Key Points
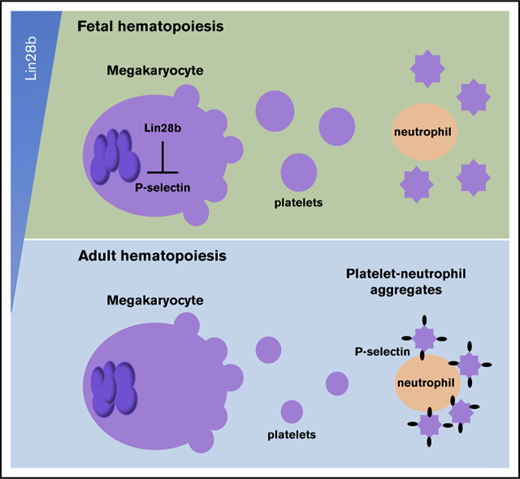
Fetal murine platelets have low levels of P-selectin and do not readily associate with neutrophils in vitro and in vivo.
Lin28b is highly expressed in fetal murine megakaryocytes and negatively regulates the expression of platelet P-selectin.
Abstract
Platelets are essential for hemostasis; however, several studies have identified age-dependent differences in platelet function. To better understand the origins of fetal platelet function, we have evaluated the contribution of the fetal-specific RNA binding protein Lin28b in the megakaryocyte/platelet lineage. Because activated fetal platelets have very low levels of P-selectin, we hypothesized that the expression of platelet P-selectin is part of a fetal-specific hematopoietic program conferred by Lin28b. Using the mouse as a model, we find that activated fetal platelets have low levels of P-selectin and do not readily associate with granulocytes in vitro and in vivo, relative to adult controls. Transcriptional analysis revealed high levels of Lin28b and Hmga2 in fetal, but not adult, megakaryocytes. Overexpression of LIN28B in adult mice significantly reduces the expression of P-selectin in platelets, and therefore identifies Lin28b as a negative regulator of P-selectin expression. Transplantation of fetal hematopoietic progenitors resulted in the production of platelets with low levels of P-selectin, suggesting that the developmental regulation of P-selectin is intrinsic and independent of differences between fetal and adult microenvironments. Last, we observe that the upregulation of P-selectin expression occurs postnatally, and the temporal kinetics of this upregulation are recapitulated by transplantation of fetal hematopoietic stem and progenitor cells into adult recipients. Taken together, these studies identify Lin28b as a new intrinsic regulator of fetal platelet function.
Introduction
Platelets are anucleate, terminally differentiated blood cells that are shed from mature megakaryocytes in the bone marrow and lung.1,2 During steady state hemostasis, circulating platelets adhere to sites of vascular injury to prevent bleeding and promote inflammation and immunity.3-6 Although platelet function in adult hemostasis has been well characterized, less is known about age-dependent differences in megakaryocyte and platelet function.7,8 Specifically, platelets from preterm human neonates are hyporeactive to the platelet agonists collagen, thrombin, adenosine 5′-diphosphate, and thromboxane.9 In mice, thrombus formation is unstable in yolk sac vessels at embryonic day 13.5 (E13.5), and becomes progressively more stable as gestation proceeds.10 In addition, murine fetal platelets have reduced expression of integrin-activating proteins and a significant reduction in the total expression of the cell adhesion molecule P-selectin.10 Together, these studies provide evidence that platelets have altered function during embryogenesis, with reduced expression of crucial proteins that support platelet function in hemostasis.
During embryonic development, platelet production arises from overlapping waves of primitive and definitive hematopoietic progenitors that arise in the yolk sac before the emergence of hematopoietic stem cells (HSCs).11,12 Whereas platelets are generated from temporally distinct progenitor populations, these fetal progenitors cumulatively share a core transcriptional program.13 Recently, a fetal-specific transcriptional network has been identified in myelo-erythroid progenitors in the fetal liver that is regulated by the Lin28b-let7-Hmga2 axis.14 Lin28b is an RNA binding protein that confers a fetal-specific program in multiple hematopoietic lineages, including HSCs,15 T cells,16 B cells,17 and erythroid cells.18 In mammals, Lin28b is highly expressed during fetal development and prevents the biogenesis of let-7 microRNAs.19 Mature let-7 inhibits the expression of several transcription factors, including MYC, RAS, cyclin D, and HMGA2, to promote cellular differentiation.20 To date, the Lin28b-let7-Hmga2 axis has not been evaluated in the megakaryocyte and platelet lineage; however, its expression in fetal myelo-erythroid progenitors suggests it may contribute to the prenatal platelet-forming lineages. Consistent with a possible role for Lin28b in fetal megakaryopoiesis, megakaryocytes derived from human embryonic stem (ES) cells express higher levels of LIN28B when compared with adult controls.21 Taken together, these data suggest that Lin28b may also be active in the megakaryocyte lineage.
P-selectin is an abundantly expressed protein in platelets and endothelial cells, and is stored in α-granules or Weibel-Palade bodies, respectively.22 In platelets, P-selectin is translocated to the surface after activation, where it recruits and stimulates circulating neutrophils and monocytes to promote inflammation and immunity.23-27 Whereas fetal platelets have reduced total expression of P-selectin,10 the temporal expression and potential functions of P-selectin during fetal development have received little attention. Previous studies have compared the human and murine promoter sequences and identified a conserved negative regulatory Ets/HMG element,28 suggesting it may be a target of the fetal Lin28b-let-7-Hmga2 axis.
Here, we demonstrate that Lin28b is highly expressed in fetal murine megakaryocytes and negatively regulates the expression of platelet P-selectin. Transplantation studies provide functional evidence that the postnatal upregulation of P-selectin in murine platelets is cell-intrinsic. Furthermore, neonatal platelets with low levels of P-selectin do not readily associate with neutrophils in vitro and in vivo, suggesting that platelets acquire enhanced capacity to engage inflammatory and immune responses after birth. Taken together, our studies support the concept of Lin28b as a novel mediator of a fetal-specific program of platelet function.
Methods
Integrin activation and P-selectin surface expression
One million platelets were incubated with 0.1 μg anti-mouse CD41 E450, Ter119 APC, 5 μL JonA PE, and 0.25 μg CD62P fluorescein isothiocyanate and activated with 0.5 U/mL human α-thrombin for 10 minutes at room temperature in the presence of 1 mM CaCl2. Samples were fixed with 1% formaldehyde and diluted with phosphate-buffered saline before running on an LSRII flow cytometer (BDbiosciences). Analysis of integrin activation and P-selectin surface expression was performed using FlowJo software.
Detection of leukocyte-platelet aggregates in vivo
Adult or neonatal whole blood from outbred ICR mice was stained with 0.1 μg anti-mouse CD45 E450, 0.2 μg GR-1 AF488, and 0.2 μg CD41 PE for 15 minutes at room temperature, and then fixed and lysed with 1X fix/lyse buffer (ebioscience). Samples were washed and stained with 5 μM DRAQ5, and images were acquired at 40× on an ImageStreamX imaging flow cytometer (EMDmillipore). Granulocytes were gated based on CD45 and Gr-1 positivity, and evaluated for bound platelets.
Neutrophil-platelet rosettes
A total of 1 × 106 washed adult or neonatal platelets were activated with 0.2 units/mL thrombin for 10 minutes at 37°C. Activation was stopped using 1 U/mL hirudin (Sigma), and platelets were mixed 10:1 with neutrophils isolated from the bone marrow and incubated in the presence of anti-mouse CD41 PE, GR-1 fluorescein isothiocyanate, CD45 E450 for 30 minutes at room temperature. Rosettes were fixed in 1% formaldehyde, diluted with phosphate-buffered saline, and stained with 5 μM DRAQ5 before imaging flow cytometric analysis. Focused single neutrophils double-positive for CD45 and GR-1 were used to identify rosettes.
Immunocytochemistry
Resting platelets were fixed with 2% formaldehyde and allowed to settle onto Bond-rite microscope slides for up to 4 hours. Immunocytochemistry was performed as previously described.29 Samples were stained with antibodies for Vascular Endothelial Growth Factor (VEGF), von Willebrand Factor (VWF), SELP, or COL18A1, detected with secondary antibodies conjugated with Alexa Fluors 555 or 647 (Invitrogen), and visualized on a Nikon 80i Optiphot microscope with widefield fluorescence, using a 100× Plan Fluor (NA 1.3) objective. Z-stacks were captured and images deconvolved using the Landweber methodology, and then processed to generate maximum-intensity projections, using Nikon AR software (version 4.3).
Cultured megakaryocytes
Hematopoietic progenitor cells (HPCs; Kit+) were enriched from E12.5 fetal liver and adult bone marrow by incubation with 0.5 μg anti-mouse biotinylated CD117 (ebiosciences, clone 2B8) and Streptavidin-conjugated magnetic particles (BD bioscience) according to the manufacturer’s protocol. Progenitors were differentiated into megakaryocytes by plating 2 × 105 live cells per well of a 6-well tissue culture plate containing 3 mL megakaryocyte media, and increased in CD41 expression (IMDM, 100 ng/mL thrombopoietin, 20% BIT, 2 mM GlutaMAX, 0.1% 2-ME) over the course of 3 days. Megakaryocytes were isolated by incubation with 1 μg anti-mouse biotinylated CD41 selection before RNA extraction, using an RNAeasy kit (Qiagen).
Transplantation of hematopoietic stem and progenitor cells
Dissociated whole bone marrow or E14.5 fetal livers were stained and sorted using lineage markers (anti-mouse Ter119, B220, CD3E, Gr-1, CD16/32, CD71), as well as Sca1, c-kit, and PI. At least 1 × 104 ubiquitin C (UBC)–green fluorescent protein (GFP)+ lin−sca−kit+ (HPCs) and 3000 UBC-GFP+ lin−sca+kit+ (hematopoietic stem and progenitor cells [HSPCs]) were retro-orbitally injected into individual C57/B6 recipients that were preconditioned with 4 Gy total body irradiation 2.5 to 3 days before transplantation.
Platelet analysis of transplants
Whole-blood platelet activation was performed according to manufacturer’s instructions of JON/A PE antibody (Emfret). Platelets were activated with 0.5 U/mL thrombin in the presence of anti-mouse CD41 E450, Ter119 PE-Cy7, JON/A PE, and CD62P AF647 for 10 minutes at room temperature, diluted, and analyzed immediately by flow cytometry. FlowJo software was used to identify GFP+ and GFP− platelets and to calculate the median fluorescence intensity of integrin activation (Jon/A PE) and P-selectin surface expression (CD62P AF647).
Statistics
Statistics were performed using Prism (GraphPad Software). Groups of 2 or fewer were analyzed using 2-tailed unpaired Student t test; P < .05 was considered significant. Groups of 2 or more with 1 variable were analyzed using a 1-way analysis of variance (ANOVA), followed by a Bonferroni posttest; P < .05 was considered significant. Groups of 2 or more with 2 variables were analyzed using a 2-way ANOVA followed by a Bonferroni posttest; P < .05 was considered significant.
Results
Reduced P-selectin content in embryonic and fetal platelets correlates with reduced transcripts for P-selectin in fetal megakaryocytes
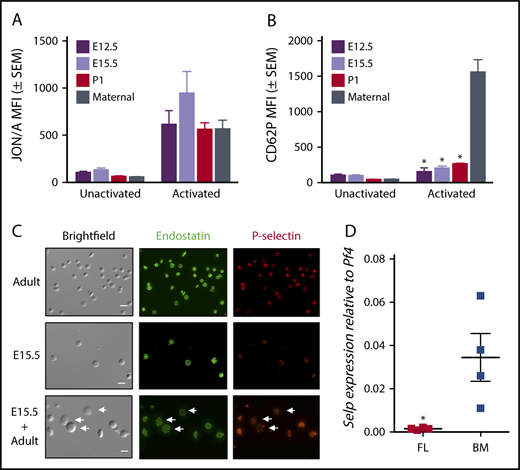
It has recently been reported that murine embryonic platelets have significantly reduced levels of P-selectin compared with adult platelets; however, the mechanism underlying the developmental regulation of P-selectin is unknown.10 Thrombin-mediated platelet activation results both in a change in conformation of the platelet-specific integrin αIIbβ3 and in α-granule secretion, which leads to the surface expression of P-selectin.30 Thrombin-induced activation of platelets isolated from E12.5 and E15.5 murine embryos, postnatal day 1 (P1) pups, and adult dams resulted in a significant change in conformation of integrin αIIbβ3 (JON/A median fluorescence intensity; Figure 1A). In contrast, parallel analysis of P-selectin surface expression revealed no significant increase with the activation of fetal and neonatal platelets, which is significantly reduced in comparison with P-selectin surface expression on platelets from adult dams (Figure 1B). These data indicate that despite robust thrombin-mediated activation, fetal and neonatal platelets display extremely low levels of P-selectin on their surface. Next, we sought to evaluate the intracellular levels of P-selectin. Immunofluorescence microscopy revealed similar expression levels of Endostatin in punctae in both adult and E15.5 platelets, but only rare punctae of P-selectin–positive granules in fetal platelets (Figure 1C). The differential content of P-selectin was evident in costained mixes of embryonic and adult mouse platelets distinguished by their difference in size (Figure 1C, bottom).
Reduced P-selectin content in embryonic and fetal platelets correlates with reduced transcripts for P-selectin in fetal megakaryocytes. (A) Integrin (αIIbβ3) activation in unactivated and thrombin-activated primary platelets isolated from E12.5 and E15.5 murine embryos, P1 pups, and adult female dams. Mean ± standard error of the mean (SEM). (B) Simultaneous analysis of samples in panel A for surface expression of P-selectin (CD62P) in unactivated and thrombin-activated primary platelets. Significance was determined using a 2-way ANOVA followed by a Bonferroni posttest. *P < .001 vs activated maternal sample, n ≥ 3. (C) Immunofluorescence microscopy of fetal (E15.5) and adult platelets reveals fewer punctae of P-selectin–positive granules in fetal platelets relative to adult controls. Deconvolved merged maximum intensity projection images are shown. Scale bar, 5 μm. Similar patterns were observed in 3 independent experiments. (D) Transcript levels of P-selectin (Selp) relative to platelet factor 4 (Pf4) in primary megakaryocytes isolated from E15.5 fetal livers, and adult bone marrow. Extremely low levels of P-selectin transcripts were found in primary megakaryocytes harvested from E15.5 fetal liver. Significance was determined using an unpaired 2-tailed Student t test, *P < .05; n = 4. MFI, median fluorescence intensity.
Reduced P-selectin content in embryonic and fetal platelets correlates with reduced transcripts for P-selectin in fetal megakaryocytes. (A) Integrin (αIIbβ3) activation in unactivated and thrombin-activated primary platelets isolated from E12.5 and E15.5 murine embryos, P1 pups, and adult female dams. Mean ± standard error of the mean (SEM). (B) Simultaneous analysis of samples in panel A for surface expression of P-selectin (CD62P) in unactivated and thrombin-activated primary platelets. Significance was determined using a 2-way ANOVA followed by a Bonferroni posttest. *P < .001 vs activated maternal sample, n ≥ 3. (C) Immunofluorescence microscopy of fetal (E15.5) and adult platelets reveals fewer punctae of P-selectin–positive granules in fetal platelets relative to adult controls. Deconvolved merged maximum intensity projection images are shown. Scale bar, 5 μm. Similar patterns were observed in 3 independent experiments. (D) Transcript levels of P-selectin (Selp) relative to platelet factor 4 (Pf4) in primary megakaryocytes isolated from E15.5 fetal livers, and adult bone marrow. Extremely low levels of P-selectin transcripts were found in primary megakaryocytes harvested from E15.5 fetal liver. Significance was determined using an unpaired 2-tailed Student t test, *P < .05; n = 4. MFI, median fluorescence intensity.
Because most α-granule contents are packed into nascent platelets during megakaryocyte maturation, we next asked whether primary murine fetal megakaryocytes contain P-selectin transcripts. As shown in Figure 1D, extremely low levels of P-selectin transcripts normalized to Platelet Factor 4 (PF4) transcripts were found in primary megakaryocytes isolated from E15.5 fetal livers compared with megakaryocytes isolated from adult bone marrow. Taken together, these data support the concept that little P-selectin is expressed at the transcript or protein level in the megakaryocyte lineage during murine embryogenesis.
Fetal/neonatal platelets contain α-granules and secrete PF4 on stimulation
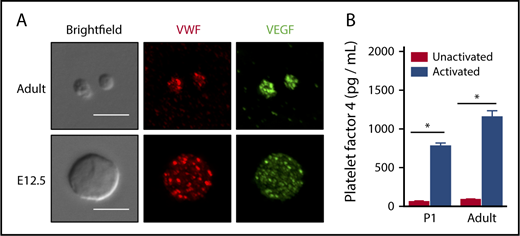
We had previously determined that embryonic murine platelets contain α-granules; however, given the low levels of P-selectin expressed on fetal platelets, we chose to investigate granule secretion in fetal and adult platelets.11 Adult platelets contain a variety of bioactive molecules in their α-granules beyond P-selectin, including VEGF, PF4, and VWF.31,32 Immunocytochemical analysis revealed that both E12.5 fetal and adult platelets each contain punctate patterns of VEGF and VWF expression (Figure 2A). To determine whether neonatal platelets can secrete α-granule proteins on activation,33,34 we stimulated primary newborn (P1) and adult platelets with thrombin, which resulted in significant increases in the concentration of PF4 in platelet supernatants (Figure 2B). These results demonstrate that P-selectin is selectively downregulated in embryonic and fetal platelets that contain and secrete known α-granule proteins after activation.
Fetal/neonatal platelets contain a-granules and secrete PF4 on stimulation. (A) Immunofluorescence microscopy of VWF and VEGF protein reveals punctate patterns of expression in resting adult and fetal (E15.5) platelets. Deconvolved merged maximum intensity projection images are shown. Scale bar, 5 μm. Similar patterns were observed in 3 independent experiments. (B) Significant amounts of Pf4 were released after activation of P1 and adult platelets. Platelet activation with thrombin (0.5 U/mL) increases the concentration of Pf4 similarly in supernatants from P1 and adult platelets when evaluated by enzyme-linked immunosorbent assay. Significance was determined using a 2-way ANOVA followed by a Bonferroni posttest, *P < .05 between unactivated and activated samples per age group. Mean ± standard error of the mean shown; n = 4.
Fetal/neonatal platelets contain a-granules and secrete PF4 on stimulation. (A) Immunofluorescence microscopy of VWF and VEGF protein reveals punctate patterns of expression in resting adult and fetal (E15.5) platelets. Deconvolved merged maximum intensity projection images are shown. Scale bar, 5 μm. Similar patterns were observed in 3 independent experiments. (B) Significant amounts of Pf4 were released after activation of P1 and adult platelets. Platelet activation with thrombin (0.5 U/mL) increases the concentration of Pf4 similarly in supernatants from P1 and adult platelets when evaluated by enzyme-linked immunosorbent assay. Significance was determined using a 2-way ANOVA followed by a Bonferroni posttest, *P < .05 between unactivated and activated samples per age group. Mean ± standard error of the mean shown; n = 4.
Neonatal platelets do not readily associate with neutrophils in vivo or in vitro
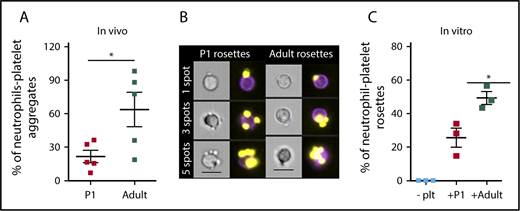
The exposure of P-selectin on the surface of adult platelets results in the generation of circulating neutrophil-platelet aggregates.25,35,36 Because activated fetal and neonatal platelets have very low levels of P-selectin on their surface, we hypothesized that fewer neutrophil-platelet aggregates would be found in the neonatal vs the adult circulation. In support of this hypothesis, significantly fewer platelet-bound neutrophils were identified in neonatal whole blood when compared with adult controls (Figure 3A). Because fewer circulating neutrophil-platelet aggregates in neonatal circulation could be a result of lower levels of circulating platelets found in the murine neonatal circulation, or impaired platelet function, we evaluated neutrophil-platelet association in vitro. Enriched bone marrow neutrophils from adult mice were mixed with activated neonatal or adult platelets in a 1:10 ratio.36,37 Rosettes, defined as single (CD45+/GR-1+) neutrophils with 2 or more (CD41+) platelets attached, were analyzed and quantified using imaging flow cytometry, as shown in Figure 3B. Despite the addition of similar numbers of platelets, significantly fewer neutrophil-platelet rosettes were formed after incubation with neonatal platelets when compared with adult platelets (Figure 3C). Taken together, these data indicate that primary neonatal platelets have a decreased ability to interact with neutrophils when compared with adult platelets.
Neonatal platelets do not readily associate with granulocytes in vivo or in vitro. (A) Significantly fewer endogenous neutrophil-platelet aggregates are found in the circulation of neonates compared with adults. n = 5. Significance determined using unpaired Student t test P < .05. Leukocytes were defined as CD45+, granulocytes were defined as CD45+/GR-1+ double-positive, and platelets were CD41+. (B) Representative images of neutrophil-platelet rosettes with 1, 3, and 5 platelet spots. CD41 PE is shown in yellow, and GR-1 fluorescein isothiocyanate is shown in purple. Images were collected at 40× magnification. Scale bar, 10 μm. (C) Fewer rosettes were formed when neutrophils were mixed with activated platelets from P1 pups (+P1) when compared with rosette formation with activated platelets from adults (+Adult). Platelets were activated with 0.5 units/mL thrombin before mixing in a 10:1 ratio with bone marrow-enriched neutrophils. Rosettes were defined as a single neutrophil with 2 or more platelets attached. Significance was determined using an unpaired 2-tailed Student t test *P < .05; n = 3.
Neonatal platelets do not readily associate with granulocytes in vivo or in vitro. (A) Significantly fewer endogenous neutrophil-platelet aggregates are found in the circulation of neonates compared with adults. n = 5. Significance determined using unpaired Student t test P < .05. Leukocytes were defined as CD45+, granulocytes were defined as CD45+/GR-1+ double-positive, and platelets were CD41+. (B) Representative images of neutrophil-platelet rosettes with 1, 3, and 5 platelet spots. CD41 PE is shown in yellow, and GR-1 fluorescein isothiocyanate is shown in purple. Images were collected at 40× magnification. Scale bar, 10 μm. (C) Fewer rosettes were formed when neutrophils were mixed with activated platelets from P1 pups (+P1) when compared with rosette formation with activated platelets from adults (+Adult). Platelets were activated with 0.5 units/mL thrombin before mixing in a 10:1 ratio with bone marrow-enriched neutrophils. Rosettes were defined as a single neutrophil with 2 or more platelets attached. Significance was determined using an unpaired 2-tailed Student t test *P < .05; n = 3.
Fetal megakaryocytes express high transcript levels of Lin28b and Hmga2 compared with adult megakaryocytes
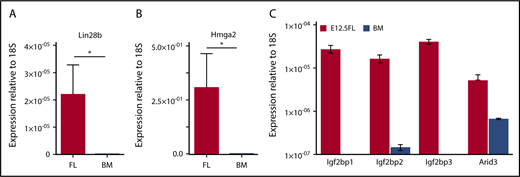
Transcripts for LIN28B are highly expressed in human fetal megakaryocytes compared with adult controls.21 We therefore asked whether murine fetal megakaryocytes also contain elevated transcript levels of Lin28b. As shown in Figure 4A-B, megakaryocytes derived from E12.5 livers expressed significantly higher levels of Lin28b and Hmga2 transcripts relative to adult bone marrow-derived megakaryocytes. In addition, 4 other known RNA targets of Lin28b/let7 signaling are also present at significantly higher transcript levels in fetal vs adult megakaryocytes (Figure 4C). Because these fetal and adult megakaryocytes were cultured under identical in vitro conditions, our findings suggest that the Lin28b-Hmga2 signaling axis may be intrinsically active in fetal megakaryopoiesis.
Fetal megakaryocytes express high levels of Lin28B and Hmga2 transcripts, as well as several other known Lin28b/Let-7 target genes. (A) Transcript levels of Lin28B relative to small ribosomal subunit 18S (18S) in cultured megakaryocytes from E12.5 fetal livers or adult bone marrow. Significance was determined using an unpaired 2-tailed Student t test *P < .0001; n = 3. (B) Transcript levels of high-mobility group AT-hook 2 (Hmga-2) relative to 18S in cultured megakaryocytes from embryonic day 12.5 fetal livers or adult bone marrow. Significance was determined using an unpaired 2-tailed Student t test *P < .0001; n = 3. (C) Transcript levels of Igf2bp1, Igf2bp2, Igf2bp3, and Arid3 relative to 18S in cultured megakaryocytes from E12.5 fetal livers or adult bone marrow. Significance was determined using 2-way ANOVA. *P < .0001; n = 3.
Fetal megakaryocytes express high levels of Lin28B and Hmga2 transcripts, as well as several other known Lin28b/Let-7 target genes. (A) Transcript levels of Lin28B relative to small ribosomal subunit 18S (18S) in cultured megakaryocytes from E12.5 fetal livers or adult bone marrow. Significance was determined using an unpaired 2-tailed Student t test *P < .0001; n = 3. (B) Transcript levels of high-mobility group AT-hook 2 (Hmga-2) relative to 18S in cultured megakaryocytes from embryonic day 12.5 fetal livers or adult bone marrow. Significance was determined using an unpaired 2-tailed Student t test *P < .0001; n = 3. (C) Transcript levels of Igf2bp1, Igf2bp2, Igf2bp3, and Arid3 relative to 18S in cultured megakaryocytes from E12.5 fetal livers or adult bone marrow. Significance was determined using 2-way ANOVA. *P < .0001; n = 3.
Induced expression of LIN28B in adult mice significantly reduces platelet P-selectin expression
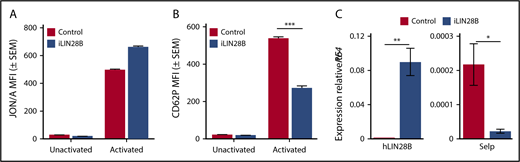
Because embryonic platelets are characterized by low levels of P-selectin and the P-selectin promoter contains HMGA binding sites, we hypothesized that Lin28b may negatively regulate P-selectin expression. A fetal program of gene expression can be reactivated by the ectopic expression of Lin28b in adult cells.14-16,18 We therefore evaluated platelet P-selectin expression in a previously published mouse model genetically engineered to overexpress human LIN28B (iLIN28B).14 Thrombin stimulation of platelets from control and iLIN28B resulted in significant amounts of integrin αIIbβ3 activation (Figure 5A). However, analysis of these same platelets revealed a significant decrease in the amount of P-selectin on the surface of iLIN28B platelets relative to control platelets (Figure 5B). These findings were also duplicated using a mouse model that specifically expresses LIN28B in the hematopoietic system (iLIN28B vav-cre) (supplemental Figure 1).14 Megakaryocytes cultured from these mice confirmed the increase in LIN28B, as well as decrease in P-selectin message (Figure 5C). Taken together, these results demonstrate that Lin28b is a negative regulator of platelet P-selectin expression.
Induction of human LIN28B in adult mice reduces platelet P-selectin transcript and cell surface expression. (A) Integrin activation in unactivated and thrombin-activated platelets from mice with doxycycline-induced expression of LIN28B (iLIN28B) and control mice. (B) Surface expression of P-selectin (CD62P) in thrombin-activated platelets from iLIN28B and control mice. Significance was determined using a 2-way ANOVA followed by a Bonferroni posttest ***P < .001; n ≥ 6. (C) Transcript levels of human LIN28B and murine Selp relative to murine PF4 in cultured megakaryocytes from mice with doxycycline-induced expression of human LIN28B (iLIN28B) and control mice. Significance was determined using 2-way ANOVA. *P < .05; **P < .005; n = 3.
Induction of human LIN28B in adult mice reduces platelet P-selectin transcript and cell surface expression. (A) Integrin activation in unactivated and thrombin-activated platelets from mice with doxycycline-induced expression of LIN28B (iLIN28B) and control mice. (B) Surface expression of P-selectin (CD62P) in thrombin-activated platelets from iLIN28B and control mice. Significance was determined using a 2-way ANOVA followed by a Bonferroni posttest ***P < .001; n ≥ 6. (C) Transcript levels of human LIN28B and murine Selp relative to murine PF4 in cultured megakaryocytes from mice with doxycycline-induced expression of human LIN28B (iLIN28B) and control mice. Significance was determined using 2-way ANOVA. *P < .05; **P < .005; n = 3.
Expression of P-selectin in platelets is intrinsically regulated by fetal hematopoietic progenitors
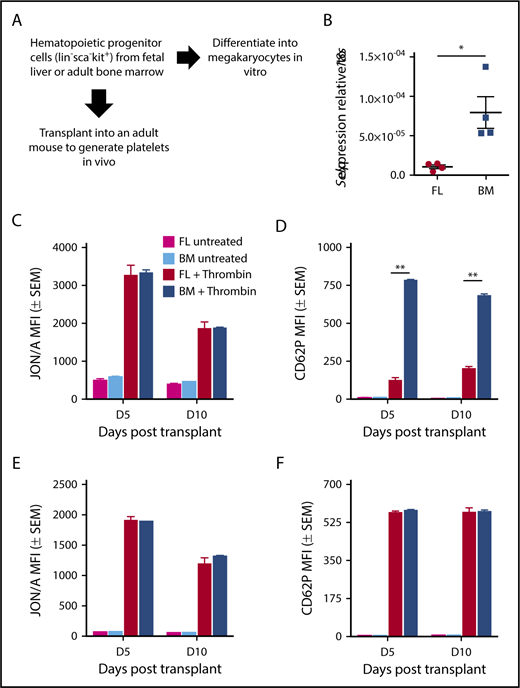
Megakaryocyte maturation and subsequent platelet production during much of embryogenesis occurs in the liver.11 This is in contrast to megakaryocyte maturation and platelet production during steady state adult hemostasis, which takes place primarily in the bone marrow and the lung. To investigate whether these differences in niche contribute to the regulation of P-selectin transcription, we isolated lineage−, kit+, sca− cells, which are enriched for HPCs, from E14.5 livers and adult bone marrow, and differentiated them into megakaryocytes in vitro (Figure 6A). P-selectin transcript levels were significantly lower in cultured fetal megakaryocytes compared with adult megakaryocytes (Figure 6B).
P-selectin is intrinsically regulated in fetal hematopoietic progenitors. (A) Experimental scheme. Kit-positive hematopoietic progenitors were derived from E12.5 fetal liver or adult bone marrow and cultured for 3 days in the presence of TPO. Megakaryocytes were isolated by CD41 selection. For transplant studies, lin−sca−kit+ (HPC) cells were sorted from E14.5 fetal livers and from adult bone marrow of UBC-GFP mice and transplanted into GFP− recipients. Whole blood from recipients was activated on days 5 and 10 posttransplant, with 0.5 U/mL thrombin in the presence of antibodies for activated αIIbβ3 (JON/A) and surface P-selectin (CD62P). Donor (GFP+) and recipient (GFP−) platelet populations were evaluated individually for activated integrin αIIbβ3 and P-selectin surface expression. (B) Transcript levels of P-selectin (Selp) relative to 18S in cultured megakaryocytes. Significance was determined using an unpaired 2-tailed Student t test *P < .05; n = 5. (C) Integrin activation (αIIbβ3) in donor (GFP+) platelets. Analysis was performed using 2-way ANOVA with Bonferroni posttest, n ≥ 3. (D) P-selectin surface expression in donor (GFP+) platelets. Significance was determined using a 2-way ANOVA followed by a Bonferroni posttest, **P < .001; n ≥ 3. (E) Integrin activation (αIIbβ3) in donor (GFP−) platelets. Analysis was performed using 2-way ANOVA with Bonferroni posttest, n ≥ 3. (F) P-selectin surface expression in donor (GFP−) platelets. No significance was detected using a 2-way ANOVA with Bonferroni posttest, n ≥ 3.
P-selectin is intrinsically regulated in fetal hematopoietic progenitors. (A) Experimental scheme. Kit-positive hematopoietic progenitors were derived from E12.5 fetal liver or adult bone marrow and cultured for 3 days in the presence of TPO. Megakaryocytes were isolated by CD41 selection. For transplant studies, lin−sca−kit+ (HPC) cells were sorted from E14.5 fetal livers and from adult bone marrow of UBC-GFP mice and transplanted into GFP− recipients. Whole blood from recipients was activated on days 5 and 10 posttransplant, with 0.5 U/mL thrombin in the presence of antibodies for activated αIIbβ3 (JON/A) and surface P-selectin (CD62P). Donor (GFP+) and recipient (GFP−) platelet populations were evaluated individually for activated integrin αIIbβ3 and P-selectin surface expression. (B) Transcript levels of P-selectin (Selp) relative to 18S in cultured megakaryocytes. Significance was determined using an unpaired 2-tailed Student t test *P < .05; n = 5. (C) Integrin activation (αIIbβ3) in donor (GFP+) platelets. Analysis was performed using 2-way ANOVA with Bonferroni posttest, n ≥ 3. (D) P-selectin surface expression in donor (GFP+) platelets. Significance was determined using a 2-way ANOVA followed by a Bonferroni posttest, **P < .001; n ≥ 3. (E) Integrin activation (αIIbβ3) in donor (GFP−) platelets. Analysis was performed using 2-way ANOVA with Bonferroni posttest, n ≥ 3. (F) P-selectin surface expression in donor (GFP−) platelets. No significance was detected using a 2-way ANOVA with Bonferroni posttest, n ≥ 3.
In the adult, platelet P-selectin is partially dependent on circulating fibrinogen levels.38 Because many hematological parameters can be altered during fetal/neonatal development, we sought to determine whether the intrinsic differences between fetal liver and adult bone marrow megakaryocytes are maintained in vivo. Therefore, we transplanted HPCs sorted from E14.5 livers or from adult bone marrow of UBC-GFP mice into sublethally irradiated GFP− recipient mice (Figure 6A). Donor platelet production was equally robust from fetal liver-derived and from bone marrow-derived HPCs (supplemental Figure 3A). Donor (GFP+; Figure 6C-D) and recipient (GFP−; Figure 6E-F) platelets were analyzed both for integrin activation and for P-selectin surface expression, with representative flow plots shown in supplemental Figure 2. Recipient platelets at rest and after activation had comparable levels of Integrin αIIbβ3 activation on all the days analyzed after transplantation (Figure 6E). Similar results were found with P-selectin surface expression on recipient platelets (Figure 6F). Donor platelets derived both from bone marrow and from fetal liver HPCs had comparable amounts of integrin activation at rest and after activation on days 5 and 10 posttransplantation (Figure 6C). In contrast, activated donor platelets from fetal liver HPCs had lower levels of P-selectin surface expression when compared with donor platelets from bone marrow HPCs on days 5 and 10 (Figure 6D). These results indicate that the low levels of P-selectin expression on embryonic and neonatal platelets are not a result of differences between the fetal liver or adult bone marrow or lung “niches,” or potential differences in circulating fibrinogen levels, but rather, are a result of intrinsic differences between fetal and adult megakaryocytes.
Expression of P-selectin increases postnatally on the surface of activated platelets
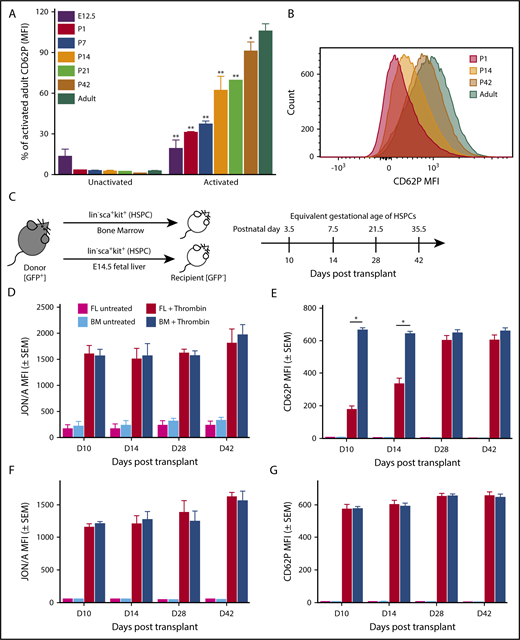
To determine when P-selectin surface expression reaches adult levels, we analyzed platelets from E12.5 mouse fetuses and from mice at days 1, 7, 14, 21, and 42 after birth. P-selectin surface expression on activated platelets gradually increased from 25% in fetal platelets at E12.5 to 70% at postnatal day 21, reaching adult levels by 8 weeks after birth (Figure 7A). We do not observe a combination of high- and low-expressing platelets at intermediate points (Figure 7B). This observation, along with the prolonged time frame, the gradual change, and the short platelet lifespan, is not consistent with the change in P-selectin expression being a result of replacement of fetal megakaryocyte progenitor–derived platelets with adult progenitor-derived platelets. Instead, it suggests that new megakaryocytes generated from postnatal HSCs progressively upregulate P-selectin expression.
The onset of P-selectin expression occurs postnatally and is regulated by a developmental transition in HSPCs. (A) P-selectin surface expression in unstimulated and stimulated platelets isolated from various gestational ages postnatally. Values shown are expressed as a percent of CD62P MFI on activated adult platelets. Platelets harvested from murine embryos (E12.5), pups (days 1, 7, 14, 21, and 42 after birth), and non-pregnant adult mice, were activated with thrombin (0.5 units/mL) in the presence of antibodies to detect activated αIIbβ3 (JON/A) and surface P-selectin (CD62P). Significance was determined using a 2-way ANOVA followed by a Bonferroni posttest *P < .01, ** P < .001 versus activated adult P-selectin surface expression; n ≥ 3. (B) Representative histogram of MFI of CD62P (P-selectin) on activated platelets from P1, P14, P42, and adult. Postnatally, a single platelet population transitions from expression low levels of P-selectin (P1) to higher levels of P-selectin (Adult). (C) Experimental scheme. Lin−sca+kit+ (HSPCs) cells were sorted from E14.5 fetal livers and adult bone marrow of UBC-GFP mice and transplanted into sublethally irradiated GFP− recipients. Whole blood from recipients was activated on days 10, 14, 28, and 42 posttransplantation with 0.5 U/mL thrombin for 10 minutes in the presence of antibodies for activated αIIbβ3 (JON/A) and surface P-selectin (CD62P). HSPCs were taken from E14.5 fetal livers; therefore, days 10 (D10), D14, D28, and D42 posttransplant have equivalent gestational ages of P3.5, P7.5, P21.5, and P35.5, respectively. Donor (GFP+) and recipient (GFP−) platelet populations were evaluated individually for activated integrin αIIbβ3 and P-selectin surface expression. (D) Integrin activation (αIIbβ3) in untreated and thrombin-treated donor (GFP+) platelets. Comparable amounts of integrin activation were seen in platelets produced from both bone marrow (BM)–derived and fetal liver (FL)–derived donor HSPCs on days 10, 14, 28, and 42 after transplantation. Analysis performed using 2-way ANOVA with Bonferroni posttest, n ≥ 3. (E) P-selectin surface expression in untreated and thrombin-treated donor (GFP+) platelets. On days 10 and 14 posttransplant, FL-derived HSPCs produce platelets with low levels of P-selectin surface expression after activation relative to activated donor platelets from BM-derived HSPCs. By D28 posttransplant, platelets produced from either BM-derived HSPCs or FL-derived LSKs have comparable P-selectin expression after activation. Platelets produced by BM-derived HSPCs maintain comparable levels of P-selectin surface expression on all days after transplantation, suggesting developmental changes in FL HSPC’s result in the production of platelets with gradually increasing levels of P-selectin surface expression. Significance was determined using a 2-way ANOVA followed by a Bonferroni posttest *P < .001; n ≥ 3. (F) Integrin activation (αIIbβ3) in untreated and thrombin-treated recipient (GFP−) platelets. Comparable amounts of integrin activation were found on platelets of the recipient mouse after transplantation of bone marrow or fetal-liver derived LSKs. (G) P-selectin surface expression in untreated and thrombin-treated recipient (GFP−) platelets. Comparable amounts of P-selectin surface expression were found on platelets of the recipient mouse after transplantation of BM- or FL-derived LSK (Lin−sca+kit+). No significance was detected using a 2-way ANOVA with Bonferroni posttest, n ≥ 3.
The onset of P-selectin expression occurs postnatally and is regulated by a developmental transition in HSPCs. (A) P-selectin surface expression in unstimulated and stimulated platelets isolated from various gestational ages postnatally. Values shown are expressed as a percent of CD62P MFI on activated adult platelets. Platelets harvested from murine embryos (E12.5), pups (days 1, 7, 14, 21, and 42 after birth), and non-pregnant adult mice, were activated with thrombin (0.5 units/mL) in the presence of antibodies to detect activated αIIbβ3 (JON/A) and surface P-selectin (CD62P). Significance was determined using a 2-way ANOVA followed by a Bonferroni posttest *P < .01, ** P < .001 versus activated adult P-selectin surface expression; n ≥ 3. (B) Representative histogram of MFI of CD62P (P-selectin) on activated platelets from P1, P14, P42, and adult. Postnatally, a single platelet population transitions from expression low levels of P-selectin (P1) to higher levels of P-selectin (Adult). (C) Experimental scheme. Lin−sca+kit+ (HSPCs) cells were sorted from E14.5 fetal livers and adult bone marrow of UBC-GFP mice and transplanted into sublethally irradiated GFP− recipients. Whole blood from recipients was activated on days 10, 14, 28, and 42 posttransplantation with 0.5 U/mL thrombin for 10 minutes in the presence of antibodies for activated αIIbβ3 (JON/A) and surface P-selectin (CD62P). HSPCs were taken from E14.5 fetal livers; therefore, days 10 (D10), D14, D28, and D42 posttransplant have equivalent gestational ages of P3.5, P7.5, P21.5, and P35.5, respectively. Donor (GFP+) and recipient (GFP−) platelet populations were evaluated individually for activated integrin αIIbβ3 and P-selectin surface expression. (D) Integrin activation (αIIbβ3) in untreated and thrombin-treated donor (GFP+) platelets. Comparable amounts of integrin activation were seen in platelets produced from both bone marrow (BM)–derived and fetal liver (FL)–derived donor HSPCs on days 10, 14, 28, and 42 after transplantation. Analysis performed using 2-way ANOVA with Bonferroni posttest, n ≥ 3. (E) P-selectin surface expression in untreated and thrombin-treated donor (GFP+) platelets. On days 10 and 14 posttransplant, FL-derived HSPCs produce platelets with low levels of P-selectin surface expression after activation relative to activated donor platelets from BM-derived HSPCs. By D28 posttransplant, platelets produced from either BM-derived HSPCs or FL-derived LSKs have comparable P-selectin expression after activation. Platelets produced by BM-derived HSPCs maintain comparable levels of P-selectin surface expression on all days after transplantation, suggesting developmental changes in FL HSPC’s result in the production of platelets with gradually increasing levels of P-selectin surface expression. Significance was determined using a 2-way ANOVA followed by a Bonferroni posttest *P < .001; n ≥ 3. (F) Integrin activation (αIIbβ3) in untreated and thrombin-treated recipient (GFP−) platelets. Comparable amounts of integrin activation were found on platelets of the recipient mouse after transplantation of bone marrow or fetal-liver derived LSKs. (G) P-selectin surface expression in untreated and thrombin-treated recipient (GFP−) platelets. Comparable amounts of P-selectin surface expression were found on platelets of the recipient mouse after transplantation of BM- or FL-derived LSK (Lin−sca+kit+). No significance was detected using a 2-way ANOVA with Bonferroni posttest, n ≥ 3.
Expression of P-selectin is temporally regulated by a developmental transition in hematopoietic stem and progenitor cells
Lin28b is associated with a developmentally timed switch that alters the transcriptional landscape of murine embryonic HSCs as they transition to an adult-like program around 4 weeks after birth.15,39-41 To determine whether developmentally regulated changes in HSCs alter P-selectin surface expression in platelets, we transplanted donor HSPCs (lineage−, Kit+, Sca+ cells) from E14.5 livers and from bone marrow of adult UBC-GFP mice into sublethally irradiated adult (GFP−) recipients (Figure 7C). HSPCs are a mixed population of cells containing multipotent progenitors, as well as short-term and long-term HSCs.42,43 Recipient platelets at rest and after activation had comparable levels of Integrin αIIbβ3 activation and P-selectin expression on all days examined after transplantation (Figure 7F-G). Donor platelet production was equally robust from fetal liver-derived and bone marrow-derived HSPCs (supplemental Figure 3B). Donor platelets both from bone marrow HSPCs and from fetal liver HSPCs had comparable amounts of integrin activation on days 10, 14, 28, and 42 posttransplantation (Figure 7D). In contrast, activated donor platelets from fetal liver HSPCs had low levels of P-selectin surface expression when compared with donor platelets from bone marrow HSPCs on days 10 and 14 after transplant, and achieved comparable levels of adult P-selectin surface expression between 2 and 4 weeks after transplantation (Figure 7E). These data suggest that the gradual onset of P-selectin surface expression on platelets reflects an intrinsic developmental transition in HSPCs.
Discussion
Platelet function is well characterized in adult hematopoiesis; however, the regulation of age-dependent differences in platelet function has not been elucidated. Lin28B has emerged in recent years as an important mediator of fetal-specific programs in multiple hematopoietic lineages. Induced expression of LIN28B in adult HSCs elevates the self-renewal potential of adult HSCs, thus making them more similar to fetal HSCs.15 Similarly, overexpression of LIN28B increases the number of myelo-erythroid progenitors in adult mice, recapitulating the myelo-erythroid dominance found in fetal hematopoiesis.14 Cumulatively, these studies and others in the lymphoid lineage14,17 support a model in which Lin28B serves a master regulator and determinant of fetal hematopoiesis. Here, we demonstrate that Lin28b is also active in the megakaryocyte/platelet lineage and regulates the expression of the platelet-specific protein P-selectin in the mouse.
The circulation of platelets with low levels of P-selectin between E12.5 and birth, together with our fetal HSC transplantation studies, indicate that both HSC-independent yolk sac hematopoiesis and fetal HSCs give rise to platelets containing low levels of P-selectin. Recently, a close relationship between HSCs and the megakaryocyte lineage has become more apparent. Subpopulations of HSCs have been identified in adult mice, including platelet-biased stem cells primed to synthesize platelet proteins and megakaryocyte progenitors with an HSC immunophenotype that are active only under stress.44,45 HSCs and megakaryocytes share transcriptional programs, and recent studies support the concept that the megakaryocyte lineage emerges directly from fetal HSCs.46,47 Fetal HSCs are highly proliferative, outcompeting adult stem cells for engraftment after transplantation, and have a distinct transcriptional landscape relative to HSCs from adult bone marrow.48,49 These fetal HSC characteristics are not confined to the fetus, but are also found in murine bone marrow HSCs during the first 3 to 4 weeks postnatally, and are associated with a persistent, but decreasing, expression of Lin28B and Hmga2.50 This transition in Lin28b and Hmga2 expression also coincides temporally with the upregulation of P-selectin expression on the surface of activated postnatal platelets (Figure 7A) and raises the possibility that differences between fetal and adult megakaryopoiesis are associated with differences between their HSCs. Consistent with this conjecture, we found that the transplantation of fetal HSPCs into adult recipients resulted in the production of platelets with gradually increasing levels of P-selectin surface expression normalizing to adult levels by 4 weeks (Figure 7E).
In adult hemostasis, activated platelets support inflammation and immunity by recruiting neutrophils and monocytes to sites of vascular injury.3-6 Our studies suggest that sites of platelet activation during embryogenesis with lower levels of P-selectin would have reduced leukocyte recruitment. It is plausible that in utero there is not much risk for pathogen invasion at sites of vascular injury. However, platelet activation does occur at discrete sites of lymphatic-venous separation and cerebrovascular patterning, as well as subsequent closure of the ductus arteriosus after birth.51-53 Recruitment of neutrophils to these sites of platelet activation may, in fact, not be beneficial, and could cause unwanted inflammation in a rapidly developing vasculature. Leukocyte recruitment is facilitated not only by platelet P-selectin but also by endothelial P-selectin.54 Similar to platelets, endothelial cells of late fetal yolk sac vessels have been reported to have very low levels of P-selectin expression.55 This phenomenon is also observed during human development, where umbilical cord endothelial cells from preterm infants express significantly less P-selectin compared with term infants.56,57 Notably, studies on murine leukocyte populations have not revealed any significant differences in the expression of P-selectin glycoprotein ligand-1 on circulating GR-1+ cells during the neonatal period.55 Cumulatively, our findings and the results of previous studies support the concept that the downregulation of P-selectin both in fetal platelets and in endothelial cells is beneficial for early development.
Our study clearly identifies Lin28b as a novel transcriptional regulator of P-selectin during murine development; however, the function of this pathway in human megakaryocytes and platelets remains unclear. Analysis of developmental changes in human megakaryopoiesis has demonstrated that LIN28B is most highly expressed in megakaryocytes derived from ES cells, and is the lowest in adult-derived megakaryocytes.21 However, human neonatal and adult human platelets have similar levels of intracellular P-selectin,58 which contrasts with our findings in the mouse. Together these studies suggest that the human neonatal period may be difficult to recapitulate in the mouse, potentially because of species-specific differences in the transition from embryonic to fetal, and fetal to adult, hematopoiesis. Studies of HMGA2 highlight this complexity, as Hmga2 levels in mice peak early in embryogenesis and gradually fall during the transition to adult hematopoiesis.15 In humans, HMGA2 is alternatively spliced, with HMGA2-L being the dominant isoform in fetal HSCs, which is sensitive to degradation by let-7. However, newborn HSCs express the let-7-resistant isoform HMGA2-S.59 At this time, it is unclear whether alternative splicing of HMGA2 effects P-selectin expression. Although our mouse model does not recapitulate all the differences in human neonatal platelet function, our results clearly suggest that LIN28B may have previously unrecognized roles in megakaryocyte development and thus platelet function.
Because Lin28b expression is restricted to fetal hematopoiesis, our findings may have implications for donor-independent platelets derived from ES and induced pluripotent stem (iPS) cells. LIN28B is highly expressed in human ES cell–derived megakaryocytes when compared with adult controls, suggesting that platelets derived from ES cells are likely to have fetal characteristics.21 Quantitative P-selectin surface expression on platelet-like particles derived from ES or iPS cells has not been compared with primary adult platelets, making it difficult to evaluate similarities or differences to fetal platelets.60,61 Furthermore, although platelets are currently being pharmed in vitro, several studies have also described the generation of platelets in vivo by transplanting megakaryocytes derived from ES/iPS cells.62 Because fetal hematopoietic progenitors transplanted into an adult niche continue to make platelets with low levels of P-selectin, it is likely that the platelets generated from ES-cell-derived megakaryocytes will also resemble embryonic platelets. We propose that Lin28b represents a novel target, the expression of which, once reduced in ES/iPS cell-derived megakaryocytes, may lead to the generation of more adult-like platelets for future use in transfusion medicine.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the Flow Cytometry Core Facility at the University of Rochester Medical Center for technical support.
This work was supported by funding from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (K08 DK114527 [R.G.R.], R24 DK092760 [G.Q.D.], and R01 DK098251 [J.P.]).
Authorship
Contribution: M.C.S. designed, performed, and analyzed experiments and wrote the manuscript; S.C.C. and K.F. assisted with megakaryocyte and platelet RNA expression experiments and analysis; P.D.K. performed and analyzed immunocytochemistry of platelet α-granules; R.G.R and G.Q.D provided mice with inducible LIN28B expression; A.D.K. and L.V. irradiated mice for transplantation, managed timed pregnancies, and maintained animal colonies; S.C.C. provided technical support; and K.E.M. and J.P. contributed to experimental design, analysis, and manuscript preparation.
Conflict-of-interest disclosure: G.Q.D. receives sponsored research support from Megakaryon (Japan), a firm developing platelet products from pluripotent stem cells, and is a founder of and equity holder in 28/7 Therapeutics, a company focused on developing anticancer drugs targeting the LIN28/let-7 pathway. The remaining authors declare no competing financial interests.
Correspondence: James Palis, Center for Pediatric Biomedical Research, Department of Pediatrics, University of Rochester Medical Center, 601 Elmwood Ave, Rochester, NY 14642; e-mail: james_palis@urmc.rochester.edu.