Key Points
A heteroclitic WT1 peptide vaccine is well tolerated and induces immunologic responses in most acute myeloid leukemia patients post-CR1.
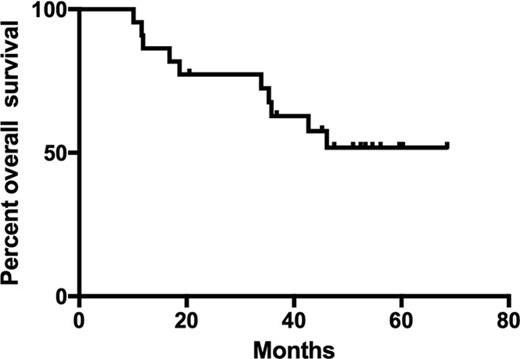
Median overall survival for the group of patients vaccinated was not reached but is poised to reach or exceed 67.6 months.
Abstract
A National Cancer Institute consensus study on prioritization of cancer antigens ranked the Wilms tumor 1 (WT1) protein as the top immunotherapy target in cancer. We previously reported a pilot study of a multivalent WT1 peptide vaccine (galinpepimut-S) in acute myeloid leukemia (AML) patients. We have now conducted a phase 2 study investigating this vaccine in adults with AML in first complete remission (CR1). Patients received 6 vaccinations administered over 10 weeks with the potential to receive 6 additional monthly doses if they remained in CR1. Immune responses (IRs) were evaluated after the 6th and 12th vaccinations by CD4+ T-cell proliferation, CD8+ T-cell interferon-γ secretion (enzyme-linked immunospot), or the CD8-relevant WT1 peptide major histocompatibility complex tetramer assay (HLA-A*02 patients only). Twenty-two patients (7 males; median age, 64 years) were treated. Fourteen patients (64%) completed ≥6 vaccinations, and 9 (41%) received all 12 vaccine doses. Fifteen patients (68%) relapsed, and 10 (46%) died. The vaccine was well tolerated, with the most common toxicities being grade 1/2 injection site reactions (46%), fatigue (32%), and skin induration (32%). Median disease-free survival from CR1 was 16.9 months, whereas the overall survival from diagnosis has not yet been reached but is estimated to be ≥67.6 months. Nine of 14 tested patients (64%) had an IR in ≥1 assay (CD4 or CD8). These results indicated that the WT1 vaccine was well tolerated, stimulated a specific IR, and was associated with survival in excess of 5 years in this cohort of patients. This trial was registered at www.clinicaltrials.gov as #NCT01266083.
Introduction
Immunotherapy is on the forefront of oncologic clinical research and patient care, with an ever increasing number of cancer immunotherapeutics entering the clinic.1,2 Historically, this modality has long been appreciated to be effective therapy for acute myeloid leukemia (AML) patients as evidenced by the improved outcomes for individuals undergoing allogeneic hematopoietic stem cell transplantation (HSCT).3,4 Several investigators have attempted to mimic the effects of HSCT without actually performing the transplant using cytokines, interferons, genetically engineered chimeric antigen receptor T cells, and various vaccines.5-7
We have previously reported the results from a pilot study investigating the potential application of a multivalent heteroclitic Wilms tumor 1 (WT1) peptide vaccine (galinpepimut-S [GPS]) in the treatment of adult AML patients.8 The heteroclitic peptides within the vaccine bear a single amino acid substitution in key residues that are specifically designed to both enhance antigenicity against and mitigate immune tolerance toward the corresponding native WT1 peptide sequences expressed in tumor cells and recognized by the host’s immune system.8 A second pilot study using the same vaccine conducted in AML patients in second complete remission (CR) as well as 2 other studies conducted in patients with malignant pleural mesothelioma all showed promising activity.9-11 This phase 2 study investigates the safety and efficacy of GPS treatment in adult AML patients in first complete remission (CR1).
Materials and methods
Trial design
This was a phase 2 open-label study evaluating the safety and efficacy of GPS in 22 patients with AML (www.clinicaltrials.gov #NCT01266083). Patients were required to have histologic confirmation of the diagnosis at Memorial Sloan Kettering Cancer Center according to standard criteria and to have WT1-positive disease as assessed by a quantitative real-time reverse-transcription polymerase chain reaction (RT-PCR) assay for WT1 at the time of enrollment on study. All patients were required (1) to be in CR1, (2) to be within 2 years of achieving CR1, and (3) to have completed all planned chemotherapy with adequate recovery of hematologic counts.12,13 The protocol was reviewed and approved by the Memorial Hospital Institutional Review Board and was conducted under a US Food and Drug Administration investigational new drug application. All patients gave written informed consent prior to enrolling in the study.
Vaccine formulation
The GPS formulation has previously been described in detail.8,14 Briefly, the vaccine consists of 4 WT1-derived peptides: a synthetic heteroclitic short peptide to stimulate CD8+ responses (WT1-A1), 2 native long peptides (331 and 427) to stimulate CD4+ responses, and a synthetic heteroclitic long peptide to stimulate both CD4+ and CD8+ cells (122A1). Immediately prior to administration, the peptides were mixed at a 1:1 ratio with Montanide ISA 51 VG (Seppic, Fairfield, NJ), an immune adjuvant.
Treatment plan
Patients received 6 vaccinations subcutaneously biweekly over a 10 week period. Vaccination sites were rotated between extremities. Injection sites were also pre-stimulated with 70 μg granulocyte-macrophage colony stimulating factor (Sargramostim; Bayer Healthcare Pharmaceuticals, Seattle, WA) injected subcutaneously on days −2 and 0 of each vaccination. Routine toxicity assessments were conducted throughout the trial and were graded in accordance with the National Cancer Institute Common Toxicity Criteria for Adverse Events version 4.0. Bone marrow aspirates were examined for morphology and were assessed following the 6th vaccination and end of study or as clinically indicated. Potential effects on minimal residual disease (MRD) were assessed at baseline and week 12 or at relapse via RT-PCR for WT1 in bone marrow aspirates. Patients who remained in CR were eligible to receive up to 6 more vaccinations (for a total of 12) administered monthly.
Efficacy assessments
Clinical measures
The protocol assumed a 3-year historical overall survival (OS) rate of 20% to 25% in AML patients.15-17 It was hypothesized the actual OS rate at 3 years from first vaccine treatment would be ≥34% (ie, an absolute improvement of 9% to 14% or a relative benefit of 36% to 70%). This was the prespecified efficacy threshold to justify further clinical investigations using the vaccine to treat CR1 AML patients (ie, the primary end point of the study). Median (actuarial) disease-free survival (DFS) and OS times (in months), as well as landmark actuarial DFS and OS rates (%) at 6 and 9 months post-GPS were secondary end points.
Immune responses
Immune responses (IRs) were evaluated at baseline, after 6 vaccinations, following completion of each patient’s extended vaccination period (ie, between 7 and 12 total vaccinations for those patients who received >6 vaccinations), or upon discontinuing therapy. Response was assessed by 1 or more of the following methods: CD4+ T cell proliferation, CD3/CD8+ T cell interferon γ (IFN-γ) release measured by enzyme-linked immunospot (ELISPOT) assay, or WT1/HLA-A*02:01 tetramer staining to measure the CD8+ T-cell response in HLA-A*02–positive patients.
CD4+ T-cell response
CD4+ T-cell response was measured using a standard CD4+ lymphocyte proliferation assay (supplemental Methods).8,18 The readout of this assay, which used tritiated thymidine, was measured counts/min, and represented mean values of quadruplicate microwell cultures per each sample analyzed.
CD8+ T-cell response
To detect specific CD8+ T cell responses, 2 rounds of in vitro stimulations of CD3+ T cells were performed.8,14,19 Following stimulation and incubation, CD8+ responses were then measured by IFN-γ secretion of the cells was examined by ELISPOT assay and/or tetramer staining (supplemental Methods).
Measurement of WT1 transcript by RT-PCR
Measurement of WT1 transcript was accomplished through methods previously described.8,14,20
Statistics
Descriptive statistics were used to summarize continuous data variables (eg, mean, standard deviation [SD], median, minimum, and maximum). Categorical data were summarized using counts and percentages. All statistical tests were carried out at the 5% significance level with 95% confidence intervals (CIs). Kaplan-Meier plots were used to illustrate trends in OS, event-free survival (EFS), and DFS and time to event analyses were compared using the log rank analysis methods calculated using either the LIFETEST procedure of the Statistical Analysis System (Cary, NC) or GraphPad Prism 7 software (La Jolla, CA).
Results
Patient demographics and baseline characteristics
A total of 22 patients (7 males, 15 females) were enrolled on the study and were evaluable for response (Table 1). The median age was 64 years (range, 25-76 years). Fifty percent (11/22) of study patients had a normal karyotype. Ten of these patients (91%) had further molecular testing, and 2 were found to have mutated NPM1 without a FLT-3 internal tandem duplication mutation. Using cytogenetic and molecular characteristics, risk was stratified according to the European LeukemiaNet (ELN) prognostic scoring system13 ; 8 (36%) were favorable, 10 (45%) were intermediate, 3 (14%) were adverse, and 1 (5%) had an undetermined risk status. Because 2 peptides in the GPS vaccine directly activate exclusively CD8+ T cells in an HLA-dependent context, HLA genotyping data were obtained for all patients prior to vaccination. Nine (41%) patients were positive for the HLA-A*02:01 serotype, and these were the only patients subsequently tested for CD8+ IR. Overall, 95% of patients received an anthracycline-cytarabine induction regimen, with 41% and 55% of patients receiving 1 or 2 and 3 or 4 postremission treatments, respectively. All patients completed any planned upfront antileukemic therapy prior to study enrollment and were in CR1 according to standard criteria.12,13 All patients had measurable WT1 transcript levels in bone marrow cells at study initiation. The median time in CR1 prior to receiving the GPS vaccine was 8 months, with a range of 2 to 22 months.
Demographics and baseline characteristics
| Parameter . | Values . |
|---|---|
| Patients treated | 22 (100) |
| Male/female | 7/15 |
| Age, median (range), y | 64 (25-76) |
| KPS, median (range) | 90 (80-100) |
| HLA-A* 02:01 positive | 9 (40.9) |
| Primary de novo AML | 22 (100) |
| ELN risk stratification | |
| Favorable | 8 (36) |
| Intermediate | 10 (46) |
| Adverse | 3 (14) |
| Undetermined | 1 (4) |
| Prior therapy | |
| Induction: Ara-C and anthracycline | 21 (96) |
| 7+3 (D60) | 9 (41) |
| 7+3 (D90) | 8 (37) |
| 7+3(I) | 1 (4) |
| 5+3 (I) | 3 (14) |
| Postremission therapy | 21 (96) |
| 1-2 cycles | 9 (41) |
| HiDAC based | 4 (18) |
| IDAC based | 5 (23) |
| 2-4 cycles | 13 (59) |
| HiDAC based | 8 (36) |
| IDAC based | 5 (23) |
| Time to GPS from CR1, median (range), mo | 8 (2-22) |
| Parameter . | Values . |
|---|---|
| Patients treated | 22 (100) |
| Male/female | 7/15 |
| Age, median (range), y | 64 (25-76) |
| KPS, median (range) | 90 (80-100) |
| HLA-A* 02:01 positive | 9 (40.9) |
| Primary de novo AML | 22 (100) |
| ELN risk stratification | |
| Favorable | 8 (36) |
| Intermediate | 10 (46) |
| Adverse | 3 (14) |
| Undetermined | 1 (4) |
| Prior therapy | |
| Induction: Ara-C and anthracycline | 21 (96) |
| 7+3 (D60) | 9 (41) |
| 7+3 (D90) | 8 (37) |
| 7+3(I) | 1 (4) |
| 5+3 (I) | 3 (14) |
| Postremission therapy | 21 (96) |
| 1-2 cycles | 9 (41) |
| HiDAC based | 4 (18) |
| IDAC based | 5 (23) |
| 2-4 cycles | 13 (59) |
| HiDAC based | 8 (36) |
| IDAC based | 5 (23) |
| Time to GPS from CR1, median (range), mo | 8 (2-22) |
Values are n (%) unless otherwise noted.
7+3, cytarabine (200 mg/m2) × 7 days + anthracycline × 3 days; Ara-C, cytarabine; D60, daunorubicin 60 mg/m2; D90, daunorubicin 90 mg/m2; HiDAC, high-dose cytarabine 2000 to 3000 mg/m2 for 6 to 12 doses; I, idarubicin 12 mg/m2; IDAC, intermediate-dose cytarabine 1000 to 1500 mg/m2 for 6 to 12 doses; KPS, Karnofsky performance status.
Patient outcomes
Of the 22 patients, 14 (64%) received the planned 6 vaccinations and 10 (46%) completed all 12 vaccinations. Overall, 15 (68%) patients relapsed: 10 while receiving vaccine, 4 after the entire series of 12 vaccinations, and 1 patient 13 months following discontinuation of therapy secondary to a delayed- type hypersensitivity reaction. Four of the 15 patients relapsed after 1 vaccination, which is likely an insufficient amount of time to induce an IR. Ten relapsed patients died due to complications from progressive leukemia. Five of the 15 relapsed patients underwent HSCT following successful reinduction chemotherapy. Three of these patients remained alive without evidence of recurrent disease, ranging from 11 to 31 months post-HSCT. There was no statistically significant difference in either relapse rate or OS in patients who were vaccinated prior to spending 8 months in CR1 compared with those vaccinated at or after this time point.
Survival from diagnosis, CR1, and first GPS treatment
From the first GPS treatments, 19 of 22 (86%) patients were evaluable for survival at 3 years, and 9 of these 19 evaluable patients (47%) were alive for ≥3 years. Consequently, the study met its prespecified end point of ≥34% OS at 3 years, warranting additional clinical investigations of the GPS vaccine in the treatment of AML.
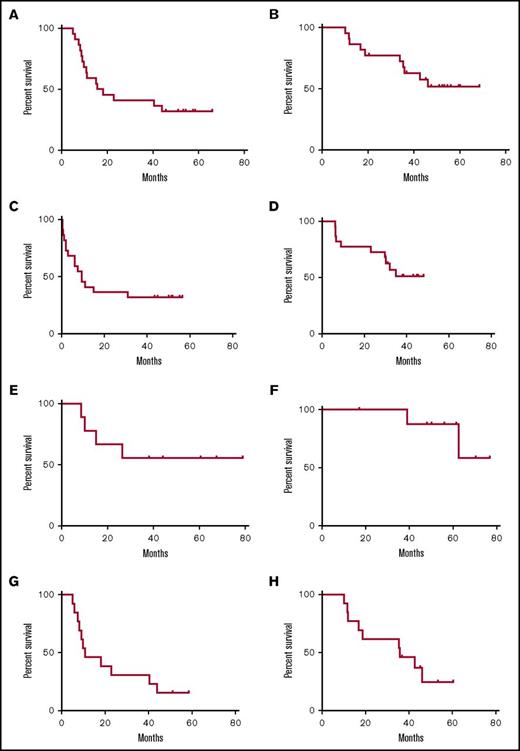
From CR1, the median DFS was 16.9 months (Figure 1A). The median OS from diagnosis (Figure 1B) was not reached but is poised to reach or exceed 67.6 months (5.6 years by log-rank analysis). The median EFS from the time of first GPS vaccination was 9.4 months, whereas the median OS from the time of vaccination has not been reached (Figure 1C-D). The probabilities of EFS at 6 and 9 months postvaccine were 64% (CI, 40%, 80%) and 54% (CI, 32%, 72%), respectively. Likewise, the probabilities of OS at 6 and 9 months postvaccine were 100% (CI, 100%, 100%) and 77% (CI, 54%, 90%), respectively.
Survival curves for vaccinated patients. (A) DFS (from time of CR) for entire cohort. (B) OS (from time of diagnosis) for entire cohort. (C) EFS (from time of first vaccination). (D) OS (from time of first vaccination). (E) DFS (from time of CR) for patients <60 years. (F) OS (from time of diagnosis) for patients <60 years. (G) DFS (from time of CR) for patients ≥60 years. (H) OS (from time of diagnosis) for patients ≥60 years.
Survival curves for vaccinated patients. (A) DFS (from time of CR) for entire cohort. (B) OS (from time of diagnosis) for entire cohort. (C) EFS (from time of first vaccination). (D) OS (from time of first vaccination). (E) DFS (from time of CR) for patients <60 years. (F) OS (from time of diagnosis) for patients <60 years. (G) DFS (from time of CR) for patients ≥60 years. (H) OS (from time of diagnosis) for patients ≥60 years.
Survival in subgroups
For patients <60 years of age (n = 9), neither the median DFS nor OS time was reached (Figure 1E-F). However, for patients in the older cohort (age ≥60 years; n = 13), median DFS from CR1 was 10.8 months, and median OS time postdiagnosis was 35.8 months (Figure 1G-H). Likewise, from the first GPS treatment, the median EFS and OS times in the older cohort were 7.8 and 30.2 months, respectively. Lastly, landmark EFS in the older cohort at 6 and 9 months after the first GPS treatment was 54% (CI, 25%, 76%) and 46% (CI, 19%, 70%), respectively, whereas OS 6 and 9 months after the first GPS were 100% (CI, 100%, 100%) and 62% (CI, 31%, 82%).
Stratifying OS from diagnosis according to ELN risk categories yielded median OS times that were not reached in the favorable (n = 8) and the intermediate (n = 10) groups and 11.9 months in the adverse group (n = 3). Median DFS from CR1 was 27.7, 20.5, and 9.1 months for the favorable, intermediate, and adverse groups, respectively. The median time for follow-up from diagnosis was 43 months, with a range of 10-68 months.
MRD
Baseline WT1 transcript measurements varied among the patients (reported as absolute copy number; range, 1.37-252.9; mean, 64.9 ± 76.11). Nineteen of 22 of the trial patients (86%) had serial RT-PCR measurements available for analysis. There was no correlation between baseline WT1 transcript levels and clinical outcomes. Increases in WT1 transcript levels over baseline was seen in 67% (8/12) of relapsed patients measured with a >1 log increase in 50% (6/12) of these patients at the time of or shortly before clinical relapse these. Although there was some variability among transcript levels measured serially in the patients who remain in CR1, 6 of 7 (86%) had either decreasing or stable (≤1 log change) transcripts compared with baseline at relatively low levels of expression (supplemental Data; Figure 1).
All 22 patients had baseline immunophenotyping preformed using a standard 4- to 6-color panel, which included myeloid, T-cell, and B-cell markers. Only 1 of the 22 patients tested (4%) had a flow cytometric evidence of aberrancy (supplemental Data).
IRs
Immunologic correlative data were available from 14 of the 22 patients (64%) accrued to the study.
CD4+ response
Nine patients were tested for a CD4+ IR, and 4 of these (44%) had a detectable IR. One patient responded to all 4 peptides, 3 patients responded to the long peptide 331, and 3 patients responded to the heteroclitic peptide 1221A1. Representative data of the CD4+ T-cell proliferation assay from patient 4 are shown (Figure 2). Of the 5 patients who were negative for CD4+ response, 2 were tested at relapse following 1 vaccination. One other patient was tested following vaccine dose 6; the results were negative, and this patient subsequently relapsed following vaccine dose 9. Another patient was tested following vaccine dose 6; the results were negative and remained negative at repeat testing upon relapse following vaccine dose 11. No clear correlation with HLA-DR subtype was seen among the responders, although the number of patients expressing individual haplotypes was too small to draw conclusions. Interestingly, none of the patients who had a positive CD4+ response in this assay relapsed.
CD4+T cell proliferation. CD4+ T cells from prevaccination (time 0), post-6 GPS vaccinations (time 6), and post-12 GPS vaccinations (time 12) from patient 4 were incubated with indicated peptides at 20 µg/mL (“20”) or 50 µg/mL (“50”) for 5 days, and 1 µCi [3H]-thymidine was added to the cultures for 20 hours. The cell proliferation was determined by [3H]-thymidine incorporation. Data are mean ± SD from quadruplicate cultures. Negative controls were also used (incubation with no peptide present and with irrelevant peptides [B2A2 long fragment of BCR-ABL]). After 6 vaccinations, cell proliferation increased sixfold to 331, eightfold to 427, 11-fold to 122A1, and 13-fold to 122A (P = .008) There was no significant dose dependency of the peptides, and the CD4+ T-cell response was sustained through the period of vaccination, although the degree varied among the individual peptides.
CD4+T cell proliferation. CD4+ T cells from prevaccination (time 0), post-6 GPS vaccinations (time 6), and post-12 GPS vaccinations (time 12) from patient 4 were incubated with indicated peptides at 20 µg/mL (“20”) or 50 µg/mL (“50”) for 5 days, and 1 µCi [3H]-thymidine was added to the cultures for 20 hours. The cell proliferation was determined by [3H]-thymidine incorporation. Data are mean ± SD from quadruplicate cultures. Negative controls were also used (incubation with no peptide present and with irrelevant peptides [B2A2 long fragment of BCR-ABL]). After 6 vaccinations, cell proliferation increased sixfold to 331, eightfold to 427, 11-fold to 122A1, and 13-fold to 122A (P = .008) There was no significant dose dependency of the peptides, and the CD4+ T-cell response was sustained through the period of vaccination, although the degree varied among the individual peptides.
CD8+ response
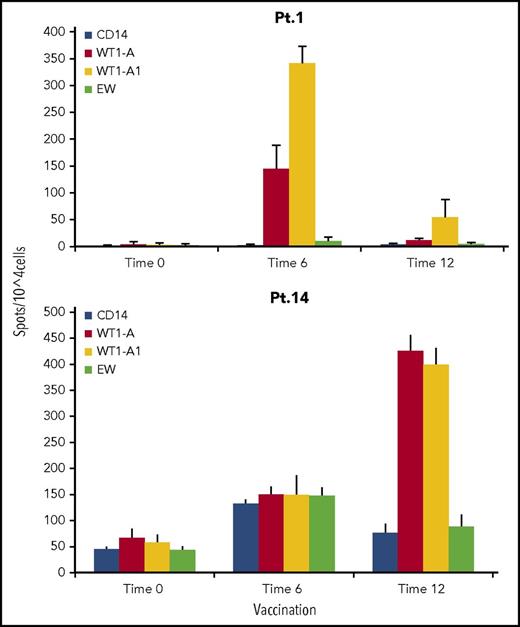
Seven of the 9 patients who were HLA-A*02 positive were tested for a CD8+ T-cell response to the native WT1-A peptide, as well as the synthetic heteroclitic WT1-A1 peptide, by IFN-γ ELISPOT assay or tetramer staining. Six of 7 patients (86%) had a positive CD8+ IRs. These assays also evaluated whether the vaccinating heteroclitic peptide (WT1-A1) generated IRs against the native peptide (WT1-A). This cross-reactivity is crucial to vaccine strategy, as cytotoxic T cells that are induced need to recognize antigens in the form it exists in the leukemia cells of the patient. All 5 patients tested with the ELISPOT assay had significant increases in IFN-γ secreting cells to the native WT1-A peptide following vaccination. Representative responses from 2 patients are shown in Figure 3.
IFN-γ secretion by CD8+cells. CD3+ T cells from patient 1 (Pt. 1) 1 and patient 14 (Pt. 14) were stimulated with WT1-A (native) peptide. IFN-γ–secreting T cells were measured by ELISPOT assay after challenge with indicated peptides. Controls were no peptide (only CD14+ antigen-presenting cells) or irrelevant Ewing sarcoma–derived peptide (EW). Data are mean ± SD from quadruplicate cultures from before GPS administration (time 0), after 6 GPS vaccinations (time 6), and after 12 GPS vaccinations (time 12). A positive response was defined as meeting all of the following criteria: (1) a twofold increase in the IFN-γ–secreting cells and in frequencies of CD8+ WT1-A tetramer–positive cells over the controls, (2) P < .05, and (3) >30 spots per 105 cells. Results indicate that a WT1-A–specific response can be generated by challenge to both native and heteroclitic peptide, suggesting processing and presentation of the antigen. In patient 1, a stronger response was seen after 6 vaccinations and then faded. In patient 14, a stronger response was seen after 12 vaccinations.
IFN-γ secretion by CD8+cells. CD3+ T cells from patient 1 (Pt. 1) 1 and patient 14 (Pt. 14) were stimulated with WT1-A (native) peptide. IFN-γ–secreting T cells were measured by ELISPOT assay after challenge with indicated peptides. Controls were no peptide (only CD14+ antigen-presenting cells) or irrelevant Ewing sarcoma–derived peptide (EW). Data are mean ± SD from quadruplicate cultures from before GPS administration (time 0), after 6 GPS vaccinations (time 6), and after 12 GPS vaccinations (time 12). A positive response was defined as meeting all of the following criteria: (1) a twofold increase in the IFN-γ–secreting cells and in frequencies of CD8+ WT1-A tetramer–positive cells over the controls, (2) P < .05, and (3) >30 spots per 105 cells. Results indicate that a WT1-A–specific response can be generated by challenge to both native and heteroclitic peptide, suggesting processing and presentation of the antigen. In patient 1, a stronger response was seen after 6 vaccinations and then faded. In patient 14, a stronger response was seen after 12 vaccinations.
The frequency of WT1-A–specific CD8+ T cells was assessed by tetramer staining in 5 patients. Two of the HLA-A*02–positive patients tested demonstrated a small number of CD8+ tetramers present at baseline. Overall, 4 of 5 patients tested (80%) demonstrated increased WT1-A tetramer-positive cells in the CD8+ population after vaccination. Representative tetramer data from 2 patients are illustrated in Figure 4. Three patients in the above group were also tested via ELISPOT, and all 3 had a positive result with both assays.
Tetramer assays. CD3+ T cells from patient 1 (A) and patient 14 (B) were stained with WT1-A/HLA-A*02:01 tetramer with CD8+ and other T-cell markers. Percentage of tetramer-positive CD8+ T cells (number shown in the top right of each histogram and highlighted in bold print) were gated on CD3+ events after establishing a lymphocyte gate. Cells from before vaccine (time 0 [T0]), after 6 GPS vaccinations (T6), and after 12 GPS vaccinations (T12) are shown. (A) CD8+ cells only from patient 1 are shown. Cultures were preincubated with native (WT1-A) and heteroclitic (WT1-A1) peptides. The data are controls (no peptide [−]) on the left followed by representative staining from triplicate cultures following antigen exposure (+) on the right. Before vaccination, there were low percentages of tetramer-positive cells in the CD8+ population. (B) Both CD8+ and CD8− cells from patient 14 are shown. Cultures are incubated with WT1-A peptides. Bottom row of histograms show concurrent testing with irrelevant tetramers. After vaccination, a large increase in the percentage of WT1-A–specific CD8+ T cells was noted in primed cultures indicating that vaccination with the heteroclitic peptide can induce T cells that recognize the native sequence.
Tetramer assays. CD3+ T cells from patient 1 (A) and patient 14 (B) were stained with WT1-A/HLA-A*02:01 tetramer with CD8+ and other T-cell markers. Percentage of tetramer-positive CD8+ T cells (number shown in the top right of each histogram and highlighted in bold print) were gated on CD3+ events after establishing a lymphocyte gate. Cells from before vaccine (time 0 [T0]), after 6 GPS vaccinations (T6), and after 12 GPS vaccinations (T12) are shown. (A) CD8+ cells only from patient 1 are shown. Cultures were preincubated with native (WT1-A) and heteroclitic (WT1-A1) peptides. The data are controls (no peptide [−]) on the left followed by representative staining from triplicate cultures following antigen exposure (+) on the right. Before vaccination, there were low percentages of tetramer-positive cells in the CD8+ population. (B) Both CD8+ and CD8− cells from patient 14 are shown. Cultures are incubated with WT1-A peptides. Bottom row of histograms show concurrent testing with irrelevant tetramers. After vaccination, a large increase in the percentage of WT1-A–specific CD8+ T cells was noted in primed cultures indicating that vaccination with the heteroclitic peptide can induce T cells that recognize the native sequence.
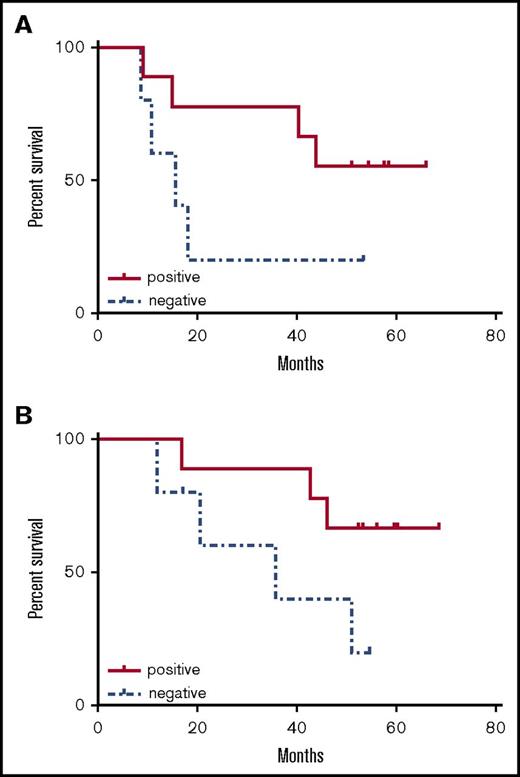
Clinical outcomes were examined in the patients who had either a CD4+ or CD8+ immunologic response (n = 9) vs patients with no response (n = 5) in the correlative assays. There was no difference in immunologic response between patients who had received 0 to 2 vs 3 or 4 cycles of postremission chemotherapy. Comparison of the survival curves between the immunologic responders and nonresponders show clear separation between groups for both DFS and OS (Figure 5), although the small sample size precludes detecting a statistically significant difference.
Survival curves according to immunologic response. (A) DFS (from time of CR; median not reached vs 15.6 months, P = .11). (B) OS (from time of diagnosis; median not reached vs 35.8 months, P = .08).
Survival curves according to immunologic response. (A) DFS (from time of CR; median not reached vs 15.6 months, P = .11). (B) OS (from time of diagnosis; median not reached vs 35.8 months, P = .08).
Safety and toxicity
The vaccine was generally well tolerated and the toxicity profile consistent with other WT1 vaccine–adjuvant combinations21-25 (Table 2). The montanide adjuvant is a known irritant,26 and many of the most frequent toxicities consisted of mild to moderate local reactions and inflammation: injection site reaction (46%), fatigue (32%), skin induration (32%), and injection site pruritus (27%). These toxicities were self-limited and responded to local supportive measures and analgesics. Several transient occurrences of decreased white blood cell, neutrophil, lymphocyte, and platelet counts were noted; these resolved (often on the same day of testing) and resulted in no significant infectious complications or supportive transfusions. None of the patients developed significant hepatic or renal insufficiency, and no episodes of systemic anaphylaxis were observed. Other common toxicities are summarized in Table 2.
Treatment-related toxicity
| Toxic events . | Number of patients (N = 22), n (%) . | ||||
|---|---|---|---|---|---|
| Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | All grades . | |
| Total number of toxic events | 144 | 6 | 61 | 33 | 244 |
| Patients with ≥1 toxic events | 9 (40.9) | 1 (4.5) | 8 (36.4) | 3 (13.6) | 21 (95.5) |
| Injection site reaction | 9 (40.9) | 1 (4.5) | — | — | 10 (45.5) |
| Skin induration | 6 (27.3) | 1 (4.5) | — | — | 7 (31.8) |
| Fatigue | 7 (31.8) | — | — | — | 7 (31.8) |
| Pruritus | 5 (22.7) | 1 (4.5) | — | — | 6 (27.3) |
| Lymphocyte count decreased | — | — | 4 (18.2) | 2 (9.1) | 6 (27.3) |
| Neutrophil count decreased | — | — | 4 (18.2) | 2 (9.1) | 6 (27.3) |
| White blood cell count decreased | — | — | 5 (22.7) | 1 (4.5) | 6 (27.3) |
| Platelet count decreased | — | — | 3 (13.6) | 1 (4.5) | 4 (18.2) |
| Pain | 3 (13.6) | — | — | — | 3 (13.6) |
| Pain in extremity | 3 (13.6) | — | — | — | 3 (13.6) |
| Flushing | 2 (9.1) | — | 1 (4.5) | — | 3 (13.6) |
| Dry skin | 2 (9.1) | — | — | — | 2 (9.1) |
| Rash maculopapular | 2 (9.1) | — | — | — | 2 (9.1) |
| Bone pain | — | 1 (4.5) | 1 (4.5) | — | 2 (9.1) |
| Muscular weakness | 2 (9.1) | — | — | — | 2 (9.1) |
| Skin infection | 2 (9.1) | — | — | — | 2 (9.1) |
| Upper respiratory tract infection | 2 (9.1) | — | — | — | 2 (9.1) |
| Urinary tract infection | 1 (4.5) | 1 (4.5) | — | — | 2 (9.1) |
| Headache | 2 (9.1) | — | — | — | 2 (9.1) |
| Toxic events . | Number of patients (N = 22), n (%) . | ||||
|---|---|---|---|---|---|
| Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | All grades . | |
| Total number of toxic events | 144 | 6 | 61 | 33 | 244 |
| Patients with ≥1 toxic events | 9 (40.9) | 1 (4.5) | 8 (36.4) | 3 (13.6) | 21 (95.5) |
| Injection site reaction | 9 (40.9) | 1 (4.5) | — | — | 10 (45.5) |
| Skin induration | 6 (27.3) | 1 (4.5) | — | — | 7 (31.8) |
| Fatigue | 7 (31.8) | — | — | — | 7 (31.8) |
| Pruritus | 5 (22.7) | 1 (4.5) | — | — | 6 (27.3) |
| Lymphocyte count decreased | — | — | 4 (18.2) | 2 (9.1) | 6 (27.3) |
| Neutrophil count decreased | — | — | 4 (18.2) | 2 (9.1) | 6 (27.3) |
| White blood cell count decreased | — | — | 5 (22.7) | 1 (4.5) | 6 (27.3) |
| Platelet count decreased | — | — | 3 (13.6) | 1 (4.5) | 4 (18.2) |
| Pain | 3 (13.6) | — | — | — | 3 (13.6) |
| Pain in extremity | 3 (13.6) | — | — | — | 3 (13.6) |
| Flushing | 2 (9.1) | — | 1 (4.5) | — | 3 (13.6) |
| Dry skin | 2 (9.1) | — | — | — | 2 (9.1) |
| Rash maculopapular | 2 (9.1) | — | — | — | 2 (9.1) |
| Bone pain | — | 1 (4.5) | 1 (4.5) | — | 2 (9.1) |
| Muscular weakness | 2 (9.1) | — | — | — | 2 (9.1) |
| Skin infection | 2 (9.1) | — | — | — | 2 (9.1) |
| Upper respiratory tract infection | 2 (9.1) | — | — | — | 2 (9.1) |
| Urinary tract infection | 1 (4.5) | 1 (4.5) | — | — | 2 (9.1) |
| Headache | 2 (9.1) | — | — | — | 2 (9.1) |
For toxicity grading, National Cancer Institute Common Toxicity Criteria for Adverse Events version 4.0 was used. Table is ordered by decreasing proportions of total patients having at least 1 toxic event. Toxic events occurring in only 1 patient were excluded.
N, total number of patients; n, number of patients meeting specified criteria; TEAE, treatment-emergent adverse event.
Two patients discontinued therapy due to probable hypersensitivity reactions that were believed to be related to vaccination. One patient developed 2 episodes of a maculopapular rash (grade 1) and then developed, on a separate occasion, an episode of flushing (grade 3) immediately following the second vaccine administration. The patient remained hemodynamically stable and developed no signs of anaphylaxis, but, given the severity of the episode, he discontinued further vaccinations. The patient subsequently relapsed and died of complications of recurrent AML 29 months after discontinuing vaccine therapy. The second patient developed bone pain, dyspnea, flushing, and noncardiac chest pain (grade 3) leading to hospitalization immediately following the fourth vaccine dose. The patient responded to standard supportive measures, and the symptoms resolved the same day without further incident. The patient recovered without sequelae and remains alive as of the last study follow-up (∼36 months after discontinuing GPS).
Discussion
The WT1 protein has been highly rated as an immunologic target by a National Cancer Institute prioritization project.27 Several groups have vaccinated patients with WT1 peptides and reported both immunologic and clinical responses.21-23,28 Generally, these studies have used HLA-restricted class I peptides (either HLA-A*02:01 or HLA-A*24:02) to elicit CD8+ cytotoxic T lymphocyte (CTL) responses. Potential therapeutic activity has been demonstrated by both clinical response and decreases in measurable MRD (WT1) transcript levels. These studies have supported the hypothesis that the WT1 antigen is a validated immunologic target that can be used to effectively treat myeloid malignancies.
Several challenges, however, exist for generating effective responses to oncofetal self-antigens, such as WT1. Although WT1 is expressed at higher levels in neoplastic cells of a variety of types, there may be low level expression in normal cells.20 The generation of low-avidity CTLs may be favored as part of the normal physiologic process of developing immunologic tolerance. As a consequence, high-avidity CTLs, which should be more effective in responding to tumor, are deleted from the T-cell repertoire early in development and unavailable for recruitment in response to leukemia. Such a scenario is supported in a study by Rezvani et al, where the effect of serial vaccine boosters failed to generate the high-avidity CTLs needed to establish long-term memory and a significant antileukemia effect.24 Alternatively, Nakae et al have described decreased responses among patients who harbor a population of effector memory T cells, which they describe as having an “activated” state according to gene expression profiling.29 T cells that are constitutively activated may represent an exhausted state and are therefore unable to respond to an exogenous priming stimulus like a vaccine peptide resulting in relative anergy.
Several strategies were adopted in designing the WT1 vaccine to potentially address these issues. The vaccine contains both major histocompatibility complex (MHC) class I and II peptides with the synthetic heteroclitic analog WT1-A1 peptide designed primarily for class I HLA-A*02:01, a type commonly expressed in the US population. In addition, MHC peptide-binding algorithms predict that multiple class I and II epitopes may also be found within the sequences of the 4 peptides, if they are appropriately processed.14,19 These include HLA -A*02:01, HLA-A*03, HLA-A*24:02, HLA-B*15:01, HLA-B*39:01, HLA-B*07:02, and HLA-B*08,-B27:05, among others, and a wide array of HLA-DRB1 types covering much of the US population. The patients who made responses to the vaccine in the 3 AML trials, including this trial, were characterized by a widely heterogeneous group of HLA types and subtypes.8,9 The substitution of a single amino acid in the native WT1 sequence improves the immunogenicity of the vaccine peptide by virtue of its improved binding affinity to HLA on antigen-presenting cells, resulting in a more stable MHC/peptide complex on the cell surface.19,30 The longer peptides were designed to stimulate a broad range of class II molecules, allowing for more widespread application across the population tested. While the WT1-A1 peptide is designed to induce an enhanced CD8+ CTL response, the class II peptides will primarily stimulate CD4+ helper cells. The induction and maintenance of a memory CTL response requires CD4+ T cell help, and by providing for activation of both T-cell subsets, we hoped to induce a more robust, long-lived IR that could translate into clinical efficacy.31,32 Lastly, peptide 122A1 contains both CD4+ and CD8+ epitopes known to be immunogenic within the same sequence.
With regard to safety in this study, a total of 22 patients were treated and evaluable for response. Overall, WT1 vaccinations in AML patients were safe and well tolerated, with a majority of TEAEs being of mild or moderate severity (150/244 [61.5%]). There were no reported deaths, and a majority of grade 3/4 TEAEs were unrelated to study treatment. The majority of injection site reactions, skin induration, and pruritus were easily managed with supportive care. A single patient experienced a significant adverse event that led to study discontinuation, and 1 additional patient discontinued the study due to probable hypersensitivity reactions.
Although this is a relatively small phase 2 study enrolling 22 patients, several clinical observations regarding the potential for therapeutic efficacy can be made. The study met its prespecified end point of ≥34% actual OS rate at 3 years, justifying future clinical investigations of this WT1 vaccine. The actual OS rate of 47.4% at 3 years postvaccine treatment exceeded historical published data of 20% to 25% by 2.4- to 1.9-fold (or 240% to 190%), respectively. In addition, 11 of the 22 patients (50%) were alive at the time of their last assessment. Nine of these remained in CR1, while 3 relapsed during or following vaccine administration and were successfully salvaged with HSCT. The median DFS from CR1 was 16.9 months, with the median OS not reached, but poised to be at least 67.6 months. These survival outcomes are superior to published results for similar patients treated with conventional postremission therapies and compare favorably to subsets of patients treated with HSCT.33-41
Six of the 7 HLA-A*02:01-type patients (86%) had a CD8+ response as evidenced by a positive result in either the IFN-γ ELISPOT (5 of 5; 100%) or WT1-A tetramer assay (4 of 5; 80%). Four of the 9 patients tested (44%) for CD4+ proliferative response had a positive result. Seven of these patients were not HLA-A*02:01 type. There was no clear correlation of CD4+ proliferative response with HLA-DR type suggesting reactivity across multiple HLA subtypes, but the numbers are very small. Two of the patients were tested early upon relapse following vaccine 1. Although both had a negative CD4+ proliferative response, one was of HLA-A*02:01 type and had an increase in WT1-A tetramer staining to CD8+ T cells. Despite the generation of CD8+ CTLs, the patient had overt clinical relapse, again suggesting either that the duration of exposure to the vaccine was inadequate to generate a clinically sufficient response or that even early CD8+ responses alone are inadequate to generate an antileukemia effect. The one other HLA-A*02:01–positive patient who underwent both CD4+ and CD8+ testing had positive results in both assays and is alive without evidence of disease 71 months since diagnosis. One other patient with a negative result remains in CR1 35 months after achieving remission.
Immunologic responses may translate into improved clinical outcomes, as there is a suggestion of improved DFS and OS in patients who had a positive result in one of the correlative assays. Although this difference is not statistically significant given the small number of patients treated in this study, the observation is intriguing enough to warrant further study in the context of a larger randomized clinical trial.
There are, however, several potential caveats in interpreting the results of this trial with regard to therapeutic efficacy. The study group is relatively heterogeneous, with several different prognostic groups contained within the data set, which could skew outcomes.42 The patients enrolled in this study may represent those with the best possible response in that they not only were able to achieve CR1 but also remained in CR1 for a median of 8 months prior to vaccination. The immunologic correlates provide information regarding biologic effect but are not surrogates for clinical response. Several patients did not exhibit an IR yet still did well. We do not know whether the negative results were false negatives, as the assays are not sensitive or directed at most relevant key epitopes, and not all technical issues regarding testing of this type have been resolved to date.43
Despite these potentially confounding factors, this trial supports the continued investigation of WT1 vaccination as a strategy for AML postremission therapy. The vaccine can be administered on an outpatient basis with minimal toxicity in most patients. This cohort of patients appears to do well compared with historical outcome data, and the immunologic correlates that have been used in this study show the generation of an IR in most tested patients. The ultimate question of clinical efficacy, however, will need to be addressed in a larger trial in a more homogeneous patient population. A pivotal randomized study addressing this question is planned in the near future.
Acknowledgments
This research was funded in part through the National Institutes of Health, National Cancer Institute Cancer Center Support Grant P30 CA008748, National Institutes of Health, National Cancer Institute grant P01 CA 23766, and the Sellas Life Sciences Group (P.G.M.).
Authorship
Contribution: P.G.M. designed the study, treated patients on study, analyzed the data, and wrote the manuscript; T.D., R.Z, M.F., and M.E.A. performed correlative laboratory studies; Y.B., R.J.B., and S.M.C. were involved in data collection and patient follow-up; M.F., T.R., J.G.J., R.R., J.H.P., D.D., and M.S.T. treated patients and provided follow-up data; L.K. and N.S. were involved with data analysis; D.A.S. designed the study and wrote the manuscript; and all authors contributed to review of the manuscript.
Conflict-of-interest disclosure: P.G.M. receives research funding from Sellas Life Sciences. L.K. and N.S. are employees of Sellas Life Sciences Group and receive salary and stock options as compensation for their employment. D.A.S. is inventor of the vaccine and a consultant to Sellas. The remaining authors declare no competing financial interests.
The current affiliation for R.Z., M.F., T.R., and J.G.J. is Division of Hematology/Oncology, Department of Medicine, Columbia University Medical Center, New York, NY.
Correspondence: Peter G. Maslak, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: maslakp@mskcc.org.
The full-text version of this article contains a data supplement.

![Figure 2. CD4+ T cell proliferation. CD4+ T cells from prevaccination (time 0), post-6 GPS vaccinations (time 6), and post-12 GPS vaccinations (time 12) from patient 4 were incubated with indicated peptides at 20 µg/mL (“20”) or 50 µg/mL (“50”) for 5 days, and 1 µCi [3H]-thymidine was added to the cultures for 20 hours. The cell proliferation was determined by [3H]-thymidine incorporation. Data are mean ± SD from quadruplicate cultures. Negative controls were also used (incubation with no peptide present and with irrelevant peptides [B2A2 long fragment of BCR-ABL]). After 6 vaccinations, cell proliferation increased sixfold to 331, eightfold to 427, 11-fold to 122A1, and 13-fold to 122A (P = .008) There was no significant dose dependency of the peptides, and the CD4+ T-cell response was sustained through the period of vaccination, although the degree varied among the individual peptides.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/2/3/10.1182_bloodadvances.2017014175/3/m_advances014175f2.jpeg?Expires=1767701827&Signature=oMUWzxk9nVpZYjj04lwF4q7bi80w84y8iinSIJXijo-iHJknWvjV71cQNncl6PR0wstpm1B-k3jG3GlbU2Ukpf9x7d7iyJAREjZsG3niSLGEVmFnXEoADjQjALtUsma9m56BX73EEFv0RPA5zO8qTE3VACzHLBnDLHtb0x2y0AeRc0SE66K42xXCRRsmarzO~NrxHOVyYLIsfSLT-PKuGRo~sqLsshMMiuioQKtTtNUOqYiHTV44dhlyEQj1pCW8hktA5eREEyiMcJlI4-8cfU5g8nJ-k4wPrmijHD-MYupXilEdmIPdOB-P8v8pXw1AII-Hf5S16D6JuH~qWj3VnA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Tetramer assays. CD3+ T cells from patient 1 (A) and patient 14 (B) were stained with WT1-A/HLA-A*02:01 tetramer with CD8+ and other T-cell markers. Percentage of tetramer-positive CD8+ T cells (number shown in the top right of each histogram and highlighted in bold print) were gated on CD3+ events after establishing a lymphocyte gate. Cells from before vaccine (time 0 [T0]), after 6 GPS vaccinations (T6), and after 12 GPS vaccinations (T12) are shown. (A) CD8+ cells only from patient 1 are shown. Cultures were preincubated with native (WT1-A) and heteroclitic (WT1-A1) peptides. The data are controls (no peptide [−]) on the left followed by representative staining from triplicate cultures following antigen exposure (+) on the right. Before vaccination, there were low percentages of tetramer-positive cells in the CD8+ population. (B) Both CD8+ and CD8− cells from patient 14 are shown. Cultures are incubated with WT1-A peptides. Bottom row of histograms show concurrent testing with irrelevant tetramers. After vaccination, a large increase in the percentage of WT1-A–specific CD8+ T cells was noted in primed cultures indicating that vaccination with the heteroclitic peptide can induce T cells that recognize the native sequence.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/2/3/10.1182_bloodadvances.2017014175/3/m_advances014175f4.jpeg?Expires=1767701827&Signature=DipBRrZMciwAh38m90mBBMz1rdzE3Nmt-RMI30SFXh6Rjsnj7ES2~r0T7lS89O6J-3-QPwzYvVfWkekwbPW8rnUMo9QkYkRrZ0nHSIyCBVldvwXhmE6K4ng~sLTM1MqTjChcSiVZyIs2l17FZI8o4L-WqkTJb5eu9CkxPew4PePiLFbuGjcJ8AUh2zkiKZvIT8jEF0RtPyZYuYWBEGIZJ2HVbIMnrd0gRnrsodKovcEAzRdaf4CzeKuABGIu-tPhsAI3vOR9EsoUSkvK6-14GNpL1wYA17YNi9kudCb55zZ9pQ7VsjnIEl7EajRBFS9M5xRJerINJ7yT89cboWCTTw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)