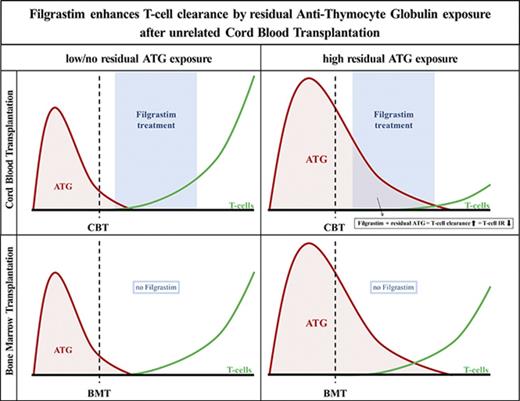
Key Points
Residual ATG exposure delays CD4+ T-cell reconstitution more severely after CBT than after BMT.
Filgrastim (G-CSF), given early after CBT, enhances ATG-mediated T-cell clearance in patients with residual ATG exposure.
Abstract
Residual antithymocyte globulin (ATG; Thymoglobulin) exposure after allogeneic hematopoietic (stem) cell transplantation (HCT) delays CD4+ T-cell immune reconstitution (CD4+ IR), subsequently increasing morbidity and mortality. This effect seems particularly present after cord blood transplantation (CBT) compared to bone marrow transplantation (BMT). The reason for this is currently unknown. We investigated the effect of active-ATG exposure on CD4+ IR after BMT and CBT in 275 patients (CBT n = 155, BMT n = 120; median age, 7.8 years; range, 0.16-19.2 years) receiving their first allogeneic HCT between January 2008 and September 2016. Multivariate log-rank tests (with correction for covariates) revealed that CD4+ IR was faster after CBT than after BMT with <10 active-ATG × day/mL (P = .018) residual exposure. In contrast, >10 active-ATG × day/mL exposure severely impaired CD4+ IR after CBT (P < .001), but not after BMT (P = .74). To decipher these differences, we performed ATG-binding and ATG-cytotoxicity experiments using cord blood– and bone marrow graft–derived T-cell subsets, B cells, natural killer cells, and monocytes. No differences were observed. Nevertheless, a major covariate in our cohort was Filgrastim treatment (only given after CBT). We found that Filgrastim (granulocyte colony-stimulating factor [G-CSF]) exposure highly increased neutrophil-mediated ATG cytotoxicity (by 40-fold [0.5 vs 20%; P = .002]), which explained the enhanced T-cell clearance after CBT. These findings imply revision of the use (and/or timing) of G-CSF in patients with residual ATG exposure.
Introduction
Pediatric patients with primary immune deficiencies (PIDs), metabolic disorders, or refractory hematological malignancies often receive an allogeneic hematopoietic (stem) cell transplantation (HCT) as last-resort treatment. T-cell immune reconstitution (IR) after HCT is pivotal for disease control and reduces the probability of transplantation-related mortality.1-7 To prevent rejection of the graft and graft-versus-host disease (GVHD), antithymocyte globulin (ATG; Thymoglobulin, Genzyme) was introduced to conditioning regimens. ATG has a half-life of up to ∼30 days8 and is often still present during the first weeks after HCT. It has been shown that this can result in a delayed T-cell IR,9-12 which is associated with an increased risk of relapse and viral reactivations and subsequently with lower survival chances.1-7
In a recent ATG pharmacokinetic/pharmacodynamic analysis, we found that CD4+ T-cell IR (CD4+ IR) after cord blood transplantation (CBT) was affected more by residual ATG than CD4+ IR after bone marrow transplantation (BMT).10 Nevertheless, in patients undergoing a CBT without ATG in the conditioning, very rapid T-cell reconstitution associated with very low incidences of viral reactivations and relapse was observed.13,14 Although the lower T-cell dose in cord blood (CB) grafts does not explain the higher effect of ATG on IR,15 other possible covariates that may influence T-cell reconstitution, such as steroid-treated acute GVHD (aGVHD) after HCT, have not yet been evaluated in these analyses. Therefore, the underlying mechanism for the suggested higher impact of ATG on CD4+ IR after CBT is not yet understood.
Understanding the biological mechanisms is important when investigating differences in ATG cytotoxicity on CB- or bone marrow (BM)–derived target cells. Thymoglobulin consists of polyclonal immunoglobulin G (IgG) antibodies generated against human thymus cells. After binding to its targets, ATG mediates its cytotoxicity either through direct apoptosis via the Fas/FasL pathway, complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC) by natural killer (NK) cells or neutrophils, and antibody-dependent cellular phagocytosis (ADCP) by monocytes and macrophages.16-20 ATG affects early T-cell reconstitution by depleting graft-derived T cells that are important for T-cell recovery through homeostatic peripheral expansion. The CB-graft cells, most of which are naive, might contain more epitopes for ATG, which may make them more susceptible to ATG-mediated cytotoxicity than BM-graft cells. Nevertheless, the effect of ATG levels after HCT on the reconstitution of immune cell subsets, or on CB/BM-graft–derived immune cells, has not yet been evaluated.
In this study, we aim to identify a biological explanation why CD4+ IR is affected more by residual ATG exposure after CBT than after BMT. We performed multivariate analysis to evaluate the effect of residual ATG exposure on CD4+ IR after pediatric CBT and BMT, while correcting for other covariates affecting CD4+ IR. Furthermore, we studied the effect of residual ATG exposure after HCT on lymphocyte, T-cell, B-cell, NK-cell, monocyte, and neutrophil reconstitution in vivo and compared ATG binding and ATG cytotoxicity between CB- and BM-graft immune cells in vitro. The results of this study may have direct treatment-related implications to improve T-cell IR and subsequently outcome after HCT.
Methods
Patients and treatment
We performed a retrospective cohort analysis on prospective data from consecutive pediatric patients receiving their first allogeneic HCT between January 2008 and September 2016 at the University Medical Center Utrecht, The Netherlands. Active ATG (level of target-binding ATG) was measured retrospectively in EDTA blood plasma. Patients and donors were enrolled, and data were collected and registered prospectively only after written informed consent in accordance with the Helsinki Declaration. The study was approved by the local ethical committee (trial numbers 05-143 and 11-063k).
Conditioning regimens were applied according to standard protocols. For PID and non-PID benign disorders, the conditioning was Fludarabine 160 mg/m2 + exposure-targeted busulfan (to 90 mg × h/L); for malignant indications, it was fludarabine 40 mg/m2 + clofarabine 120 mg/m2 + exposure-targeted busulfan (to 90 mg × h/L); and for BM failure patients, cyclophosphamide (120 mg/kg) + fludarabine (150 mg/m2) was used. Conditioning and supportive care was homogeneous among CBT and BMT/PB cell transplant recipients. Patients who received ATG in their conditioning were given 10 mg/kg Thymoglobulin (Genzyme) from 2008 to 2010. After 2010, patients weighing >40 kg received 7.5 mg/kg. GVHD prophylaxis consisting of cyclosporin A with a target trough concentration of 200 to 250 mg/L combined with prednisolone 1 mg/kg after CBT. Prednisolone was tapered in 2 weeks starting 4 weeks after HCT in benign disorders and 1 week after engraftment in malignant disorders. Cyclosporin A was combined with methotrexate in patients receiving a BM graft (days +1, +3, and +6). Cyclosporin A was continued until 3 months (malignant disease) or 6 months (benign disorders) after HCT. In the absence of aGVHD, all patients received a steroid between 4 (malignancies) and 6 (benign conditions) weeks. Patients were prophylactically treated with acyclovir; treatment of viral reactivations of adenovirus, cytomegalovirus, and Epstein-Barr virus was started after reaching 1000 copies/mL. All patients received gut decontamination and Pneumocystis jiroveci prophylaxis according to local protocol as previously described.13 Patients were treated in high-efficiency, positive-pressure, particle-free isolation rooms. All CBT patients received 10 mg/kg granulocyte colony-stimulating factor (G-CSF; Neupogen) from day +7 after HCT until neutrophils were >2000 cells/μL.
Immunomonitoring
Blood leukocyte counts were measured prospectively, once or twice every week after HCT, until a count of 0.3 × 109 cells/L was reached. Additionally, absolute numbers of neutrophils and monocytes were measured using TruCOUNT tubes (BD Biosciences). After reaching a leukocyte count of at least 0.3 × 109 cells/L, absolute numbers of NK cells (CD3−CD16+CD56+) were additionally measured by flow cytometry at least every other week up to 12 weeks after HCT and monthly thereafter up to 6 months after HCT. Patients were randomly selected for extra staining of cells after transplantation. We determined ATG binding on recovering immune cells in CBT recipients with fluorescein isothiocyanate (FITC)–labeled donkey anti-rabbit IgG. Analysis of fluorescently labeled cells was done on a LSR Fortessa (BD Biosciences), and FlowJo (Version 10) was used for data analysis.
Cell sources
For the in vitro assays, fresh CB and BM samples were obtained from healthy donors. Peripheral blood from healthy volunteers was used as a source for neutrophils (Histo-Paque 1119; Sigma-Aldrich) and peripheral blood mononuclear cells (PBMCs) (Fico-II-Paque; BD Biosciences). PBMCs were used as source for NK cells (CD3−CD56+) and monocytes (CD14+). After isolation, target CB cells, BM cells, and CD14− PBMCs were frozen at −80°C in fetal calf serum (Bodinco) containing 10% dimethyl sulphoxide (Sigma-Aldrich). G-CSF–stimulated neutrophils were derived from the blood of patients and healthy volunteers who received 10 mg/kg Filgrastim (G-CSF) per day for a minimum of 5 days. Nonstimulated neutrophils were obtained from healthy volunteers.
ATG-binding assay
CB cells and BM cells were Fc-blocked with mouse serum and incubated with 0 to 100 μg/mL rabbit-ATG (Thymoglobulin; Genzyme) for 20 minutes at 4°C. ATG binding was detected with a FITC-conjugated donkey anti-rabbit antibody (BD Biosciences). The panel for staining subsets included CD3-AF700, CD4-PerCP-Cy5.5, CD8-phycoerythrin (PE)-Cy7, CD14-BV510, CD19-allophycocyanin (APC)-eFluor780, and CD56-PE. The second panel included CCR7-APC, CD28-BV421, CD27-BV510, CD45RO-BV711, γδTCR-PE, CD4-PerCP-Cy5.5, CD8-Pe-Cy7, and CD3-AF700.
CDC assay
CB or BM cells were incubated with 0 to 100 μg/mL ATG for 30 minutes at room temperature. Without washing, 100 μL of 15.5% serum (pooled from 8 healthy donors) or phosphate-buffered saline was added for a 30-minute incubation at 37°C. After incubation, killing of specific cell subsets was evaluated by staining the cells with fluorescent markers (CD14-BV510, CD19-APC-eFluor780, CD56-APC, CD4-BV711, CD3-AF700, and CD8-Pe-Cy7). Propidium iodide (PI; TACS, Trevigen) was used to identify percentages of dead cells. ATG-mediated CDC was evaluated by subtracting background killing by ATG (the percentage of PI+ target cells exposed to ATG only) from the percentage of PI+ target cells exposed to ATG in presence of complement.
NK and neutrophil ADCC assays
For effector NK-cell isolation, fresh PBMCs were stained with CD3-AF700 and CD56-APC and sorted for CD3−CD56+ using a FACSAria II (Becton Dickinson). NK cells were stimulated with interleukin 2 (IL-2) and IL-15 (Sino Biological) overnight at 37°C. CB and BM cells were stained with CellTrace-Violet (CTV; Life Technologies) and sorted by magnetic-activated cell sorting Miltenyi Biotech) into CD14+ monocytes and CD14− target cells for neutrophil ADCC. Target cells were incubated with allogeneic NK cells at an effector/target ratio of 1:1 for NK ADCC, and CD14− target cells were incubated at 2:1 with neutrophils for 4 hours at 37°C. ATG was added at 0 to 100 μg/mL. Prior to incubation, cells were centrifuged for 2 minutes at 1200 rpm. After incubation, cells for NK ADCC were stained with CD14-APC-eFluor780, CD19-PerCP-Cy5.5, CD56-APC, CD107a-FITC, CD4-BV711, CD3-AF700, and CD8-Pe-Cy7 and for neutrophil ADCC with CD19-PerCP-Cy5.5, CD56-APC, CD4-APC-eFluor780, CD3-AF700, CD8-Pe-Cy7, CD27-PE, and CD45RO-BV711. 7-AAD in PerCP-Cy5.5 (BD Pharmingen) was used to stain dead cells. ADCC was evaluated by subtracting background (the percentage 7-AAD+ target cells exposed to ATG only) from the percentage of 7-AAD+ target cells exposed to both ATG and effector cells.
Monocyte/macrophage ADCP assay
Monocytes/macrophages were isolated from PBMCs by magnetic-activated cell sorting for CD14+ cells. Allogeneic CD14− CB and BM cells were stained with CTV and pHRodo-RED (Thermo Fisher Scientific) and incubated with freshly isolated monocytes at an effector/target ratio of 2:1 for 4 hours at 37°C in the absence/presence of ATG (0-100 μg/mL). Prior to incubation, cells were centrifuged for 2 minutes at 1200 rpm. After incubation, cells were stained with CD14-APC-eFluor780, CD19-PerCP-Cy5.5, CD56-APC, CD4-BV711, CD3-AF700, and CD8-Pe-Cy7. 7-AAD was used to stain dead cells and silicate beads (Invitrogen) were added to enumerate the cells. To evaluate monocyte ADCP per subset, the remaining amount of CTV+ target cells was calculated, with the percentage of CTV+ CD14+ cells and the percentage of CTVlow/pHRodohigh CD14+ cells providing important controls for phagocytosis.
G-CSF–treated neutrophil ADCP assay
Freshly isolated neutrophils were incubated with allogeneic CD14− PBMCs stained with CTV and pHRodo-RED at an effector/target ratio of 2:1 for 2 hours at 37°C in the absence/presence of ATG (0-100 μg/mL). For ADCC, 7-AAD was used to stain dead cells and analyzed as described above. Neutrophil ADCP was evaluated by the percentage of CTV+ neutrophils and by the percentage of CTVlow/pHRodohigh neutrophils. In addition, control neutrophils where stained with CD11b-AF700, CD62L-PE-Cy7, CD66b-BV421, CD63-FITC, CD64-APC, CD35-PE, and CD16-BV510.
Confocal microscopy
Confocal images were made of neutrophils derived from healthy donors who were treated or not with 10 mg/kg Filgrastim for 5 days. These neutrophils were incubated for 2 hours or overnight with allogeneic CTV-labeled CD14− PBMCs at a 2:1 ratio in the presence of 20 μg/mL ATG at 37°C. After incubation, neutrophils were stained with CD64-APC antibody, and cells were cytospun for 5 minutes at 500 rpm (Cytospin3, Shandon). After fixation with 4% paraformaldehyde and adding Mowiol (Sigma-Aldrich), cells were visualized using a confocal microscope (Zeiss LSM). For quantification of confocal data, 100 subsequent neutrophils (CD64+) were counted twice to determine the amount of target cells within neutrophils. Intracellular localization was confirmed using z-stack evaluation. Pictures were processed using Volocity Version 6.1.1 (PerkinElmer) software.
Data analysis and statistics
We compared the probability of CD4+ T-cell reconstitution, previously defined as having ≥50 × 106 cells/L in 2 consecutive measurements within the first 100 days after HCT, according to cell source (BM vs CB) and ATG exposures. Cox proportional hazard models were used to identify covariates affecting CD4+ IR, including age, sex, diagnosis (malignancy, PID, BM failure syndromes, or benign non-PID), treatment period (before or after 2014), steroid-treated (grade II-IV) aGVHD, and ATG exposure after HCT. Variables were statistically significant (P > .05) in univariate analysis were evaluated as covariates using the multivariate log-rank test for comparing the effect of multiple ATG exposure groups on CD4+ IR. Cumulative incidence curves were plotted, and statistical tests were performed in R 3.3 using the packages cmprsk, mgcv, and survival.21 Duration of follow-up was defined as the time from HCT to last contact or death. For in vivo evaluation of the effect of ATG exposure on subset recovery within 100 days after HCT, LOESS-regression curves were made using R 3.3, with linear-mixed effects models for statistical analysis. GraphPad Prism 7 (GraphPad Software) was used to produce graphs and perform statistical analysis; 2-tailed unpaired Student t tests were applied to evaluate differences between ATG-binding and ATG-mediated cytotoxicity of CB and BM subsets per ATG concentration, as well as differences between neutrophils from G-CSF–treated and nontreated individuals. P < .05 is regarded as statistically significant.
Results
Effect of ATG after HCT on immune cell subset recovery in children
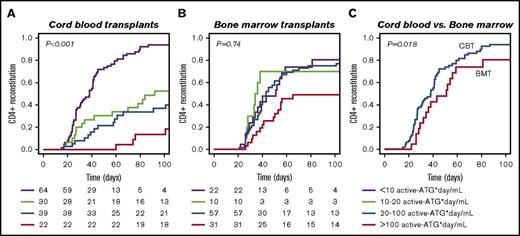
A total of 275 consecutive patients were included (Table 1). According to univariate analysis, ATG exposure, steroid-treated aGVHD, and transplantation period are variables affecting CD4+ IR probability (Table 2). Figure 1 illustrates the differential effect of residual ATG exposure on IR after CBT (P < .001) or BMT (P = .74), as tested in multivariate analysis correcting for steroid-treated aGVHD and transplantation period as covariates. No clinical covariates could thus explain the differential effect of ATG exposure on CD4+ IR.
Patient characteristics
| . | All patients . |
|---|---|
| Number of patients | 275 |
| Male/female | 174/101 |
| Age at transplant, median (range), y | 7.8 (0.16-19.2) |
| Stem cell source | |
| BM | 116 |
| CB | 155 |
| PBSCs | 4 |
| ATG exposure groups, active ATG × day/mL | 275 |
| AUC <10 | 86 |
| AUC 10-20 | 40 |
| AUC 20-100 | 96 |
| AUC >100 | 53 |
| Conditioned with ATG | 189 |
| Treated with G-CSF | 155 |
| BM | 0 |
| CB | 155 |
| Diagnosis | |
| Malignancy | 144 |
| PID | 53 |
| Benign non-PID | 47 |
| BM failure | 31 |
| Follow-up, days (range) | 1050 (12-3591) |
| . | All patients . |
|---|---|
| Number of patients | 275 |
| Male/female | 174/101 |
| Age at transplant, median (range), y | 7.8 (0.16-19.2) |
| Stem cell source | |
| BM | 116 |
| CB | 155 |
| PBSCs | 4 |
| ATG exposure groups, active ATG × day/mL | 275 |
| AUC <10 | 86 |
| AUC 10-20 | 40 |
| AUC 20-100 | 96 |
| AUC >100 | 53 |
| Conditioned with ATG | 189 |
| Treated with G-CSF | 155 |
| BM | 0 |
| CB | 155 |
| Diagnosis | |
| Malignancy | 144 |
| PID | 53 |
| Benign non-PID | 47 |
| BM failure | 31 |
| Follow-up, days (range) | 1050 (12-3591) |
Values represent number of patients, unless otherwise indicated.
AUC, area under the curve.
Univariate analysis of variables possibly affecting CD4+ T-cell reconstitution
| Variable . | P . | Significance level . |
|---|---|---|
| Age | .72 | NS |
| Sex | .073 | NS |
| Diagnosis | .72 | NS |
| HCT year | .02 | * |
| ATG exposure | .004 | ** |
| Steroid treated aGVHD | .025 | * |
| Variable . | P . | Significance level . |
|---|---|---|
| Age | .72 | NS |
| Sex | .073 | NS |
| Diagnosis | .72 | NS |
| HCT year | .02 | * |
| ATG exposure | .004 | ** |
| Steroid treated aGVHD | .025 | * |
Cox proportional hazard models were used for univariate analyses.
NS, not significant (P ≥ .05).
P < .05; **P < .01.
The effect of residual ATG exposure on CD4+ IR after CBT and BMT. (A-B) Residual ATG exposure affects CD4+ T-cell reconstitution (defined as ≥50 × 106 CD4+ T-cells/L in 2 consecutive measurements after HCT) more in CB recipients (A; n = 155, P < .001) than in BM/PB recipients (B; n = 120, P = .74). (C) When ATG exposure is low, CD4+ IR is faster after CBT compared with BMT (P = .018). P values are for comparisons among all 4 groups (multivariate log-rank test), with correction for covariates.
The effect of residual ATG exposure on CD4+ IR after CBT and BMT. (A-B) Residual ATG exposure affects CD4+ T-cell reconstitution (defined as ≥50 × 106 CD4+ T-cells/L in 2 consecutive measurements after HCT) more in CB recipients (A; n = 155, P < .001) than in BM/PB recipients (B; n = 120, P = .74). (C) When ATG exposure is low, CD4+ IR is faster after CBT compared with BMT (P = .018). P values are for comparisons among all 4 groups (multivariate log-rank test), with correction for covariates.
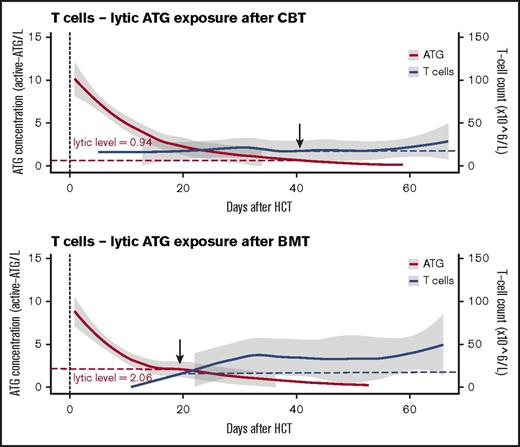
In patients with no residual ATG exposure (defined as <10 active ATG × day/mL) IR was faster after CBT than after BMT (Figure 1C; P = .018). In patients with high post-HCT ATG exposure (>100 active ATG × day/mL), especially T-cell recovery (absolute number in time) was severely affected in both BMT and CBT patients (supplemental Figure 1B; P = .02). Remarkably, after BMT, T cells were able to recover faster in the presence of higher plasma levels of circulating ATG than after CBT; this “lytic” ATG level (level above which no T-cell IR was observed) was 0.94 active-ATG/L after CBT but 2.06 active ATG/L after BMT (Figure 2). Recovery of total lymphocytes (supplemental Figure 1A; P = .44), B cells (supplemental Figure 1C; P = .47), NK cells (supplemental Figure 1D; P = .17), monocytes (supplemental Figure 1E; P = .18), and neutrophils (supplemental Figure 1F; P = .22) was not affected.
Differential lytic effect of residual ATG exposure on T-cell IR in CB or BM recipients. The lytic effect of residual ATG exposure on T cells is depicted in patients who received CBT or BMT. ATG exposure is depicted as area under the curve after HCT with 95% confidence intervals (red line, gray area), with T-cell reconstitution evaluated as mean cell amounts over time with 95% confidence intervals (blue line, gray area).
Differential lytic effect of residual ATG exposure on T-cell IR in CB or BM recipients. The lytic effect of residual ATG exposure on T cells is depicted in patients who received CBT or BMT. ATG exposure is depicted as area under the curve after HCT with 95% confidence intervals (red line, gray area), with T-cell reconstitution evaluated as mean cell amounts over time with 95% confidence intervals (blue line, gray area).
Because no clinical covariates could explain the differential effect of ATG exposure on CD4+ IR between CBT and BMT recipients, we evaluated other potentially important differences between CBT and BMT recipients. One major difference is the fact that CBT patients commonly receive G-CSF in many (if not all) centers, whereas BMT patients do not get it routinely (Table 1). Another difference is the origin and composition of the graft cells; CB-graft T cells are generally naive and might thus express more epitopes for ATG (generated against thymocytes) and/or be more sensitive to ATG-mediated cytotoxicity than BM-derived T cells. We further investigated these variables in the context of the biological mechanisms of ATG-mediated cytotoxicity.
Filgrastim treatment enhances neutrophil-mediated ATG cytotoxicity
G-CSF is known to enhance immunoglobulin-mediated phagocytosis by neutrophils.22-28 We therefore hypothesized that Filgrastim (G-CSF) might affect IR through enhanced ATG-mediated cytotoxicity. G-CSF administration usually starts within the first week after CBT and lasts until neutrophil engraftment occurs (mostly within 3-4 weeks). At this time, residual ATG is still present in many patients.9,10 The low abundance of cells coated with ATG in the peripheral blood after CBT (not shown) did not allow ex vivo analyses; the clearance of ATG-coated cells is apparently fast. We therefore compared ATG-mediated cytotoxicity by neutrophils from individuals who did or did not receive Filgrastim treatment (Figures 3 and 4).
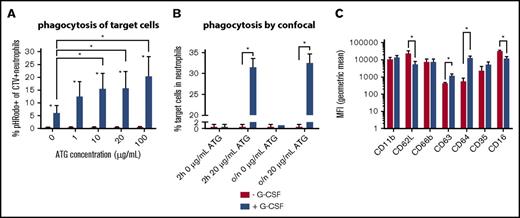
Higher ATG-mediated cytotoxicity by neutrophils after in vivo G-CSF treatment. Neutrophils were derived from healthy volunteers not receiving G-CSF (n = 5) or from donors who received 10 mg/kg G-CSF for at least 5 days (n = 5). (A) Percentage of pHRodo+CTV+ neutrophils as evaluated with flow cytometry; difference between neutrophils with and without G-CSF per ATG concentration tested: 0 μg/mL, P = .005; 1 μg/mL, P = .005; 10 μg/mL, P = .002; 20 μg/mL, P = .005; 100 μg/mL, P = .002. (B) The percentage of neutrophils that phagocytized a target cell as evaluated by confocal microscopy, no G-CSF vs G-CSF: 0 μg/mL 2-hour incubation, P = .99; 20 μg/mL 2-hour incubation, P < .001; 0 μg/mL overnight incubation, P = .90; 20 μg/mL overnight incubation, P = .001. Z-stack analyses were applied to ensure target cells were contained within neutrophils. (C) The geometric mean fluorescent intensity (MFI) of CD11b, CD62L (P = .03), CD66b, CD63 (P = .003), CD64 (P < .001), CD35, and CD16 (P = .03) on neutrophils, with (blue) and without (red) G-CSF treatment. Statistically significant differences (P < .05) are indicated with asterisks.
Higher ATG-mediated cytotoxicity by neutrophils after in vivo G-CSF treatment. Neutrophils were derived from healthy volunteers not receiving G-CSF (n = 5) or from donors who received 10 mg/kg G-CSF for at least 5 days (n = 5). (A) Percentage of pHRodo+CTV+ neutrophils as evaluated with flow cytometry; difference between neutrophils with and without G-CSF per ATG concentration tested: 0 μg/mL, P = .005; 1 μg/mL, P = .005; 10 μg/mL, P = .002; 20 μg/mL, P = .005; 100 μg/mL, P = .002. (B) The percentage of neutrophils that phagocytized a target cell as evaluated by confocal microscopy, no G-CSF vs G-CSF: 0 μg/mL 2-hour incubation, P = .99; 20 μg/mL 2-hour incubation, P < .001; 0 μg/mL overnight incubation, P = .90; 20 μg/mL overnight incubation, P = .001. Z-stack analyses were applied to ensure target cells were contained within neutrophils. (C) The geometric mean fluorescent intensity (MFI) of CD11b, CD62L (P = .03), CD66b, CD63 (P = .003), CD64 (P < .001), CD35, and CD16 (P = .03) on neutrophils, with (blue) and without (red) G-CSF treatment. Statistically significant differences (P < .05) are indicated with asterisks.
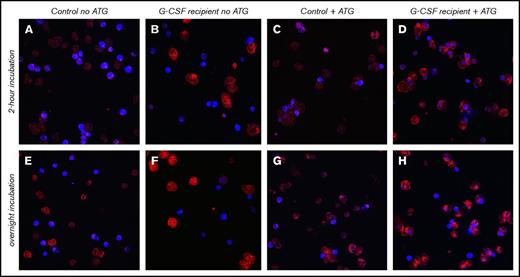
Confocal microscopy confirms higher cytotoxicity through ATG-mediated phagocytosis by neutrophils after G-CSF treatment. Neutrophils were obtained from a healthy donor who received 10 mg/kg Filgrastim for 5 days and from a healthy donor who served as control. Neutrophils were incubated with CD14− allogeneic PBMCs in the absence or presence of 20 μg/mL ATG for 2 hours or overnight. Neutrophils were stained with CD64− APC (red), and target cells were CTV+ labeled (blue) for confocal microscopy visualization. Pictures were taken with same Z-resolution (in 1 slice). Original magnification ×65. (A,E) Control neutrophils and target cells without ATG, incubated for 2 hours (A) or overnight (E). (B,F) Neutrophils from G-CSF recipient and target cells without ATG, incubated for 2 hours (B) or overnight (F). (C,G) Control neutrophils and target cells with ATG, incubated for 2 hours (C) or overnight (G). (D,H) Neutrophils from G-CSF recipient and target cells with ATG, incubated for 2 hours (D) or overnight (H). For neutrophils from the G-CSF recipient, shown are target cells within neutrophils. Z-stack analyses were applied to ensure target cells were contained within neutrophils.
Confocal microscopy confirms higher cytotoxicity through ATG-mediated phagocytosis by neutrophils after G-CSF treatment. Neutrophils were obtained from a healthy donor who received 10 mg/kg Filgrastim for 5 days and from a healthy donor who served as control. Neutrophils were incubated with CD14− allogeneic PBMCs in the absence or presence of 20 μg/mL ATG for 2 hours or overnight. Neutrophils were stained with CD64− APC (red), and target cells were CTV+ labeled (blue) for confocal microscopy visualization. Pictures were taken with same Z-resolution (in 1 slice). Original magnification ×65. (A,E) Control neutrophils and target cells without ATG, incubated for 2 hours (A) or overnight (E). (B,F) Neutrophils from G-CSF recipient and target cells without ATG, incubated for 2 hours (B) or overnight (F). (C,G) Control neutrophils and target cells with ATG, incubated for 2 hours (C) or overnight (G). (D,H) Neutrophils from G-CSF recipient and target cells with ATG, incubated for 2 hours (D) or overnight (H). For neutrophils from the G-CSF recipient, shown are target cells within neutrophils. Z-stack analyses were applied to ensure target cells were contained within neutrophils.
We observed a strikingly higher capacity of G-CSF–exposed neutrophils to phagocytize ATG-targeted cells, as confirmed by both flow cytometry (Figure 3A) and confocal microscopy (Figure 3B). We stained target cells with pHRodo, which increases in fluorescent intensity after lysosomal processing, as a measure for processing after phagocytosis. The increase of pHRodo is negligible in control neutrophils but increases dose-dependently up to 20% with G-CSF–stimulated neutrophils (P = .002). Neutrophil ADCC was comparable (supplemental Figure 2), suggesting that G-CSF provides an important additional killing mechanism through phagocytosis. Furthermore, expression of CD11b, CD66b, and CD35 was comparable, with lower expression of CD62L (P = .025) and CD16 (P = .025), whereas CD63 (P = .006) and CD64 (P < .001) were highly upregulated in neutrophils treated with G-CSF (Figure 3C). Confocal microscopy visualizes the enhanced capacity of neutrophils from G-CSF recipients to phagocytize target cells (Figure 4A-D), which was not found in nontreated neutrophils after either 2 hours (P < .001) or overnight incubation (P < .001).
Comparable ATG binding and cytotoxicity in CB- and BM-graft lymphocytes
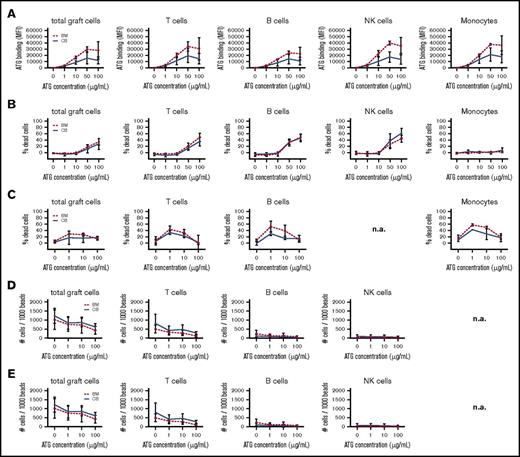
In vitro ATG binding (Figure 5A; supplemental Figure 3A), CDC (Figure 5B; supplemental Figure 3B), NK ADCC (Figure 5C; supplemental Figure 3C), neutrophil ADCC (Figure 5D; supplemental Figure 3D), and monocyte/macrophage ADCP (Figure 5E; supplemental Figure 3E) was generally dose dependent. Note that the concentrations indicate total ATG levels, whereas in vivo active ATG is determined to be only ∼9% of total ATG.9 Clinically relevant ATG levels after HCT range between 0.1 and 10 μg active ATG/mL blood, which is ∼1 to 100 μg total ATG/mL.9,10 ATG binding was detected on the surface of T cells, CD4+ T cells, CD8+ T cells, γδT cells, NK cells, monocytes, and B cells. Binding of naive and effector/memory BM-graft–derived T cells was comparable (data not shown) but was not evaluated for CB grafts, as these do not contain adequate amounts of effector/memory T cells. CDC was only observed with ATG >10 μg/mL. ATG-mediated ADCC and ADCP showed comparable cytotoxicity in all subsets tested, at the clinically relevant concentrations of 1 and 10 μg/mL ATG, with lower toxicity at 100 μg/mL. Overall, we did not find significant differences in ATG binding or cytotoxicity between BM- and CB-graft lymphocytes.
ATG-binding and ATG-mediated cytotoxicity of CB and BM cells. ATG-binding and ATG-mediated cytotoxicity in cells from CB (n = 3) and BM grafts (n = 3). (A) Binding of ATG to graft cells after exposure to 0, 1, 10, 50, and 100 μg/mL rabbit ATG, as evaluated using a FITC-labeled donkey anti-rabbit antibody (total cells, T cells, B cells, NK cells, and monocytes). (B) CDC is evaluated as the percentage of 7-AAD+ immune cells after exposure to 0, 1, 10, 50, and 100 μg/mL rabbit ATG (total cells, T cells, B cells, NK cells, and monocytes). ATG-mediated ADCC is evaluated as the percentage of 7-AAD+ immune cells after exposure to 0, 1, 10, and 100 μg/mL rabbit ATG. (C) NK ADCC (total cells, T cells, B cells, and monocytes). (D) Neutrophil ADCC (total cells, T cells, B cells, and NK cells). (E) Monocyte/macrophage ADCP is evaluated as the amount of remaining target cells/1000 beads after exposure to 0, 1, 10, and 100 μg/mL rabbit ATG (total cells, T cells, B cells, and NK cells). No significant differences were observed. n.a., not applicable.
ATG-binding and ATG-mediated cytotoxicity of CB and BM cells. ATG-binding and ATG-mediated cytotoxicity in cells from CB (n = 3) and BM grafts (n = 3). (A) Binding of ATG to graft cells after exposure to 0, 1, 10, 50, and 100 μg/mL rabbit ATG, as evaluated using a FITC-labeled donkey anti-rabbit antibody (total cells, T cells, B cells, NK cells, and monocytes). (B) CDC is evaluated as the percentage of 7-AAD+ immune cells after exposure to 0, 1, 10, 50, and 100 μg/mL rabbit ATG (total cells, T cells, B cells, NK cells, and monocytes). ATG-mediated ADCC is evaluated as the percentage of 7-AAD+ immune cells after exposure to 0, 1, 10, and 100 μg/mL rabbit ATG. (C) NK ADCC (total cells, T cells, B cells, and monocytes). (D) Neutrophil ADCC (total cells, T cells, B cells, and NK cells). (E) Monocyte/macrophage ADCP is evaluated as the amount of remaining target cells/1000 beads after exposure to 0, 1, 10, and 100 μg/mL rabbit ATG (total cells, T cells, B cells, and NK cells). No significant differences were observed. n.a., not applicable.
Together, these data show that cells regenerating from CB or BM have similar ATG-binding characteristics and susceptibility for ATG-mediated killing in the absence of G-CSF. The use of G-CSF, however, dramatically increases the killing of ATG-coated cells after CBT.
Discussion
T-cell immune reconstitution after HCT is pivotal for survival.1-7 Hence, it is important to understand which factors affect IR and what mechanisms are involved. To our knowledge, we are the first to show IR in context of in vivo active-ATG levels after HCT. Here, we show in a pediatric cohort of patients that residual ATG exposure has a higher impact on CD4+ IR after CBT than after BMT. This is not because of clinical covariates hampering CD4+ IR or differences in susceptibility of ATG-mediated killing mechanisms between CB and BM cells. However, G-CSF treatment, which is very commonly administered after CBT (and not routinely after BMT), potentiates the impairment of T-cell IR due to enhanced T-cell clearance by residual ATG after HCT. These data provide an explanation for the historical observation that T-cell IR after CBT seems inferior to that of BMT. Moreover, we report that in absence of ATG exposure, T-cell IR is better after CBT than after BMT.
Previous studies support our finding that G-CSF enhances cytotoxicity of monoclonal antibodies by neutrophils,22-28 which is partly explained by strong induction of the high-affinity IgG receptor (FcγRI; CD64).28,29 For instance, G-CSF treatment was shown to enhance the antitumor effects of rituximab in CD20+ models and of trastuzumab in HER2+ models.24 Also in the clinical setting, the combination of G-CSF and rituximab resulted in improved efficacy in patients at risk for non-Hodgkin lymphoma relapse, ascribed to greatly enhanced neutrophil-mediated rituximab cytotoxicity by G-CSF treatment.25 Because neutrophils can be detected as soon as 3 days after HCT, the overlap in the timing of neutrophil recovery, residual ATG exposure, and Filgrastim treatment provides a cytotoxic formula for T cells.
Other factors, such as GVHD and subsequent steroid treatment, may affect T-cell reconstitution,30-32 although the incidence of GVHD is generally higher after BMT than after CBT.33-35 Until now, such factors had not been analyzed as covariates to evaluate the effect of ATG exposure on CD4+ IR. The multivariate analysis performed in the current study strongly indicates that ATG exposure predicts CD4+ IR differently after CBT or BMT, despite covariates such as steroid-treated aGVHD. Since our CBT patients received different GVHD prophylaxis (prednisolone) than BMT recipients (methotrexate), it was not possible to correct for this. Nevertheless, we show that CD4+ IR is better after CBT than after BMT, despite prophylaxis with prednisolone after CBT. Therefore, if prednisolone is (partly) responsible for the delay in CD4+ IR after CBT, the rate of CD4+ IR after CBT (with low ATG exposure) might be underestimated in our cohort. Furthermore, a recent report showed that the amount of T cells within the graft does not affect ATG clearance.15 The generally lower amount of T cells within CB grafts thus also does not provide an explanation for the higher impact of ATG on T-cell IR after CBT. In addition, we showed that CB immune cells are not more sensitive to ATG cytotoxicity than BM cells. ATG binds similarly to CB- and BM-derived immune cell subsets, and our data were similar to that in studies evaluating ATG affinity on PBMCs.36 This was unexpected, because ATG (developed against [naive] thymus cells) was expected to higher bind to the generally naive CB-derived T cells as opposed to the generally less naive BM-derived T cells. In accordance with the increased level of ATG binding, T cells were most susceptible to ATG-mediated cytotoxicity. These findings indicate that the difference in ATG cytotoxicity on T-cell IR after CBT or BMT are not explained by graft differences.
One way to enhance T-cell IR after ATG-conditioned HCT might be by decreasing residual ATG levels via individualized ATG conditioning and/or therapeutic drug monitoring of ATG.9,13 Another way would be to adjust (or delay) timing of G-CSF treatment to prevent coexposure with ATG. G-CSF treatment might even be abandoned in some patients with residual ATG exposure. A large study of 2719 HCT patients within the database of the Center for International Blood and Marrow Transplant Research showed that G-CSF treatment after HCT had no clinical or survival benefits.37 Its purpose was to improve neutropenia after CBT, but improved CB-graft quality (higher cell doses) and availability in recent years had already decreased neutropenia time. It was also suggested that G-CSF treatment skews toward Th2 T-cell recovery,38-40 and induces myeloid-derived suppressor cells,40 making G-CSF treatment undesirable, because it might hamper T-cell immunity against viruses or cancer cells. On the other hand, introducing G-CSF treatment after HCT in patients receiving an ATG-containing conditioning might potentiate ATG-mediated depletion of residual lymphoid leukemia blasts.41,42 The net effect may, however, be counterbalanced by higher nonrelapse mortality due to poor IR. Prospective trials are needed to study the benefit of personalized conditioning regimens and post-HCT strategies, such as G-CSF, to increase T-cell reconstitution and improve HCT outcomes.
In conclusion, poor T-cell reconstitution after CBT in pediatric patients with residual ATG exposure is likely due to enhanced ATG-mediated T-cell clearance by G-CSF treatment. Without G-CSF treatment, no differences in ATG-mediated cytotoxicity were found between CB and BM lymphocytes. In the context of ATG-containing conditioning regimens, the use of G-CSF treatment should be revisited to achieve predictable, optimal T-cell IR and better clinical outcomes after HCT.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank D. van den Blink and K. Westinga for their help in obtaining samples from grafts and healthy volunteers, T. ten Broeke and A. Brandsma for their help setting up some of the in vitro assays, and A. Lacna and L. Jongeneel for their help with confocal microscopy.
This work was supported by Foundation Children Cancerfree (KiKa) project number 142.
The funder had no role in the design of the study.
Authorship
Contribution: C.d.K. and S.N. designed the research; C.d.K. wrote and edited the manuscript; C.d.K. and J.-A.G. performed experiments and analyzed and interpreted the data; C.d.K., J.L., and R.A. performed statistical analysis on clinical data; S.N. and J.J.B. reviewed the manuscript and provided critical comments; and all authors reviewed and approved the final report.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stefan Nierkens, Laboratory of Translational Immunology, University Medical Center Utrecht, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands, e-mail: s.nierkens@umcutrecht.nl.
References
Author notes
J.J.B. and S.N. contributed equally to this study.