Key Points
PP2A inhibition occurs in AML by 2 different pathways: CIP2A in normal karyotype patients and SETBP1 in adverse karyotype patients.
AKTS473 phosphorylation is a predictor of survival, and diagnostic levels of AKTS473 could be a novel biomarker in AML.
Introduction
Acute myeloid leukemia (AML) is curable with modern treatment including stem cell transplantation (SCT) for a small proportion of patients; however, the majority of patients will die of their disease. Protein phosphatase 2A (PP2A) is a phosphatase and a tumor suppressor, which is often inhibited in malignant cells by the inhibitory proteins cancerous inhibitor of PP2A (CIP2A), SET, and binding protein for SET (SETBP1), leading to abnormal proliferation and differentiation.1 High levels of CIP2A correlate with suppressed PP2A activity and are an adverse prognostic indicator in many malignancies.2-6 CIP2A functions by preventing PP2A-driven dephosphorylation of c-Myc, which results in stabilization.1,4,7-9 CIP2A protein is associated with increased proliferation and cellular transformation in several malignancies,3,10-11 as well as an antiapoptotic phenotype.12 In chronic myeloid leukemia (CML), a high CIP2A protein level is a predictor of subsequent progression to blast crisis.13 In AML, overexpression of the SETBP1 predicts adverse outcome in elderly patients.14 SETBP1 overexpression protects SET from protease cleavage, which results in inhibition of PP2A. However, the clinical relevance of high CIP2A protein in AML is unknown.
Methods
The levels of PP2A and network-related proteins were studied in a panel of 120 AML samples. Samples were collected in the UK AML15 (younger patients, n = 79, of whom 39 were normal karyotype and 40 adverse karyotype) and AML16 trials (n = 41, aged over 60 years). Details of the trial protocols are provided in supplemental Methods.20-21 Patients were stratified into intermediate or adverse risk, based on cytogenetics.22 Flow cytometry was performed as previously described13 for the detection of CIP2A, PP2Ac, PP2AY307 (to assess inactive PP2A), SET, SETBP1, c-Myc, E2F1, AKTT308 and AKTS473, and STAT5 (for more details, see supplemental Methods). The mean fluorescence intensity (MFI) for CIP2A protein level across all 120 patients ranged from 0 to 13.69, with an interquartile range of 1.47 to 5.11 and a median of 3. Using the same statistical approach as previously reported in CML,9 high CIP2A patients are defined as those patients with a CIP2A MFI level ≥3.
Results and discussion
AML15 normal karyotype patients have higher diagnostic CIP2A protein levels than AML15 adverse karyotype (P = .005) or AML16 normal karyotype (P ≤ .001) (Figure 1A). No relationship is seen between the CIP2A level and overall survival or relapse risk (supplemental Figure 1A-B). It was not possible to examine the relationship between CIP2A level and remission rate as all but 1 patient entered remission within 2 courses of treatment. However, in 51% of remitters who later relapsed, high CIP2A was associated with inferior survival from relapse of 56 days, compared with 303 days in patients with low CIP2A (Figure 1B). No correlation was seen between CIP2A messenger RNA levels and clinical outcome. Interestingly, the presence of an FLT3-ITD mutation was associated with higher levels of CIP2A though not with mutated NPM1 messenger RNA (P = .03; Figure 1C).
High CIP2A-expressing patients have inferior survival from relapse. (A) Diagnostic CIP2A protein level in 120 AML patients. AML15 are younger patients; AML16 are older patients. AML15 normal karyotype (N = 39); AML15 adverse karyotype (n = 40); and AML16 normal karyotype (N = 41). (B) AML15 normal karyotype patients stratified by diagnostic CIP2A level. P values are only significant where shown. Survival from relapse. The numbers of assessable cases are shown below each plot. The log-rank test was used for Kaplan-Meier estimates. (C) CIP2A level stratified by FLT3-ITD mutation status. *Denotes statistical significance when compared to normal MNC cells.
High CIP2A-expressing patients have inferior survival from relapse. (A) Diagnostic CIP2A protein level in 120 AML patients. AML15 are younger patients; AML16 are older patients. AML15 normal karyotype (N = 39); AML15 adverse karyotype (n = 40); and AML16 normal karyotype (N = 41). (B) AML15 normal karyotype patients stratified by diagnostic CIP2A level. P values are only significant where shown. Survival from relapse. The numbers of assessable cases are shown below each plot. The log-rank test was used for Kaplan-Meier estimates. (C) CIP2A level stratified by FLT3-ITD mutation status. *Denotes statistical significance when compared to normal MNC cells.
FLT3-ITD mutations induce constitutive tyrosine kinase activity and confer a poor prognosis. In analyses adjusted for FLT3-ITD mutation and censoring at SCT, the association between high CIP2A patients and shorter survival from relapse was retained (hazard ratio, 4.02; P = .04).
High CIP2A protein levels are associated with significantly higher levels of inactive PP2A (assessed by PP2AY307), SET, SETBP1, c-Myc, AKTS473, STAT5, and E2F1, shown in supplemental Figure 2, panels A-G, respectively. These data suggest that a similar CIP2A/PP2A signaling pathway exists to that previously described in CML,9 with the exception of AKTS473, which is a new association (supplemental Figure 2).
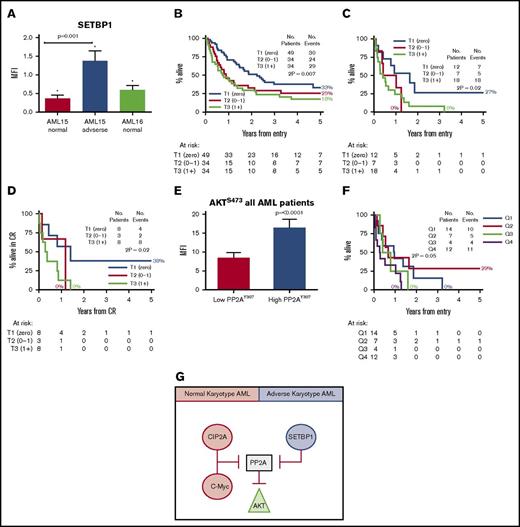
Patients with detectable SETBP1 protein at diagnosis have inferior overall survival. (A) SETBP1 protein expression is significantly elevated in younger AML patients with adverse karyotype (P = .001; Mann-Whitney U test). Error bars are standard error of mean. (B) Kaplan-Meier estimates for overall survival in all patients. All AML patients are stratified into diagnostic SETBP1 terciles: absent (T1), intermediate (T2), and high (T3). (C) Young adverse patients. (D) Relapse-free survival for younger adverse AML patients, stratified by the SETBP1 level at diagnosis. The numbers of assessable cases are shown below each plot. (E) High AKTS473 level is associated with inferior overall survival. (F) Kaplan-Meier estimates for overall survival for young adverse AML patients stratified by quartiles according to the AKTS473 level at diagnosis. High AKTS473 level is associated with inferior overall survival (P = .05; log-rank test). (G) Suggested model of PP2A inhibition in AML. In normal karyotype patients, PP2A is inhibited predominately by CIP2A whereas in adverse-risk patients, SETBP1 inhibition dominates. PP2A inhibition from either source results in high levels of AKTS473. *Denotes statistical significance when compared to normal MNC cells. CR, complete remission.
Patients with detectable SETBP1 protein at diagnosis have inferior overall survival. (A) SETBP1 protein expression is significantly elevated in younger AML patients with adverse karyotype (P = .001; Mann-Whitney U test). Error bars are standard error of mean. (B) Kaplan-Meier estimates for overall survival in all patients. All AML patients are stratified into diagnostic SETBP1 terciles: absent (T1), intermediate (T2), and high (T3). (C) Young adverse patients. (D) Relapse-free survival for younger adverse AML patients, stratified by the SETBP1 level at diagnosis. The numbers of assessable cases are shown below each plot. (E) High AKTS473 level is associated with inferior overall survival. (F) Kaplan-Meier estimates for overall survival for young adverse AML patients stratified by quartiles according to the AKTS473 level at diagnosis. High AKTS473 level is associated with inferior overall survival (P = .05; log-rank test). (G) Suggested model of PP2A inhibition in AML. In normal karyotype patients, PP2A is inhibited predominately by CIP2A whereas in adverse-risk patients, SETBP1 inhibition dominates. PP2A inhibition from either source results in high levels of AKTS473. *Denotes statistical significance when compared to normal MNC cells. CR, complete remission.
The diagnostic level of SETBP1 protein is higher in AML15 patients with adverse karyotype than in comparable normal karyotype patients (P = .001; Figure 2A). One-third of patients had no detectable SETBP1. All AML patients are stratified into diagnostic SETBP1 terciles: absent (T1), intermediate (T2), and high (T3). Patients with detectable SETBP1 protein at diagnosis (T2 and T3) have an inferior overall survival than those in whom SETBP1 protein was undetectable (T1) (P = .01; Figure 2B). This association is also shown within the younger patients with adverse karyotype (P = .02; Figure 2C), in whom a relationship between detectable SETBP1 and inferior relapse-free survival is also seen (P = .02; Figure 2D).
High SETBP1 was associated with high SET levels as it protects SET from degradation and inactivates PP2A (elevated levels of PP2AY307; P = .01). High SETBP1 was associated with elevated CIP2A (P = .02) suggesting that PP2A inhibition occurs by multiple methods. c-Myc and c-MycS62 levels were also elevated (P ≤ .001), consistent with stabilization of c-Myc in the presence of PP2A inhibition. Additionally, AKT was elevated and activated, as evidenced by elevated AKTS473 (P = .01) and AKTT308 levels (P < .0001) (supplemental Figure 3A-G). No difference in the level of E2F1 was observed in adverse karyotype patients, unlike that observed in normal karyotype patients when stratified by CIP2A. These data imply that the mode of PP2A inhibition by SETBP1 is different to the previously described CIP2A/c-Myc/E2F1 inhibition in normal karyotype and CML patients.9
AKT is a substrate for PP2A and its phosphorylation status is a marker of PP2A activity. No relationship was seen between AKTT308 phosphorylation and outcome. AKTS473 phosphorylation was significantly associated with both raised CIP2A and SETBP1 (P = .003 and P = .03; supplemental Figures 2E and 3H). PP2A inactivity was significantly correlated with AKT activation (P ≤ .0001; Figure 2E). High AKTS473 phosphorylation was significantly associated with poorer overall survival in young adverse karyotype patients (P = .05; Figure 2F). These data suggest that PP2A inhibition is a common event in AML and can occur by 2 mechanisms. CIP2A-mediated inhibition of PP2A is seen in normal karyotype patients whereas SETBP1-mediated inhibition of PP2A is observed in adverse karyotype patients. Both CIP2A and SETBP1 act to inhibit PP2A, and AKTS473 phosphorylation may therefore be acting as a readout of PP2A inhibition (Figure 2G).
Multivariable analysis using Cox regression was used to find the most important predictors of outcome in all 120 patients. Factors included the following: age, trial (AML15 vs AML16), diagnostic cytogenetic category, presentation white blood cell count, secondary leukemia or the presence of FLT3-ITD, plus the PP2A network proteins: total PP2A, PP2AY307, CIP2A, SET, SETBP1, c-Myc, c-MycS62, STAT5, AKTT308, and AKTS473. The level of AKTS473 was the third most significant factor in analysis of survival censored at SCT, after age and cytogenetic category; it was therefore the most powerful molecular marker. Multivariate analysis also showed that the CIP2A diagnostic protein level was a stronger predictor than FLT3-ITD mutation at predicting survival from relapse (hazard ratio, 4.02; P = .04).
In summary, we demonstrate 3 principal findings. First, high CIP2A protein levels correlate with poor survival from relapse in normal karyotype patients. CIP2A protein level strongly correlates with FLT3-ITD mutation. Second, SETBP1 is higher in younger patients with adverse karyotype, and detectable levels correlate with poor overall survival. Third, the phosphorylation status of AKT may be a useful novel biomarker for outcome in AML. This could be assessed as part of a diagnostic flow cytometry antibody panel, although this would require optimization/validation before being used in a routine clinical setting. Interestingly, a specific AKT inhibitor has been reported to inhibit cell proliferation and clonogenic properties and to induce apoptosis in AML cells with high-risk cytogenetics.23 Together with the present findings, this suggests that AKT phosphorylation status may represent a novel therapeutic target in high-risk AML and should be tested prospectively as part of a clinical trial.
The full-text version of this article contains a data supplement.
Acknowledgment
The authors thank Robert Harris for continuing support and advice on this work.
Authorship
Contribution: C.M.L. performed experiments, designed the study, and wrote the manuscript; C.M.L., L.J.S., N.C., and A.K.H. performed experiments; R.K.H. performed statistical analysis; R.K.H. and A.K.B. provided clinical samples; and C.M.L. and R.E.C. designed the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Claire M. Lucas, Department of Molecular and Clinical Cancer Medicine, Institute of Translational Medicine, University of Liverpool, Sherrington Building, Ashton St, Liverpool L69 3GE, United Kingdom; e-mail: cml@liv.ac.uk.