CD33-ADC compared with CLL1-ADC or CLL1-ADC compared with IgG-ADC applied at the same concentration using the 2-tailed equal variance Student t test. (D-E) Sublethally irradiated NSG mice (6-7 animals per group) were dosed with 2 × 106 CD34+ cells (pooled from 3 healthy G-CSF–mobilized peripheral blood donors) and treated with 0.5 mg/kg IgG-ADC, CLL1-ADC, or CD33-ADC 24 hours after cell administration. (D) Fourteen days following cell dosing, overall bone marrow engraftment is shown as the percentage of human CD45+ cells and (E) myeloid engraftment is shown as the percentage of human CD45+CD33+ cells. (F) Kaplan-Meier survival analysis for 28 days following cell infusion is shown for a similar in vivo study for sublethally irradiated NSG mice (8 animals per group) dosed with 2 × 106 CD34+ cells from a pool of three different mobilized peripheral blood donors. One day following irradiation, mice were dosed with vehicle (PBS) only or CD34+ cells. Twenty-four hours after cell administration, mice were treated with PBS or 0.5 mg/kg IgG-ADC, CLL1-ADC, or CD33-ADC. *P = 0.0226 for CLL1-ADC vs CD33-ADC survival using the log-rank (Mantel-Cox) test.
To understand the in vivo differentiation of normal human CD34+ cells in the presence of the ADCs, an NSG mouse xenograft model was used.19,20 Sublethally irradiated NSG mice were each dosed with 2 × 106 CD34+ cells (>97% CD34+, 71% CD34+CD33+, and 23.2% CD34+CLL1+) and treated with 0.5 mg/kg IgG-ADC, CLL1-ADC, or CD33-ADC. Bone marrow was harvested from all animals 14 days following cell administration and engraftment was based on human CD45+ expression (Figure 5D). Engraftment was similar in the IgG-ADC and CLL1-ADC animals at 46.5% ± 9.7% and 45.9% ± 14.8%, respectively. Engraftment in the CD33-ADC–treated mice was 11.1% ± 5.2% (P < .0001 vs IgG-ADC control). The CD33-ADC likely killed a significant percentage of the CD34+ cells that were CD33+ and prevented differentiation of the cells toward the myeloid lineage. The percentage of CD45+CD33+ myeloid cells detected was 39.9% ± 8.2%, 39.9% ± 12.6%, and 2.69% ± 1.37% for the IgG-ADC, CLL1-ADC, and CD33-ADC groups, respectively (Figure 5E). The majority of the engrafted cells in the CD33-ADC–treated mice were not detected by antibodies against CD33, CD15, CD19, CD2, CD3, or CD71, which indicates that if the myeloid, lymphoid, and erythroid precursor cells were killed by CD33-ADC, the remaining cells differentiated toward an alternative lineage. Survival at 28 days following cell infusion show that although all ADC-treated groups had deaths starting at day 19, survival was lowest in the CD33-ADC–treated mice and was significantly lower (P < .05) than in CLL1-ADC–treated mice (Figure 5F). These results demonstrate that CLL1-ADC did not affect engraftment or differentiation of CD34+ cells from normal, healthy donors in an in vivo xenograft model.
Discussion
AML is a disease that stems from modification of HSCs during its normal differentiation to myeloid cells. The LSC phenotype from AML suggests repopulating activity, which most likely contributes to the relapse of patients.21 CLL1 expression is maintained during generation of LSCs and when LSCs undergo further differentiation.22,23 Considering AML is a deadly disease with a median 5-year survival of 5% for patients over 65 years of age (median age for this disease), it is important that the disease is treated with a drug that addresses the root cause of the problem, the LSC. Toward this objective, we developed CLT030 (CLL1-ADC), a humanized monoclonal ADC targeting CLL1. Although CLL1 is reliably overexpressed on the LSCs and blast cells, it has minimal to no expression on most normal HSCs or other healthy tissues. This makes it an attractive target for potentially curative therapy. An antibody targeting CLL1 linked covalently to a highly potent DNA-binding payload offers an effective mechanism to kill blast cells and LSCs. The ADC proposed here is designed to be stable in the bloodstream and to release its DNA-binding payload only upon internalization into the lysosomes of CLL-bearing tumor cells, reducing nonspecific released-payload killing. Thus, CLL1-ADC is intended to improve the therapeutic outcome for AML patients by specifically targeting LSCs and blast cells while avoiding most of the systemic toxicities inherent with conventional chemotherapeutic agents.
Cellerant’s payload (D211) attached to CLL1-ADC is a PBD dimer payload family member that specifically belongs to the subgroup of IQB dimers. PBD dimers bind in the minor groove and cross-link specific repeat sequences in the DNA through the N10 position of both monomers.24 Two of the PBD ADCs that are currently in clinical development (SGN-CD33A and Rova-T ADCs) use PBD linker-payloads, SGD-1910 and SG3249, respectively.25,26 Like the PBD payload, the D211 payload has IC50 values in the picomolar range in AML cell lines. PBD ADCs were shown to be safe in preclinical models and in clinical trials.11,25,26 Therefore, CLL1-ADC is expected to have a favorable safety profile although appropriate Investigational New Drug–enabling preclinical studies will be conducted before initiating a phase 1 trial.
Targeting CLL1 using other therapeutic modalities such as T-cell–recruiting bispecific antibody (CLL1-CD3) and CLL1–chimeric antigen receptor (CAR)–T have been recently described.27,28 The CLL1-CD3–bispecific antibody displayed activity at low as well as high copy number.27 This may be a disadvantage as normal tissues (eg, lung) with a low target copy number (100-5000 range) could be affected. A CAR–T-cell approach would be more severe where cells with low target copy number (10-1000 copies per cell) could be killed, therefore warranting the use of these technologies only when there is absolutely no or minimal expression in normal tissues.29,30 On the other hand, CLL1-ADC displayed target-specific cell killing in a copy-number–dependent manner with minimal or no activity in cells with a target copy number of <5000 copies whereas potent activity (0.5-2 ng/mL) was observed in tissues with target copy number of >30 000 and modest activity (20-400 ng/mL) with moderate copy number (10 000-30 000). ADCs have been through intensive investigation in the preclinical as well as clinical setting for the past 30 years and there are over 60 ADCs currently undergoing clinical investigation.31,32 Therefore, they have significant advantages over the less clinically validated T-cell–recruiting bispecific and CAR-T approaches.
We and others have elegantly shown that CLL1 expression is nonexistent or minimal in the most primitive HSC population among healthy, nonleukemic subjects. The expression of CLL1 has been observed in mature myeloid cells such as granulocytes and monocytes. Some of these cells, such as granulocytes, have a short half-life (8-12 hours) in a healthy individual and the body constantly replenishes them from HSCs. Expression of CLL1 is present in GMPs but not as much in more immature CMPs. Although there is potential of transient to severe depletion of neutrophils, the hematopoietic recovery should not be delayed as the HSCs are preserved. The resulting neutropenia can be managed with effective use of antimicrobials as currently practiced in AML patients.
Taken together, CLL1-ADC could become an attractive targeted therapeutic for AML. The current standard of care for AML patients is inadequate as evidenced by a low 5-year survival rate.3 Although the 7 + 3 regimen chemotherapy used in AML is usually successful at killing most of the bulk cancer cells and putting the disease into remission, relapse rates are high, resulting in an average 5-year survival rate of ∼25%.3 Therapies that are effective initially, as demonstrated by reductions in tumor burden, may have only limited or transient effectiveness if they do not effectively eliminate the LSCs. Conversely, therapeutic strategies, such as CLL1-ADC described here, aimed to eliminate LSCs within the tumors, offer the potential of reducing disease progression and providing durable responses. In addition, the use of a DNA-binding payload in CLL1-ADC is critical because such a payload gives the ADC the ability to kill both proliferative and quiescent cells, unlike conventional chemotherapy. CLL1 is widely expressed in patients across all different types of AML and within a significant proportion of AML cells within a patient. This profile, along with the absence of expression in normal hematopoietic stem and progenitor cells, makes CLL1-ADC a very compelling product candidate for the treatment of AML patients.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Len Presta and Jennifer Lu for their help with anti-CLL1 antibody humanization, Zemin Zhang for help with RNA-sequencing analysis from the TCGA portal, Gabriel Grenot for help with in vivo mouse studies, Jiang Liu for help with final manuscript figures, Sasha Lazetic for help in generating anti-idiotypic anti-CLL1 antibody, Jiping Huang for help in generating anti-D212 antibody, and Abzena/Bristol facility colleagues for their help with production of ADCs as a part of contract research organization service.
Authorship
Contribution: Y.-P.J. designed experiments, analyzed the data, and contributed to vital new reagents; B.Y.L. designed and performed experiments, analyzed the data, and wrote the manuscript; Q.Z., R.C., J.Z., M.M., J.H., T.D.-P., S.R., X.Z., and J.L. designed and performed experiments and analyzed the data; S.P. designed experiments, analyzed the data, and cowrote the manuscript; G.B. and E.D.H. designed and analyzed the data; R.M. designed experiments and cowrote the manuscript; and J.R.J. designed experiments, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: Y.-P.J., B.Y.L., Q.Z., S.P., J.Z., M.M., J.H., T.D.-P., S.R., G.B., R.M., and J.R.J. are/were employees of Cellerant Therapeutics Inc at the time this work was conducted. R.C. and J.L. are/were postdoctoral fellows at Cleveland Clinic. X.Z. and E.D.H. are employees of Cleveland Clinic.
Correspondence: Jagath R. Junutula, Cellerant Therapeutics Inc, 1561 Industrial Rd, San Carlos, CA 94070; e-mail: jagathjr@cellerant.com.
References
Author notes
Y.-P.J. and B.Y.L. contributed equally.