Key Points
M2-MΦs promote and M1-MΦs inhibit HSC self-renewal via differential expression of Arg1 and NOS2, respectively.
Coculture of hUCB CD34+ cells with M2-MΦs resulted in a significant expansion of CD34+ cells and SCID–mice repopulating cells.
Abstract
Uncovering the cellular and molecular mechanisms by which hematopoietic stem cell (HSC) self-renewal is regulated can lead to the development of new strategies for promoting ex vivo HSC expansion. Here, we report the discovery that alternative (M2)-polarized macrophages (M2-MΦs) promote, but classical (M1)-polarized macrophages (M1-MΦs) inhibit, the self-renewal and expansion of HSCs from mouse bone marrow (BM) in vitro. The opposite effects of M1-MΦs and M2-MΦs on mouse BM HSCs were attributed to their differential expression of nitric oxide synthase 2 (NOS2) and arginase 1 (Arg1), because genetic knockout of Nos2 and Arg1 or inhibition of these enzymes with a specific inhibitor abrogated the differential effects of M1-MΦs and M2-MΦs. The opposite effects of M1-MΦs and M2-MΦs on HSCs from human umbilical cord blood (hUCB) were also observed when hUCB CD34+ cells were cocultured with M1-MΦs and M2-MΦs generated from hUCB CD34− cells. Importantly, coculture of hUCB CD34+ cells with human M2-MΦs for 8 days resulted in 28.7- and 6.6-fold increases in the number of CD34+ cells and long-term SCID mice–repopulating cells, respectively, compared with uncultured hUCB CD34+ cells. Our findings could lead to the development of new strategies to promote ex vivo hUCB HSC expansion to improve the clinical utility and outcome of hUCB HSC transplantation and may provide new insights into the pathogenesis of hematological dysfunctions associated with infection and inflammation that can lead to differential macrophage polarization.
Introduction
Expansion of hematopoietic stem cells (HSCs) ex vivo can make HSC transplants available to more adult patients and improve the clinical outcome in patients transplanted with human umbilical cord blood (hUCB) HSCs.1-2 Although significant progress has been made in the last few years in identifying cells and small molecules that can promote ex vivo expansion of HSCs, rapid and efficient ex vivo HSC expansion remains a significant challenge, because the cellular and molecular mechanisms by which HSC self-renewal is regulated are not fully understood.1-2 Discovery of new cellular and molecular mechanisms that regulate HSC self-renewal has the potential to facilitate the development of novel strategies for promoting ex vivo HSC expansion and provide new insights into the pathogenesis of hematological dysfunctions.
Some of the mature progeny of HSCs are constituents of the HSC niche and can regulate HSC functions.3-4 For example, CD169+ macrophages (MΦs) modulate HSC retention, and depletion of MΦs causes HSC egress to the blood.5-6 In addition, α-smooth muscle actin–expressing MΦs regulate HSC quiescence through production of prostaglandin E2 to prevent HSC exhaustion.7 More recently, DARC-expressing MΦs maintain the dormancy of long-term HSCs through interaction with CD82/KAI1.8 However, whether MΦs have the ability to directly regulate HSC self-renewal is unknown. Activated MΦs exhibit plasticity and exert diverse functions along a spectrum between classical (M1) or alternative (M2) activation (or polarization).9-10 Although a simplistic definition based on in vitro stimulation, the spectral model of polarization has considerable value in guiding experimentation about MΦ biology in vivo, because many parallels exist between the in vitro and in vivo scenarios.11-12 M1-MΦs produce proinflammatory cytokines, reactive oxygen species, and nitric oxide (NO) and are consistent with MΦs having key roles in defense against microbial infection and cancer. In contrast, M2-MΦs express scavenging receptors and produce polyamines and various anti-inflammatory mediators that promote the resolution of inflammation and tissue repair and regeneration.12 However, the effects of MΦ polarization on HSCs have not been studied. Therefore, we investigated whether MΦs can regulate HSC self-renewal in an MΦ polarization–dependent manner using an in vitro mouse bone marrow (BM) HSC expansion model system.
Materials and methods
Animals
Male C57BL/6J (or CD45.2), B6.SJL-PtprcaPep3b/BoyJ (or CD45.1), NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG), and B6.129P2-Nos2tm1Lau/J (Nos2−/−) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). They were housed at the University of Arkansas for Medical Sciences (UAMS) or St. Jude Children’s Research Hospital, both Association for Assessment and Accreditation of Laboratory Animal Care–certified animal facilities. They received food and water ad libitum. All mice were used at ∼8-12 weeks of age. The Institutional Animal Care and Use Committee of UAMS or St. Jude Children’s Research Hospital approved all experimental procedures used in this study. Peritoneal cavity cells were harvested from Arg1 conditional-knockout mice or wild-type littermates in which the Cre driver was Tie2-Cre, as previously described.13-15
Materials
Various antibodies, cytokines, and reagents used in the studies are shown in supplemental Tables 1 and 2.
Isolation of murine Mos and MΦs from BM and MΦs from the peritoneal cavity
To isolate BM monocytes (Mos) and MΦs, BM mononuclear cells (MNCs) were stained with anti-Gr-1–phycoerythrin, anti-CD115–allophycocyanin, and anti-F4/80–fluorescein isothiocyanate on ice for 30 minutes. CD115+Gr-1low Mos, CD115+Gr-1high Mos, and CD115−Gr-1lowF4/80+SSClow MΦs were isolated with a FACSAria II cell sorter (BD Biosciences, San Jose, CA), as shown in supplemental Figure 1A.5 To isolate peritoneal MΦs, peritoneal cavity cells were harvested as described previously.16 They were allowed to adhere to plastic after the cells were cultured overnight in Dulbecco’s Modified Eagle Medium (DMEM) (Life Technologies, Grant Island, NY) with 20% fetal bovine serum (FBS) in a plastic Petri dish. Nonadherent cells were removed after the cells were washed three times with prewarmed cell culture medium. The adherent cells were harvested after the cells were incubated with cold Ca++- and Mg++-free phosphate-buffered saline (Life Technologies) without FBS for 30 minutes on ice and by repeated pipetting. The harvested adherent cells contained ∼95% F4/80+ peritoneal MΦs, as shown in supplemental Figure 1B. In addition, peritoneal cavity cells were harvested from Arg1 conditional-knockout mice or wild-type littermates in which the Cre driver was Tie2-Cre, as previously described.13-15 Tie2-Cre is used in this system because (1) LysM-Cre does not provide sufficiently efficient deletion at the floxed Arg1 locus, (2) Arg1 is predominantly expressed in MΦs, providing specificity for the deletion, and (3) MΦs from the mice were used for in vitro studies.13-15 All mice used for peritoneal cell isolation were individually tested for the deletion by culture of BM with colony-stimulating factor 1 and stimulation with interleukin-4 (IL-4) and IL-10 overnight, followed by western blotting for Arg1 protein.
Polarization of murine MΦs
Peritoneal MΦs were polarized to M1-MΦs and M2-MΦs by incubation of the cells overnight with 20 ng/mL recombinant mouse interferon-γ (INF-γ) and IL-4 (PeproTech, Rocky Hill, NJ), respectively, at 105/mL per well in a 6-well plate.
Expansion of mouse BM LSK cells in vitro
Lin−Sca1+c-kit+ (LSK) cells (2 × 103/mL per well in a 12-well plate) were cultured in a mouse HSC expansion medium (StemSpan medium; STEMCELL Technologies, Vancouver, BC, Canada) supplemented with 100 U/mL penicillin, 100 µg/mL streptomycin, 20 ng/mL recombinant mouse thrombopoietin (TPO), and stem cell factor (SCF) (PeproTech), with or without Mos or various MΦs (1×105 per well), as shown in Figure 1. The cell density of LSK cell cultures was maintained at <5 × 105/mL by adding an appropriate amount of fresh mouse HSC expansion medium every 3 days of culture. When the cells were cocultured with Mos or MΦs, nonadherent cells were harvested from the culture to remove Mos or MΦs for further analysis. For transplantation studies, LSK cells were initially cultured in mouse HSC expansion medium with or without MΦs, as shown in Figure 2, and were maintained at an appropriate density by adding the fresh mouse HSC expansion medium, as described above.
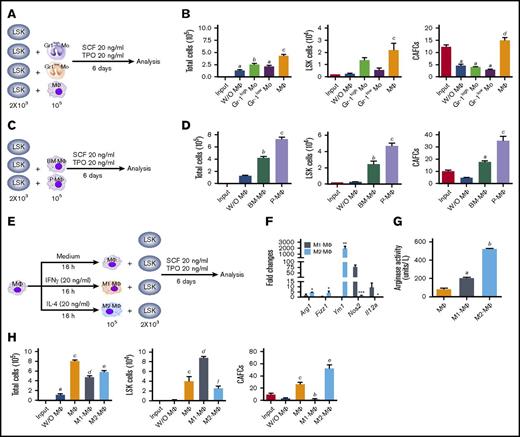
M1-MΦs and M2-MΦs have opposite effects on HSC self-renewal and expansion in vitro. (A) Diagram illustrating the design of the experiments in panel B, in which mouse BM LSK cells were cultured with sorted mouse BM CD115+Gr-1high Mos (Gr-1high Mo), CD115+Gr-1low Mos (Gr-1low Mo), or CD115−Gr-1lowF4/80+SSClow MΦs (MΦ). (B) Numbers of total cells, LSK cells, and 5-week CAFCs in the input LSK cells and the progeny from various cultures shown in panel A. aP < .05 vs Input, bP < .05 vs Input and without MΦs (W/O MΦ), cP < .05 vs all other groups, dP < .05 vs all other groups except Input. (C) Diagram illustrating the design of the experiments in panel D. MΦs were isolated from mouse BM (BM-MΦs) or peritoneal cavity (P-MΦ). (D) Numbers of total cells, LSK cells, and 5-week CAFCs in the input LSK cells and the progeny from various cultures shown in panel C. aP < .05 vs W/O MΦ, bP < .05 vs Input and W/O MΦ, cP < .05 vs all other groups. (E) Diagram illustrating the experimental design for peritoneal MΦ polarization and LSK cell cocultures. (F) Relative gene expression in M1-MΦs and M2-MΦs compared with MΦs analyzed by quantitative polymerase chain reaction. *P < .05, **P < .01, and ***P < .001 vs cells cultured with M1-MFs; unpaired Student t test. (G) Arg1 activity in MΦs, M1-MΦs, and M2-MΦs. aP < .05 vs MΦ, bP < .05 vs MΦ and M1-MΦ. (H) Numbers of total cells, LSK cells, and 5-week CAFCs in the input LSK cells and the progeny from various cultures shown in panel E. aP < .05 vs Input, bP < .05 vs MΦ, cP < .05 vs Input and W/O MΦ, dP < .05 vs Input, W/O MΦ, and MΦ, eP < .05 vs all other groups, fP < .05 vs all other groups except MΦs. All data are mean ± standard error of the mean (SEM) (n = 3 independent cultures) and were analyzed by 1-way analysis of variance (ANOVA).
M1-MΦs and M2-MΦs have opposite effects on HSC self-renewal and expansion in vitro. (A) Diagram illustrating the design of the experiments in panel B, in which mouse BM LSK cells were cultured with sorted mouse BM CD115+Gr-1high Mos (Gr-1high Mo), CD115+Gr-1low Mos (Gr-1low Mo), or CD115−Gr-1lowF4/80+SSClow MΦs (MΦ). (B) Numbers of total cells, LSK cells, and 5-week CAFCs in the input LSK cells and the progeny from various cultures shown in panel A. aP < .05 vs Input, bP < .05 vs Input and without MΦs (W/O MΦ), cP < .05 vs all other groups, dP < .05 vs all other groups except Input. (C) Diagram illustrating the design of the experiments in panel D. MΦs were isolated from mouse BM (BM-MΦs) or peritoneal cavity (P-MΦ). (D) Numbers of total cells, LSK cells, and 5-week CAFCs in the input LSK cells and the progeny from various cultures shown in panel C. aP < .05 vs W/O MΦ, bP < .05 vs Input and W/O MΦ, cP < .05 vs all other groups. (E) Diagram illustrating the experimental design for peritoneal MΦ polarization and LSK cell cocultures. (F) Relative gene expression in M1-MΦs and M2-MΦs compared with MΦs analyzed by quantitative polymerase chain reaction. *P < .05, **P < .01, and ***P < .001 vs cells cultured with M1-MFs; unpaired Student t test. (G) Arg1 activity in MΦs, M1-MΦs, and M2-MΦs. aP < .05 vs MΦ, bP < .05 vs MΦ and M1-MΦ. (H) Numbers of total cells, LSK cells, and 5-week CAFCs in the input LSK cells and the progeny from various cultures shown in panel E. aP < .05 vs Input, bP < .05 vs MΦ, cP < .05 vs Input and W/O MΦ, dP < .05 vs Input, W/O MΦ, and MΦ, eP < .05 vs all other groups, fP < .05 vs all other groups except MΦs. All data are mean ± standard error of the mean (SEM) (n = 3 independent cultures) and were analyzed by 1-way analysis of variance (ANOVA).
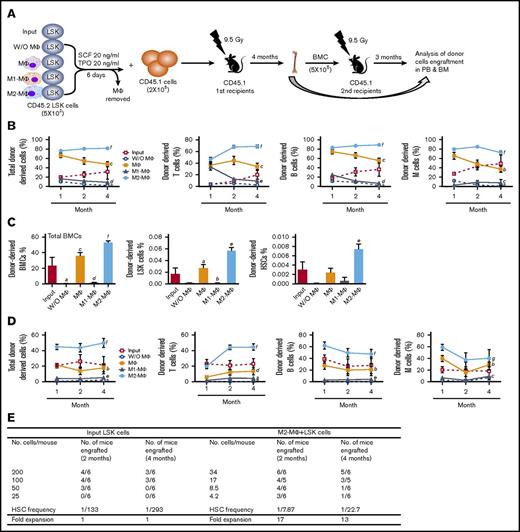
CRA and serial BM transplantation confirm the differential effects of M1-MΦs and M2-MΦs on HSC self-renewal and expansion in vitro. (A) Diagram illustrating the experimental design for LSK cell coculture with MΦs, M1-MΦs, and M2-MΦs or without MΦs (W/O MΦ) for CRA and serial BM transplantation. (B) Donor-derived total nucleate cells, T cells, B cells, and myeloid cells (M cells) in the peripheral blood of the primary recipients 1, 2, and 4 months after transplantation. All data are mean ± SEM (n = 5 recipients for input and MΦs, n = 6 recipients for W/O MΦs, and n = 8 recipients for M1-MΦs and M2-MΦ). aP < .05 vs Input, bP < 0.05 vs W/O MΦ, cP < .05 vs Input and W/O MΦ, dP < .05 vs Input and MΦ, eP < .05 vs W/O MΦ and MΦ, fP < .05 vs all other groups, 2-way ANOVA. (C) Donor-derived total BM nucleate cells (BMCs), LSK cells, and HSCs (CD150+CD48− LSK cells) in BM of the primary recipients 4 months after transplantation. Data are mean ± SEM (n = 5-8 recipients per group, as described in panel B). aP < .05 vs Input, bP < .05 vs MΦ, cP < .05 vs Input and W/O MΦ, dP < .05 vs Input and MΦ, eP < .05 vs all other groups, fP < .05 vs all other groups except MΦ (1-way ANOVA). (D) Donor-derived total nucleate cells, T cells, B cells, and myeloid cells (M cells) in the peripheral blood of the secondary recipients 1, 2, and 3 months after transplantation. Data are mean ± SEM (n = 6 recipients for input and W/O MΦ, n = 5 recipients for MΦs, and n = 7 recipients for M1-MΦs and M2-MΦs). aP < .05 vs Input, bP < .05 vs W/O MΦ, cP < .05 vs MΦ, dP < .05 vs Input and W/O MΦ, eP < .05 vs Input and MΦ, fP < .05 vs all other groups, gP < .05 vs all other groups except MΦs, 2-way ANOVA. (E) Competitive repopulation unit assay reveals that LSK cells cocultured with M2-MΦs resulted in a 13-fold expansion of long-term (4 months) repopulating HSCs compared with the input cells.
CRA and serial BM transplantation confirm the differential effects of M1-MΦs and M2-MΦs on HSC self-renewal and expansion in vitro. (A) Diagram illustrating the experimental design for LSK cell coculture with MΦs, M1-MΦs, and M2-MΦs or without MΦs (W/O MΦ) for CRA and serial BM transplantation. (B) Donor-derived total nucleate cells, T cells, B cells, and myeloid cells (M cells) in the peripheral blood of the primary recipients 1, 2, and 4 months after transplantation. All data are mean ± SEM (n = 5 recipients for input and MΦs, n = 6 recipients for W/O MΦs, and n = 8 recipients for M1-MΦs and M2-MΦ). aP < .05 vs Input, bP < 0.05 vs W/O MΦ, cP < .05 vs Input and W/O MΦ, dP < .05 vs Input and MΦ, eP < .05 vs W/O MΦ and MΦ, fP < .05 vs all other groups, 2-way ANOVA. (C) Donor-derived total BM nucleate cells (BMCs), LSK cells, and HSCs (CD150+CD48− LSK cells) in BM of the primary recipients 4 months after transplantation. Data are mean ± SEM (n = 5-8 recipients per group, as described in panel B). aP < .05 vs Input, bP < .05 vs MΦ, cP < .05 vs Input and W/O MΦ, dP < .05 vs Input and MΦ, eP < .05 vs all other groups, fP < .05 vs all other groups except MΦ (1-way ANOVA). (D) Donor-derived total nucleate cells, T cells, B cells, and myeloid cells (M cells) in the peripheral blood of the secondary recipients 1, 2, and 3 months after transplantation. Data are mean ± SEM (n = 6 recipients for input and W/O MΦ, n = 5 recipients for MΦs, and n = 7 recipients for M1-MΦs and M2-MΦs). aP < .05 vs Input, bP < .05 vs W/O MΦ, cP < .05 vs MΦ, dP < .05 vs Input and W/O MΦ, eP < .05 vs Input and MΦ, fP < .05 vs all other groups, gP < .05 vs all other groups except MΦs, 2-way ANOVA. (E) Competitive repopulation unit assay reveals that LSK cells cocultured with M2-MΦs resulted in a 13-fold expansion of long-term (4 months) repopulating HSCs compared with the input cells.
Isolation of hUCB MNCs, CD34+ cells, and CD34− cells
hUBC blood disqualified for banking due to inadequate volume and/or cell count was deidentified and provided to us by the Cord Blood Bank of Arkansas (Little Rock, AR) with the approval of the UAMS Institutional Review Board. Human MNCs were obtained by density centrifugation on Histopaque-1077 (Sigma, St. Louis, MO) and then used to isolate CD34+ cells and CD34− cells with a MACS CD34 progenitor isolation kit (Miltenyi Biotech, Auburn, CA), according to the manufacturer’s instructions. The purity of the isolated cells was routinely in the range of 80% to 95%. These cells were frozen in DMEM plus 20% FBS and 10% dimethyl sulfoxide (Sigma, St. Louis, MO) immediately and stored in a liquid nitrogen tank.
Differentiation and polarization of human MΦs
hUCB CD34− cells (5 × 106/2 mL per well in a 6-well plate) were thawed and then cultured in DMEM plus 20% FBS for 1 day. After nonadherent cells, including dead cells and cell debris, were removed from the cultures by washing the cells with prewarmed cell culture medium, they were cultured with DMEM plus 20% FBS and 50 ng/mL recombinant human SCF and MΦ colony-stimulating factor (PeproTech) for 7 days to induce MΦ differentiation, with change of 50% of the medium every 3 days of culture. To induce M1-MΦs and M2-MΦs, 20 ng/mL recombinant human interferon-γ (IFN-γ) and IL-4 (PeproTech) were added to the culture for overnight incubation, respectively.17-18
Expansion of hUCB CD34+ cells ex vivo
Freshly thawed hUCB CD34+ cells (2 × 104/2 mL per well in a 6-well plate) were cultured in a human HSC expansion medium (StemSpan medium; STEMCELL Technologies) supplemented with 100 U/mL penicillin, 100 µg/mL streptomycin, and 50 ng/mL recombinant human TPO, SCF, and Flt3 ligand (PeproTech), with or without MΦs, M1-MΦs, or M2-MΦs (∼5 × 104 per well), as shown in Figure 4A. The cell density of CD34+ cell cultures was maintained at <5 × 105/mL by adding an appropriate amount of the fresh human HSC expansion medium every 3 days of culture. When the cells were cocultured with MΦs, nonadherent cells were harvested from the culture to remove MΦs for further analysis and transplantation, as shown in Figure 4A.
Additional methods used in the studies are described in supplemental Materials and methods.
Results
MΦs, but not Mos, promote the expansion of mouse BM LSK cells and 5-week cobblestone area–forming cells (CAFCs) in vitro
Because depletion of MΦs in mice causes HSC mobilization and perturbation of HSC function,5-6 we chose an ex vivo HSC expansion model system to investigate whether MΦs can regulate HSC self-renewal in comparison with their predecessor Mos.11-12 As shown in Figure 1A-B and supplemental Figure 1A-B, resident MΦs sorted from mouse BM increased the expansion of LSK cells when they were cocultured in a serum-free medium supplemented with 20 ng/mL SCF and TPO. However, neither BM Gr-1high Mos nor Gr-1low Mos had the same effect. In addition, coculture of LSK cells with BM MΦs resulted in a slight increase in the number of 5-week CAFCs, which represent an in vitro surrogate of HSCs,19 whereas LSK cells cocultured with Mos or without MΦs showed a significant reduction in 5-week CAFCs compared with the input cells. These findings suggest that MΦs, but not Mos, promoted the expansion of LSK cells while maintaining the function of HSCs.
Because it is difficult to isolate sufficient numbers of resident BM MΦs for functional studies, we next isolated resident MΦs from the peritoneal cavity without activation by an inflammatory stimulus and compared their effect on LSK cell expansion in vitro with that of BM MΦs (supplemental Figure 1C). We found that MΦs from BM and the peritoneal cavity had the ability to promote the expansion of LSK cells and 5-week CAFCs in vitro and that peritoneal MΦs were slightly more effective than BM MΦs (Figure 1C-D; supplemental Figure 1D). Therefore, the resident peritoneal MΦs are useful as a surrogate model for resident BM MΦs.20
M1-MΦs and M2-MΦs have opposing effects on mouse BM HSC self-renewal and in vitro expansion
Because M1-MΦs and M2-MΦs have diverse functions in inflammation, tissue repair, and homeostasis,9-10,21 we next investigated whether the effects of MΦs on LSK cells and HSCs can be modulated by differential cytokine activation. As shown in Figure 1E, peritoneal MΦs were “polarized” into M1-MΦs and M2-MΦs after overnight incubation with IFN-γ and IL-4, respectively.22 M1-MΦs expressed increased Nos2 and Il12 messenger RNA (mRNA) and a lower Arg1 activity, whereas M2-MΦs expressed relatively elevated Arg1, Fizz1, and Ym1 mRNA and a higher Arg1 activity (Figure 1F-G). When the polarized MΦs were cocultured with LSK cells, M2-MΦs promoted the expansion of LSK cells and 5-week CAFCs in vitro (Figure 1H). Moreover, the effect of M2-MΦs on 5-week CAFC expansion was greater than that of nonpolarized MΦs. In contrast, M1-MΦs promoted the expansion of LSK cells to a greater degree than M2-MΦs, but they significantly reduced the number of 5-week CAFCs compared with input cells. Because 5-week CAFCs represent one of the best in vitro surrogates of HSCs,19 these findings suggest that M1-MΦs and M2-MΦs may have opposite effects on HSC self-renewal and ex vivo expansion.
We performed a competitive repopulation assay (CRA) by transplanting freshly isolated LSK cells and cells generated from the same number of LSK cells after in vitro expansion in the presence or absence of different types of MΦs into lethally irradiated recipients, along with helper/competitor cells (Figure 2A). The results from this assay showed that transplantation of input LSK cells resulted in multilineage donor cell engraftment in the peripheral blood as a function of time (from ∼20% at 1 month to ∼30% at 4 months after transplantation) (Figure 3B). LSK cells cocultured with M2-MΦs engrafted earlier and more efficiently in blood after transplantation than did all of the other cells, including the input cells (Figure 3B; supplemental Figure 2). LSK cells cocultured with MΦs also engrafted better in blood at 1 month compared with input cells, but the advantage gradually diminished to a level that was not significantly different from the blood-engraftment level of input cells 4 months after transplantation. In contrast, LSK cells cocultured with M1-MΦs or cultured without MΦs exhibited a significant reduction in short-term and long-term blood cell engraftment compared with the input cells or the cells cocultured with MΦs and M2-MΦs. Similar findings were observed in BM engraftment, including the engraftment of LSK cells and HSCs (Figure 2C; supplemental Figure 3). Moreover, the differential effects of MΦs, M1-MΦs, and M2-MΦs on blood cell engraftment could be transferred into the secondary recipients (Figure 2D; supplemental Figure 4). These results confirm that M1-MΦs and M2-MΦs have opposite effects on HSC self-renewal; consequently, M1-MΦs can inhibit, whereas M2-MΦs promote, HSC expansion. To estimate the fold expansion of HSCs after coculture with M2-MΦs, we performed a limiting-dilution assay, as shown in Figure 2E. The results from this assay showed that LSK cells cocultured with M2-MΦs for 6 days underwent a 13-fold expansion of long-term (4 months) repopulating HSCs compared with input cells, confirming the correlation between our in vitro findings and the outcomes of in vivo stem cell transfer.
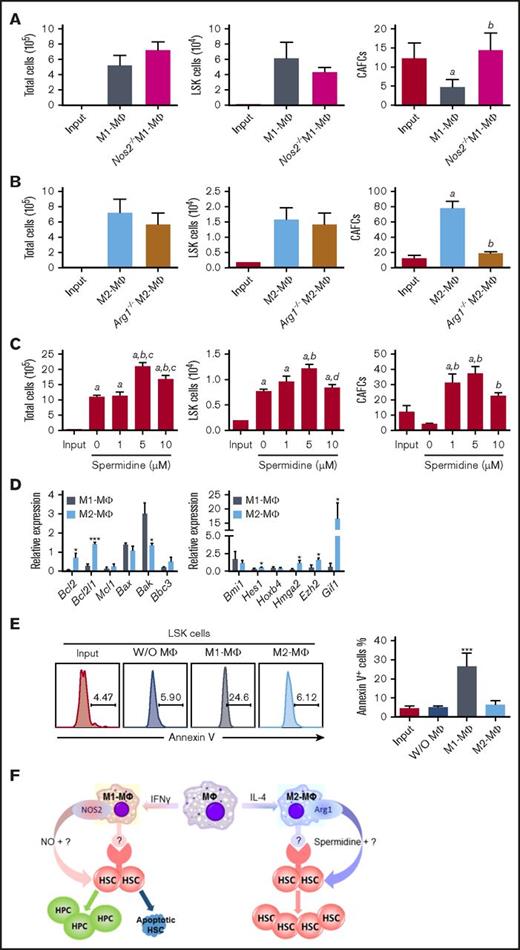
M1-MΦs and M2-MΦs differentially regulate HSC self-renewal and in vitro expansion via NOS2 and Arg-1, respectively. (A) Nos2 knockout abrogates the inhibitory effect of M1-MΦs on 5-week CAFCs. LSK cells (2 × 103) were cocultured with 1 × 105 M1-MΦs from wild-type mice and Nos2 knockout (Nos2−/−) mice in StemSpan medium supplemented with 20 ng/mL SCF and TPO for 6 days. Numbers of total cells, LSK cells, and 5-week CAFCs in the input LSK cells and the progeny from these cultures are presented as mean ± SEM (n = 3 independent cultures). aP < .05 vs Input, bP < .05 vs M1-MΦ, unpaired Student t test. (B) Arg1 knockout abrogates the promoting effect of M2-MΦs on 5-week CAFCs. LSK cells were cocultured with M2-MΦs from wild-type mice and Arg1-knockout (Arg1−/−) mice, as described in panel A. The data are mean ± SEM (n = 3 independent cultures). aP < .05 vs Input, bP < .05 vs Input and M2-MΦ, unpaired Student t test. (C) Spermidine dose dependently increases the expansion of LSK cells and 5-week CAFCs in vitro. LSK cells were cultured with increasing concentrations of spermidine, as described in panel A. Data are mean ± SEM (n = 3 independent cultures). aP < .05 vs Input, bP < .05 vs 0 µM, cP < .05 vs 1 µM, dP < .05 vs 5 µM, 1-way ANOVA. (D) Relative gene expression in LSK cells sorted from the progeny of LSK cells cultured with M1-MΦs and M2-MΦs compared with that of input LSK cells revealed that coculture with M2-MΦs upregulated the expression of several HSC self-renewal and antiapoptotic genes, whereas coculture with M1-MΦs had opposite effects and increased the expression of the proapoptotic protein Bax. Data are mean ± SEM (n = 3 independent cultures). *P < .05, ***P < .001 vs cells cultured with M1-MΦs, unpaired Student t test. (E) Representative flow cytometric analysis of apoptosis (left panel) and percentage of apoptotic cells (right panel) in input LSK cells and LSK cells after culture with M1-MΦs or M2-MΦs or without MΦs. Data are mean ± SEM (n = 2 independent cultures). ***P < .001 between the cells cultured with M1-MΦs and all other cells, unpaired Student t test. (F) Hypothetical model illustrating the role of MΦ polarization in the regulation of mouse BM HSC self-renewal and expansion in vitro.
M1-MΦs and M2-MΦs differentially regulate HSC self-renewal and in vitro expansion via NOS2 and Arg-1, respectively. (A) Nos2 knockout abrogates the inhibitory effect of M1-MΦs on 5-week CAFCs. LSK cells (2 × 103) were cocultured with 1 × 105 M1-MΦs from wild-type mice and Nos2 knockout (Nos2−/−) mice in StemSpan medium supplemented with 20 ng/mL SCF and TPO for 6 days. Numbers of total cells, LSK cells, and 5-week CAFCs in the input LSK cells and the progeny from these cultures are presented as mean ± SEM (n = 3 independent cultures). aP < .05 vs Input, bP < .05 vs M1-MΦ, unpaired Student t test. (B) Arg1 knockout abrogates the promoting effect of M2-MΦs on 5-week CAFCs. LSK cells were cocultured with M2-MΦs from wild-type mice and Arg1-knockout (Arg1−/−) mice, as described in panel A. The data are mean ± SEM (n = 3 independent cultures). aP < .05 vs Input, bP < .05 vs Input and M2-MΦ, unpaired Student t test. (C) Spermidine dose dependently increases the expansion of LSK cells and 5-week CAFCs in vitro. LSK cells were cultured with increasing concentrations of spermidine, as described in panel A. Data are mean ± SEM (n = 3 independent cultures). aP < .05 vs Input, bP < .05 vs 0 µM, cP < .05 vs 1 µM, dP < .05 vs 5 µM, 1-way ANOVA. (D) Relative gene expression in LSK cells sorted from the progeny of LSK cells cultured with M1-MΦs and M2-MΦs compared with that of input LSK cells revealed that coculture with M2-MΦs upregulated the expression of several HSC self-renewal and antiapoptotic genes, whereas coculture with M1-MΦs had opposite effects and increased the expression of the proapoptotic protein Bax. Data are mean ± SEM (n = 3 independent cultures). *P < .05, ***P < .001 vs cells cultured with M1-MΦs, unpaired Student t test. (E) Representative flow cytometric analysis of apoptosis (left panel) and percentage of apoptotic cells (right panel) in input LSK cells and LSK cells after culture with M1-MΦs or M2-MΦs or without MΦs. Data are mean ± SEM (n = 2 independent cultures). ***P < .001 between the cells cultured with M1-MΦs and all other cells, unpaired Student t test. (F) Hypothetical model illustrating the role of MΦ polarization in the regulation of mouse BM HSC self-renewal and expansion in vitro.
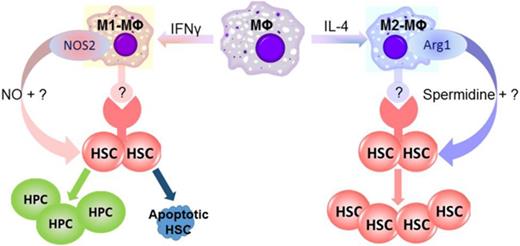
M1-MΦs and M2-MΦs regulate mouse BM HSC self-renewal and in vitro expansion in an opposite direction, in part via differential expression of NOS2 and Arg1
One of the major differences between in vitro cytokine–stimulated mouse M1-MΦs and M2-MΦs is that they use different metabolic pathways for arginine.9-10 M1-MΦs preferentially express NOS2, which catalyzes l-arginine to produce NO, whereas M2-MΦs express Arg1, which converts l-arginine into l-ornithine. The production of l-ornithine is hypothesized to contribute to the synthesis of polyamines. Consequently, M1-MΦs produce high amounts of NO, whereas M2-MΦs do not (supplemental Figure 5A). Potentially, NO derived from NOS2 can promote HSC and hematopoietic progenitor cell (HPC) proliferation and differentiation at the expense of HSC self-renewal.23-24 In addition, exposure to NO induces HSC and HPC apoptosis.25 In contrast, polyamines, such as spermidine synthesized through the arginine metabolic pathway initiated by Arg1, have the potential to promote HSC self-renewal via stimulation of autophagy.26-27 Therefore, the roles of NOS2 and Arg1 in the regulation of mouse BM HSC self-renewal and in vitro expansion by M1-MΦs and M2-MΦs, respectively, were investigated. We found that inhibition of NO production with NG-monomethyl–l-arginine or knockout of Nos2 attenuated the M1-MΦ–induced reduction in 5-week CAFCs in vitro while having no significant effect on the expansion of total and LSK cells (Figure 3A; supplemental Figure 5B-C). In contrast, inhibition of arginase activity with amino-2-borono-6-hexanoic acid or knockout of Arg1 in MΦs attenuated the promoting effect of M2-MΦs on the expansion of 5-week CAFCs in vitro while having no significant effect on the expansion of LSK cells (Figure 3B; supplemental Figure 5D). These findings suggest that M1-MΦs and M2-MΦs may regulate mouse BM HSC self-renewal and in vitro expansion, at least in part via production of NO and spermidine by NOS2 and Arg1, respectively (Figure 3F). This suggestion is in agreement with the finding that spermidine, but not l-ornithine and spermine (data not shown), was able to increase the expansion of LSK cells and 5-week CAFCs in vitro (Figure 3C). However, the effect of spermidine on the expansion of 5-week CAFCs was less pronounced than that of M2-MΦs (Figure 3B-C), suggesting that factors others than spermidine derived from M2-MΦs may also contribute to the promotion of mouse BM HSC self-renewal and in vitro expansion by M2-MΦs. This suggestion is supported by the finding that coculture of LSK cells with MΦs, M1-MΦs, and M2-MΦs, in a Transwell that prevents direct interaction between LSK cells and MΦs, attenuated the effects of MΦs, M1-MΦs, and M2-MΦs on mouse BM HSC self-renewal and in vitro expansion (supplemental Figure 6).
To further elucidate the molecular mechanisms by which mouse M1-MΦs and M2-MΦs differentially regulate mouse BM HSC self-renewal and in vitro expansion, we analyzed the expression of genes important for regulation of HSC proliferation, survival, and self-renewal in freshly isolated LSK cells, as well as LSK cells harvested from a coculture with M1-MΦs or M2-MΦs. LSK cells cocultured with M1-MΦs expressed a lower level of p21 and p27, as well as cyclin D1 and D2, compared with LSK cells cultured with M2-MΦs (supplemental Figure 5E). The mixed effects of M1-MΦs on the expression of these CDK inhibitors and cyclins in LSK cells might result in no significant changes in cell cycle distribution in LSK cells cocultured with M1-MΦs compared with LSK cells cocultured with M2-MΦs, suggesting that the increased number of LSK cells in coculture with M1-MΦs is unlikely to be attributable to the promotion of LSK cell proliferation. In contrast, we found that coculture with M1-MΦs, but not with M2-MΦs, downregulated the expression of Bcl2 and Bcl2l1 mRNA, but upregulated the expression of Bak mRNA, in LSK cells, resulting in a significant increase in apoptosis in these cells compared with freshly isolated LSK cells and LSK cells cocultured with M2-MΦs (Figure 3D-E; supplemental Figure 5F). In addition, coculture of LSK cells with M1-MΦs downregulated the expression of Hes1, Hoxb4, Hmga2, Ezh2, and Gif1 mRNA, whereas coculture of LSK cells with M2-MΦs had a lesser effect or no effect on the expression of these genes but upregulated the expression of Ezh2 and Gif1 mRNA. The differential regulation of the expression of these HSC self-renewal–related genes in LSK cells by M1-MΦs and M2-MΦs may also contribute to the opposite effect of M1-MΦs and M2-MΦs on HSC self-renewal and in vitro expansion but not on cell proliferation.
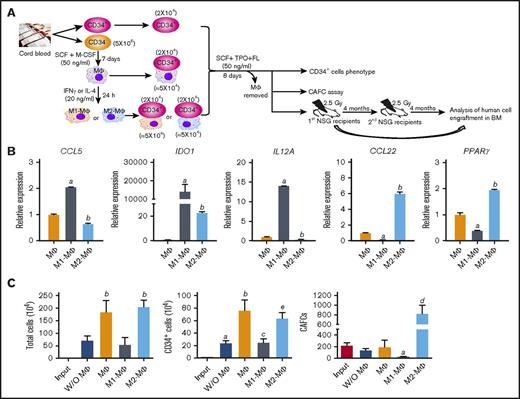
M2-MΦs promote the expansion of hUCB CD34+ cells and HSCs ex vivo
The finding that M2-MΦs can promote mouse BM HSC self-renewal and expansion in vitro prompted us to test whether M2-MΦs can also promote ex vivo expansion of hUCB CD34+ cells and HSCs. We generated MΦs from hUCB CD34− cells, as shown in Figure 4A, which exhibited the typical morphology of MΦs (supplemental Figure 7A). They were then polarized into M1-MΦs and M2-MΦs by stimulation with INF-γ and IL-4, respectively. Human M1-MΦs expressed significantly higher IDO1, IL12A, and CCL5 mRNA compared with unpolarized MΦs and M2-MΦs, whereas M2-MΦs expressed significantly higher CCL22 and PPARγ mRNA compared with unpolarized MΦs and M1-MΦs, confirming that M1-MΦs and M2-MΦs had been properly polarized (Figure 4B). When M2-MΦs were cocultured with hUCB CD34+ cells in a serum-free medium supplemented with 50 ng/mL SCF, TPO, and Flt-3 ligand for 8 days, the numbers of total cells, CD34+ cells, and 6-week CAFCs (that represent human HSCs) increased 111.0-, 49.2-, and 3.8-fold over the input values, respectively (Figure 4C; supplemental Figure 7B). In contrast, hUCB CD34+ cells cultured under the same conditions with M1-MΦs exhibited significantly less expansion of total cells and CD34+ cells compared with the cells cultured with M2-MΦs and a >85% reduction in 6-week CAFCs from the input. These findings suggest that M1-MΦs and M2-MΦs can also differentially regulate hUCB self-renewal and ex vivo expansion. To further test this hypothesis, we performed a serial transplantation experiment, as illustrated in Figure 4A. Four months after the primary transplantation, hUCB CD34+ cells cocultured with M2-MΦs produced significantly higher human cell engraftment in the BM of NSG mice than did input cells in all cell populations analyzed, including mature human blood cells (eg, CD45+ cells, CD3+ cells, CD19+ cells, CD41a+ cells, and CD33+ cells) and human HPCs and HSCs (eg, Lin−CD34+ cells, Lin−CD34+CD38− cells, and Lin−CD34+CD38−CD90+CD49f+ cells) (Figure 5A-B; supplemental Figure 7C-D). In contrast, hUCB CD34+ cells cultured without M2-MΦs generated significantly lower engraftment of human CD45+ cells, CD3+ cells, CD19+ cells, CD41a+ cells, and CD33+ cells in NSG mouse BM than did input cells 4 months after transplantation. In addition, 4 months after transplantation, the primary recipients of hUCB CD34+ cells cocultured with M2-MΦs also exhibited a significantly higher degree of human CD45+ cell engraftment in NSG mouse spleen and thymus than did the recipients transplanted with input cells and the cells cultured without M2-MΦs (supplemental Figure 8). Furthermore, coculture with M2-MΦs accelerated hUCB CD34+ cell engraftment in NSG mice, because even at 10 weeks after transplantation, the recipients of hUCB CD34+ cells cultured with M2-MΦs had a significantly higher degree of human cell engraftment in all cell populations analyzed than did those that had received transplantation of input cells and hUCB CD34+ cells cultured without M2-MΦs (supplemental Figure 9). Finally, to evaluate whether the human HSCs engrafted in the primary NSG mice represented long-term repopulating HSCs that have the ability to self-renew, we transplanted half of the BM cells harvested from the primary recipients 4 months after the first transplantation into secondary NSG recipients. As shown in Figure 5C, 4 months after the secondary transplantation, none of the secondary recipients of hUCB CD34+ cells cultured without M2-MΦs had BM engraftment of human CD45+ cells >0.5%, and only 2 of 17 secondary recipients of input cells had engraftment >0.5%. In contrast, 13 of 16 secondary recipients of hUCB CD34+ cells cultured with M2-MΦs had BM engraftment of human CD45+ cells >0.5%. The average BM engraftment of human CD45+ cells in these recipients was 5.7%. In addition, these recipients also exhibited multilineage human blood cell engraftment. Collectively, these findings suggest that culture of hUCB CD34+ cells with M2-MΦs for only 8 days promotes ex vivo expansion of human HPCs, as well as long-term human HSCs, resulting in accelerated and robust engraftment after transplantation into NSG recipients.
Coculture with M2-MΦs promotes expansion of hUCB CD34+cells and 6-week CAFCs ex vivo. (A) Diagram illustrating the experimental design for ex vivo hUCB CD34+ cell expansion and analyses. (B) Relative gene expression in M1-MΦs and M2-MΦs compared with MΦs analyzed by quantitative polymerase chain reaction. aP < .05 vs MΦ, bP < .05 vs MΦ and M1-MΦ. (C) Numbers of total cells, CD34+ cells, and 6-week CAFCs in the input cells and the progeny of hUCB CD34+ cells cultured with MΦs, M1-MΦs, or M2-MΦs or without MΦs (W/O MΦs). Data are mean ± SEM (n = 7 different units of hUCB CD34+ cells per group for total cell and CD34+ cell analyses, and n = 4 different units of hUCB CD34+ cells per group for CAFC assay). aP < .05 vs Input, bP < .05 vs Input and W/O MΦ, cP < .05 vs Input and MΦ, dP < .05 vs all other groups, eP < .05 vs all other groups except MΦ, 1-way ANOVA.
Coculture with M2-MΦs promotes expansion of hUCB CD34+cells and 6-week CAFCs ex vivo. (A) Diagram illustrating the experimental design for ex vivo hUCB CD34+ cell expansion and analyses. (B) Relative gene expression in M1-MΦs and M2-MΦs compared with MΦs analyzed by quantitative polymerase chain reaction. aP < .05 vs MΦ, bP < .05 vs MΦ and M1-MΦ. (C) Numbers of total cells, CD34+ cells, and 6-week CAFCs in the input cells and the progeny of hUCB CD34+ cells cultured with MΦs, M1-MΦs, or M2-MΦs or without MΦs (W/O MΦs). Data are mean ± SEM (n = 7 different units of hUCB CD34+ cells per group for total cell and CD34+ cell analyses, and n = 4 different units of hUCB CD34+ cells per group for CAFC assay). aP < .05 vs Input, bP < .05 vs Input and W/O MΦ, cP < .05 vs Input and MΦ, dP < .05 vs all other groups, eP < .05 vs all other groups except MΦ, 1-way ANOVA.
SRC assay confirms that M2-MΦs promote expansion of hUCB CD34+cells ex vivo. (A) Representative flow cytometric charts of analysis of donor-derived human total white blood cells (CD45+), myeloid cells (CD45+CD33+), megakaryocytes (CD45+CD41a+), B cells (CD45+CD19+), and T cells (CD45+CD3+) in BM of the primary (left panel) and secondary (right panel) NSG recipients 4 months after each transplantation. (B) Percentages of human total white blood cells (CD45+), myeloid cells (CD45+CD33+), megakaryocytes (CD45+CD41a+), B cells (CD45+CD19+), and T cells (CD45+CD3+) in BM of the primary NSG recipients. Data are mean ± SEM (n = 17 recipients of three independent transplantations with 3 different units of hUCB CD34+ cell transplants for input and M2-MΦ groups, n = 6 recipients of 1 unit of hUCB CD34+ cell transplant for without [W/O] MΦ group). aP < .05 vs Input, bP < .05 vs W/O MΦ, Mann-Whitney U test. (C) Percentages of human total white blood cells (CD45+), myeloid cells (CD45+CD33+), megakaryocytes (CD45+CD41a+), B cells (CD45+CD19+), and T cells (CD45+CD3+) in BM of the secondary NSG recipients. Data are mean ± SEM (n = 17 for input and M2-MΦ groups, n = 6 for W/O MΦ group). aP < .05 vs Input, bP < .05 vs W/O MΦ, Mann-Whitney U test. (D) Limiting-dilution analysis of the frequency of SRCs in the input CD34+ cells from 2 different units of hUCB cells and the progeny of hUCB CD34+ cells after being cultured with M2-MΦs.
SRC assay confirms that M2-MΦs promote expansion of hUCB CD34+cells ex vivo. (A) Representative flow cytometric charts of analysis of donor-derived human total white blood cells (CD45+), myeloid cells (CD45+CD33+), megakaryocytes (CD45+CD41a+), B cells (CD45+CD19+), and T cells (CD45+CD3+) in BM of the primary (left panel) and secondary (right panel) NSG recipients 4 months after each transplantation. (B) Percentages of human total white blood cells (CD45+), myeloid cells (CD45+CD33+), megakaryocytes (CD45+CD41a+), B cells (CD45+CD19+), and T cells (CD45+CD3+) in BM of the primary NSG recipients. Data are mean ± SEM (n = 17 recipients of three independent transplantations with 3 different units of hUCB CD34+ cell transplants for input and M2-MΦ groups, n = 6 recipients of 1 unit of hUCB CD34+ cell transplant for without [W/O] MΦ group). aP < .05 vs Input, bP < .05 vs W/O MΦ, Mann-Whitney U test. (C) Percentages of human total white blood cells (CD45+), myeloid cells (CD45+CD33+), megakaryocytes (CD45+CD41a+), B cells (CD45+CD19+), and T cells (CD45+CD3+) in BM of the secondary NSG recipients. Data are mean ± SEM (n = 17 for input and M2-MΦ groups, n = 6 for W/O MΦ group). aP < .05 vs Input, bP < .05 vs W/O MΦ, Mann-Whitney U test. (D) Limiting-dilution analysis of the frequency of SRCs in the input CD34+ cells from 2 different units of hUCB cells and the progeny of hUCB CD34+ cells after being cultured with M2-MΦs.
To estimate the fold expansion of human HSCs in hUCB CD34+ cells cocultured with M2-MΦs, we transplanted different numbers of input hUCB CD34+ cells or the progeny of the same numbers of hUCB CD34+ cells cultured with M2-MΦs for 8 days into NSG mice to assess the frequency of SCID mice–repopulating cells (SRCs). As shown in Figure 5D, the frequency of SRCs in input hUCB CD34+ cells from 2 separate units of hUCB was 1/1354 and 1/2119, which increased to 1/197 and 1/336, respectively, after the cells were cultured with M2-MΦs for 8 days. This result indicates that hUCB CD34+ cells cultured with M2-MΦs for 8 days led to 6.8- and 6.3-fold increases in the number of SRCs.
Discussion
MΦs play pleiotropic roles beyond host defense and regulation of various immune functions and inflammation because they are not only an essential constituent of the innate immune system but are also an important component of the stem cell niche in various tissues, including BM.5-7,9-10 Previous studies have shown that MΦs can maintain HSC quiescence and retention in the HSC niche and that their depletion causes HSC mobilization.5-7 Using an in vitro HSC-expansion model, we discovered a new function of MΦs linked to tissue and organ homeostasis: regulation of HSC self-renewal. More importantly, our study shows that the effects of MΦs on HSC self-renewal can be modulated by differential polarization. Specifically, we found that M1-MΦs activated by IFN-γ can inhibit, and M2-MΦs induced by IL-4 can promote, the self-renewal and expansion of mouse BM HSCs in vitro.
The inhibitory effect of mouse M1-MΦs on HSC self-renewal and expansion was attributable, in part, to the production of NO by NOS2, because inhibition of NOS2 activity with NG-monomethyl–l-arginine or knockout of Nos2 abrogated the inhibitory effect. This finding is in agreement with the previous observations that NO can inhibit HSC self-renewal by stimulating HSC proliferation and differentiation and induction of HSC and HPC apoptosis.23-25 Promotion of HSC self-renewal and expansion by mouse M2-MΦs is mediated, in part, by Arg1, because inhibition of Arg1 activity with amino-2-borono-6-hexanoic acid or knockout of Arg1 in purified MΦs attenuated the effect of M2-MΦs on HSC self-renewal. The mechanism by which Arg1 from M2-MΦs regulates HSC self-renewal may be attributable, at least in part, to the production of spermidine, because spermidine, but not l-ornithine and spermine, could partially replace M2-MΦs to increase the expansion of mouse BM HSCs. Spermidine is a potent inducer of autophagy, and autophagy is essential for HSC self-renewal and the maintenance of hematopoietic homeostasis.16-17 In addition, MΦs have the ability to produce other soluble factors and express a variety of adhesion molecules to regulate HSC self-renewal.28 It would be of interest to determine whether differential polarization of MΦs can alter their production and expression and whether some of these factors and molecules also contribute to M2-MΦ–mediated promotion of HSC self-renewal and expansion, because spermidine is less effective than M2-MΦs in promoting mouse BM HSC self-renewal and expansion in vitro, and preventing the direct contact between mouse BM HSCs and MΦs can attenuate the effects of MΦs on HSCs.
The finding that M2-MΦs can promote mouse BM HSC self-renewal and expansion in vitro prompted us to examine whether M2-MΦs can also promote ex vivo expansion of hUCB HSCs. Our results show that coculture of hUCB CD34+ cells with human M2-MΦs derived from hUCB CD34− cells for only 8 days resulted in a 6.6-fold expansion of SRCs measured 4 months after primary transplantation in NSG mice. More importantly, unlike human MSCs and BM mesenspheres, which promote the expansion of only human HPCs/short-term HSCs,29-30 M2-MΦs may have the ability to promote the expansion of human HPCs/short-term HSCs and long-term HSCs. This is because we found that hUCB CD34+ cells cocultured with M2-MΦs generated a more robust early (10 weeks) and long-term (4 months) multilineage engraftment in the BM, spleen, and thymus of NSG recipients after transplantation than did the noncultured hUCB CD34+ cells and hUCB CD34+ cells cultured without M2-MΦs. In fact, even 4 months after secondary transplantation, when human cells were barely detectable (average human CD45+ cell engraftment < 0.5%) in the BM of NSG mice transplanted with noncultured hUCB CD34+ cells or hUCB CD34+ cells cultured without M2-MΦs, the NSG mice transplanted with hUCB CD34+ cells cultured with M2-MΦs still showed robust multilineage engraftment of human cells in the BM. Using M2-MΦs to promote ex vivo expansion of hUCB HSCs has additional advantages over other cell types. First, human M2-MΦs are readily available by differentiation and polarization of hUCB CD34− cells in vitro. Therefore, it will be more practical and safer to expand human HSCs ex vivo by coculture of hUCB CD34+ cells with M2-MΦs than with MSCs and endothelial cells, because these cells are more difficult to harvest and require genetic engineering to promote human HSC expansion,29-32 respectively. Second, we show coculture with M2-MΦs is capable of promoting the expansion of human HPCs and long-term human HSCs, whereas coculture with MSCs and endothelial cells promotes the expansion of human HPCs but not HSCs. Finally, M2-MΦ coculture requires significantly less time to promote ex vivo expansion of hUCB HSCs than incubation with human HSC–expanding small molecules, such as SR-133 and nicotinamide34 (eg, 8 days for M2-MΦ coculture vs 21 days for SR-1 and nicotinamide). This can significantly reduce the risk for culture contamination and the costs of lengthy culture for ex vivo expansion of hUCB HSCs. Furthermore, M2-MΦ coculture may be combined with other ex vivo human HSC expansion–promoting strategies, such as “fed-batch” culture35 and culture with immobilized Notch ligand delta 1,36 pleiotrophin,37 SR-1,33 UM171,38 nicotinamide,34 and/or valproic acid39 to promote greater expansion of hUCB HSCs than individual methods. This may lead to further improvement of the utility and outcome of clinical hUCB HSC transplantation.
By comparison with murine MΦs, the mechanism by which human M2-MΦs promote hUCB HSC self-renewal and ex vivo expansion remains to be determined; unlike mouse M2-MΦs, human M2-MΦs do not strongly induce Arg1 expression in response to IL-4 following in vitro stimulation.21 Nevertheless, the overall functions of human M2-like MΦs are likely to be conserved and overlapping with rodents, because essential M2 functions like wound repair, tissue immunity, and worm expulsion are essential for rodents and primates. Thus, distinct factors and molecules produced by and expressed on MΦs may mediate the effect of human M2-MΦs on hUCB HSC self-renewal, including prostaglandin E2,7,40-41 the Wnt pathway ligands,40,42-43 and vascular cell adhesion molecule 1,44 all of which have been implicated in regulation of HSC self-renewal and hematopoiesis.
In addition, the discovery that MΦs can differentially regulate HSC self-renewal after being polarized into M1-MΦs and M2-MΦs may provide new insights into the pathogenesis of some of the hematological disorders and diseases associated with chronic infection and inflammation, because differential polarization of MΦs occurs under these pathological conditions. For example, it has been shown that bacterial infection and injection of lipopolysaccharide (LPS) and IFN-γ can lead to premature HSC exhaustion.45 Although LPS and IFN-γ may impair HSC self-renewal by directly stimulating HSC cycling via the Toll-like receptors and IFN-γ receptors expressed on HSCs,46-47 it has yet to be determined whether polarization of MΦs by LPS and IFN-γ also plays a role in the induction of premature HSC exhaustion, because LPS and IFN-γ can stimulate NO production by polarizing MΦs to M1-MΦs. Production of NO by M1-MΦs can impair HSC self-renewal by stimulation of HSC proliferation and differentiation and induction of HSC apoptosis, as shown in our studies and those reported previously. Therefore, modulation of MΦ polarization or inhibition of NO production may offer a strategy to prevent and/or ameliorate hematopoietic dysfunction resulting from chronic infection and inflammation.
The full-text version of this article contains a data supplement.
Acknowledgments
This study was supported by grants from the National Institutes of Health (NIH), National Cancer Institute (NCI) (R01 CA122023 and R01 CA211963) and the Edward P. Evans Foundation (D.Z.); NIH, NCI (P30 CA21765), the American Lebanese Syrian Associated Charities, and the Max-Planck-Gesellschaft (P.J.M.); and the NIH, National Institute of General Medical Sciences (1P20 GM109005-01A1) (D.Z. and M.H.-J.).
Authorship
Contribution: Y.L. designed, performed, and analyzed most of the experiments and wrote the manuscript; L.S., J.C., W.F., L.L., X.C., and J.Z. performed and analyzed some experiments; Y.L.L., M.H.C.-F., and P.D.E. provided some research reagents and cells, interpreted data, and revised the manuscript; M.H.-J., and I.D.B. interpreted data and revised the manuscript; P.J.M. provided mice, designed the study, analyzed and interpreted data, and revised the manuscript; D.Z., conceived, designed, and supervised the study, analyzed and interpreted data, and wrote the manuscript; and all authors discussed the results and commented on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daohong Zhou, Department of Pharmcodynamics, College of Pharmacy, University of Florida, Gainesville, FL 32611; e-mail: zhoudaohong@cop.ufl.edu.

![Figure 5. SRC assay confirms that M2-MΦs promote expansion of hUCB CD34+ cells ex vivo. (A) Representative flow cytometric charts of analysis of donor-derived human total white blood cells (CD45+), myeloid cells (CD45+CD33+), megakaryocytes (CD45+CD41a+), B cells (CD45+CD19+), and T cells (CD45+CD3+) in BM of the primary (left panel) and secondary (right panel) NSG recipients 4 months after each transplantation. (B) Percentages of human total white blood cells (CD45+), myeloid cells (CD45+CD33+), megakaryocytes (CD45+CD41a+), B cells (CD45+CD19+), and T cells (CD45+CD3+) in BM of the primary NSG recipients. Data are mean ± SEM (n = 17 recipients of three independent transplantations with 3 different units of hUCB CD34+ cell transplants for input and M2-MΦ groups, n = 6 recipients of 1 unit of hUCB CD34+ cell transplant for without [W/O] MΦ group). aP < .05 vs Input, bP < .05 vs W/O MΦ, Mann-Whitney U test. (C) Percentages of human total white blood cells (CD45+), myeloid cells (CD45+CD33+), megakaryocytes (CD45+CD41a+), B cells (CD45+CD19+), and T cells (CD45+CD3+) in BM of the secondary NSG recipients. Data are mean ± SEM (n = 17 for input and M2-MΦ groups, n = 6 for W/O MΦ group). aP < .05 vs Input, bP < .05 vs W/O MΦ, Mann-Whitney U test. (D) Limiting-dilution analysis of the frequency of SRCs in the input CD34+ cells from 2 different units of hUCB cells and the progeny of hUCB CD34+ cells after being cultured with M2-MΦs.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/2/8/10.1182_bloodadvances.2018015685/3/m_advances015685f5.jpeg?Expires=1769085486&Signature=cBsEY0mq2pmpaB8SZGot6EE76M8lrjX3o9t~3riLwKvAGx7cw-waZY~po3-QMcQ2a6FTiSjTkuP5Plq65mBEIRsWNSg3fvx7eCpPK3tsnRx2u6jhU40uSTZQevcbg0MbvrdDhVHwEz-Yvcz7RhkUGmWRREbnlaJHnUyzkr1~iX3m8~PZYYVVBlUJiKZTRv-1UrDQ-BNKVsMFOPEL38lqanN-JlQEpf1CH11PKHeTBB8fSHtEa22lYD1n1tdMXqTwtBKL7afAzeC0yzKH3duudFXZGXJTRl-Xueaos2WOKpibf~TJvBFYCey5EiTpBbsU4RVDQxAKojv4TELZWOgKNg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)