Key Points
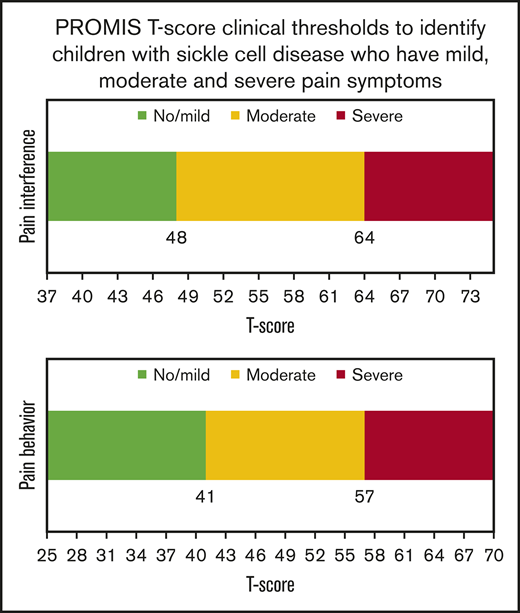
PROMIS T-scores ≤48 on pain interference and ≤41 on pain behavior characterize children with SCD who have mild symptoms.
PROMIS T-scores >64 on pain interference and >57 on pain behavior characterize children with SCD who have severe symptoms.
Abstract
The Patient Reported Outcomes Measurement Information System (PROMIS) pain interference and pain behavior domains are valid and reliable for children with sickle cell disease (SCD). However, clinical interpretation of the scores is unknown. The objective of this study was to determine the clinical meaning of PROMIS pain scores for children with SCD. We used 2 approaches to determine clinical meaning: dichotomization of item responses and T-score ranges. T-score ranges determined thresholds for no/mild, moderate, and severe pain. We compared the proportion of patients who needed pain medications among pain severity groups using χ2/Fisher’s exact tests. The study included 117 children (mean age, 11.5 years [standard deviation, 2.9 years]). Using the dichotomization approach, 43 children had pain interference T-scores ≤48 reflecting minimal pain, and 30 children had T-scores >60 reflecting substantial pain. For pain behavior, 34 children had T-scores ≤41 reflecting minimal problems, and 23 patients had T-scores >57 reflecting substantial problems with pain. Using T-score ranges, clinical thresholds of no/mild and severe pain interference were determined as ≤48.3 and >63.6, respectively. The thresholds for no/mild and severe pain behavior were ≤41.3 and >57.3, respectively. Overall, the proportion of patients who took pain medications was significantly different among those with no/mild, moderate, and severe pain as identified by pain interference (P = .002) and pain behavior domains (P = .0113). We identified T-scores for PROMIS pain domains that facilitate clinical interpretation and provide necessary information for PROMIS users in a clinical setting.
Introduction
Children with sickle cell disease (SCD) characteristically experience recurrent pain events that impacts their overall well-being.1 Pain, although a common manifestation of SCD, is currently difficult to assess given the lack of reliable and valid physiological measurements. The use of patient-reported outcomes (PROs) can enhance measurement of pain and provide a patient’s perception of how he or she is functioning.2
A considerable amount of work has already been done to develop and validate PRO measures for the pediatric population.3-12 The Patient-Reported Outcomes Measurement Information System (PROMIS) includes multiple pain domains that measure distinct aspects of patient experiences. The domain of pain interference measures the consequences of pain on relevant aspects of a child’s life and may include the extent to which pain hinders engagement with day-to-day activities. The pain behavior domain assesses a child’s behavior and his or her pain experience and expression of pain. The domains of pain interference and pain behavior have been shown to be valid and reliable for children with SCD.4,5,12 However, the adoption of these domains in clinical practice is significantly limited by the lack of clinical interpretation of scores. The pediatric PROMIS measures are scored on a T-score metric with a mean of 50 and standard deviation (SD) of 10, where 50 represents the mean of the pediatric sample in which the item response theory parameters for the domains were estimated.13 A higher score on pain domains indicates worse pain. However, we do not know the range or threshold of scores for these pain domains above or below which there is a clinical meaning for children with SCD.
The objective of this study was to determine the clinical interpretation of PROMIS pain scores for children with SCD. We used 2 approaches to add clinical meaning to T-scores and determine relevant clinical thresholds for children with mild and severe pain.
Methods
Study subjects
We recruited a convenience sample of children with SCD, age 5 to 17 years, from Children’s Hospital of Wisconsin, Milwaukee during the time period of March 2016 to May 2018. Children with SCD were eligible to self-report the PROMIS surveys if they were ≥8 years, able to read and speak English, and did not have cognitive impairments which could hamper survey comprehension. Consent/assent to participate was required to allow study participation. This study was approved by the Institutional Review Board at Children’s Hospital of Wisconsin
Health-related surveys and patient characteristics
Eligible children completed PROMIS surveys, including the validated pain domains of pain interference and pain behavior. We collected information on children’s demographic and clinical characteristics, including age, sex, race, ethnicity, SCD genotype, hydroxyurea use, and pain medication use at home and general health. Pain medication use was informed by a question answered by the child’s parent that specifically asked if the child had to take his or her strong pain medication (such as morphine or tramadol) at home in the past week for pain. Additional information that included the child’s age, sex, race, ethnicity, and hydroxyurea use were obtained by parent report using a demographic survey. The SCD genotype of the participating children was obtained via electronic health record review. A patient’s general health was assessed using the child’s response to a single question: “In general, would you say that your health is (check one box),” with the options presented as excellent, very good, good, fair, or poor.
Pediatric PROMIS pain domains
The study used a 4-item pain interference and 8-item pain behavior short form. The pain domains use a Likert response scale and have a 1-week recall period. The response options on a Likert response scale for the pain interference domain include never, almost never, sometimes, often, or almost always. The items on the pain behavior domain have an additional response option of “had no pain” along with those mentioned for the pain interference domain.
Approach 1: dichotomization of item responses
As recommended in the literature, to aid in providing clinically interpretable PRO scores,14 we dichotomized patient responses at the item level to indicate if the child-reported problems with pain. A patient was considered to have pain interference or exhibit pain behavior if he or she endorsed “sometimes, often, or almost always” for the respective items on the domains. If the patient reported “no pain, never, or almost never,” then he or she was considered to have no impairment due to pain.
Approach 2: T-score ranges to determine clinical thresholds for children with mild or severe pain
We determined the T-score range for patients who reported “no pain, never, or almost never” for all items on the pain behavior/pain interference domains and for those who reported “often or almost always” for all items on the pain interference/pain behavior domains. We classified patients into 3 pain-severity groupings (no/mild, moderate, and severe pain) based on the T-score ranges. The upper end of the T-score range for patients reporting “no pain, never, or almost never” for all items determined the threshold for mild pain. The lower end of the score range for patients reporting “often or almost always” for all items determined the threshold for severe pain. Patients with scores greater than the threshold for mild pain and lower than the threshold for severe pain were considered to have moderate pain.
Statistical analysis
For approach 1, we describe the proportion of patients who experience impairments for the specified range of T-scores. For approach 2, the thresholds were validated by comparing the distribution of patients who reported they had to take pain medications in the past week among patients in the mild, moderate, and severe pain groupings. All comparisons were done using χ2 or Fisher’s exact tests. Multiple pairwise comparisons were adjusted using the false discovery rate. All analyses were conducted using SAS 9.4.
Results
One hundred and seventeen patients completed the 2 PROMIS pain domains. Table 1 lists the demographic and clinical characteristics of study participants.
Demographic and clinical characteristics of study participants
| Patient characteristics (N = 117) . | n (%) . |
|---|---|
| Demographic characteristics | |
| Age, mean (SD), y | 11.5 (2.8) |
| Female sex | 64 (54.7) |
| Ethnicity | |
| Hispanic or Latino | 7* (6.0) |
| African American | 109 (93.2) |
| Clinical characteristics | |
| Sickle cell disease genotype | |
| Hgb SS/SB0 | 72 (61.5) |
| Hgb SC | 35 (29.9) |
| Other | 10 (8.5) |
| Currently taking hydroxyurea (yes)† | 74 (63.3) |
| Took pain medications at home in the prior week (yes) | 26 (23.2) |
| Child self-report of general health | |
| Poor/fair | 13 (11.1) |
| Good | 41 (35.0) |
| Very good | 39 (33.3) |
| Excellent | 24 (20.5) |
| Patient characteristics (N = 117) . | n (%) . |
|---|---|
| Demographic characteristics | |
| Age, mean (SD), y | 11.5 (2.8) |
| Female sex | 64 (54.7) |
| Ethnicity | |
| Hispanic or Latino | 7* (6.0) |
| African American | 109 (93.2) |
| Clinical characteristics | |
| Sickle cell disease genotype | |
| Hgb SS/SB0 | 72 (61.5) |
| Hgb SC | 35 (29.9) |
| Other | 10 (8.5) |
| Currently taking hydroxyurea (yes)† | 74 (63.3) |
| Took pain medications at home in the prior week (yes) | 26 (23.2) |
| Child self-report of general health | |
| Poor/fair | 13 (11.1) |
| Good | 41 (35.0) |
| Very good | 39 (33.3) |
| Excellent | 24 (20.5) |
Hgb SC, hemoglobin SC; Hgb SS/SB0, hemoglobin SS/Sβ0 thalassemia.
Ethnicity data were missing for 19 patients.
Hydroxyurea use data were missing for 2 patients.
Approach 1: dichotomization of item responses
Pain interference domain
For the pain interference domain, 62 patients (53%) had trouble sleeping, 58 patients (49.6%) found it hard to pay attention, 65 patients (55.6%) found it hard to run, and 54 patients (46.1%) reported interference with walking due to pain (Figure 1). There were 43 children who had pain interference T-scores ≤48, of whom 6 reported some interference. However, this interference was reported for only 1 item among the 6 patients (1 patient reported interference with sleeping, no patient reported having difficulties paying attention, 3 had trouble running, and 2 had trouble walking due to pain). Among the 31 children who had T-scores >60, 30 reported interference for all items in the domain and 1 reported interference for all items in the domain except for paying attention due to pain.
Pain interference domain. Proportion of patients reporting “sometimes, often, or almost always” (by item) having problem with pain interference.
Pain interference domain. Proportion of patients reporting “sometimes, often, or almost always” (by item) having problem with pain interference.
Pain behavior domain
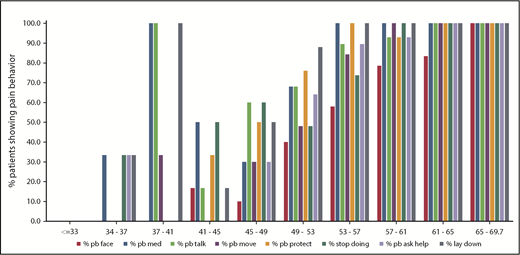
For items on the pain behavior domain, 42 of 117 patients (35.9%) endorsed pain that showed on their face, 69 patients (59.0%) asked for medicine, 66 patients (56.4%) talked about pain, 55 patients (47.0%) moved slower, 67 patients (57.3%) protected the part of the body that hurt, 59 patients (50.4%) had to stop what they were doing, 59 patients (50.4%) asked for help, and 74 patients (63.2%) lay down when in pain (Figure 2). On the low end of the T-score range, for patients with a T-score ≤33 (n = 28), no patient reported any pain-elicited behavior. For the 34 patients who had pain behavior T-scores ≤41, 5 reported some pain-elicited behavior. Among these 5 patients, none reported that pain showed on the face, 4 asked for medicine, 3 talked about their pain, 1 moved slower, none reported protecting the part of the body that hurt, 1 had to stop what he or she was doing, 1 asked for help, and 4 lay down when in pain. These 5 patients endorsed pain behavior for ≤4 of the 8 items in the measure. On the high end of the range supporting more pain behavior, there were 23 patients with pain behavior T-scores >57, of whom 18 endorsed pain behavior on all items. Of the remaining 5 patients, 3 reported that pain did not show on their face, 1 reported that pain did not show on face and he or she also did not talk about pain, and 1 reported not needing help and not protecting the part of the body that hurt.
Pain behavior domain. Proportion of patients reporting “sometimes, often, or almost always” (by item) having problem with pain behavior. % pb face, % pain showed on face; % pb med, % asked for medicine; % pb talk, % talked about pain; % pb move, % moved slower; % pb protect, % protected part of body that hurt; % pb ask help, % asked for help.
Pain behavior domain. Proportion of patients reporting “sometimes, often, or almost always” (by item) having problem with pain behavior. % pb face, % pain showed on face; % pb med, % asked for medicine; % pb talk, % talked about pain; % pb move, % moved slower; % pb protect, % protected part of body that hurt; % pb ask help, % asked for help.
Approach 2: score ranges to determine threshold scores for children with mild, moderate, and severe pain
Pain interference domain
Pain interference T-scores for all patients ranged from 36.7 to 74. There were 37 patients who reported “no pain, never, or almost never” for all items and 16 reported “often or almost always” for all items. All patients reporting “no pain, never, or almost never” for all items had scores ≤48.3. Patients reporting “often or almost always” for all items had scores ≥63.6. This resulted in patients being grouped into these 3 pain groupings: 46 patients (39.3%) with no/mild pain, 45 patients (38.5%) with moderate pain, and 26 patients (22.2%) with severe pain. There were significant differences in the proportion of patients reporting they needed to take pain medications at home in the past week among the 3 pain-severity groups (patients needing pain medications: no/mild, 6.5%; moderate, 26.7%; and severe, 42.3%; P = .002). The pairwise comparisons test showed significant differences in the proportion of patients with pain medication use between the no/mild and moderate/severe pain groups (P = .0141 and P = .0006). However, there were no significant differences in pain medication use between the moderate and severe pain groups (P = .1565).
Pain behavior domain
Pain behavior T-scores for all patients ranged from 26 to 69.7. There were 30 children who reported “no pain, never, or almost never” for all items, and 6 reported “often or almost always” for all items. All patients reporting “no pain, never, or almost never” for all items had scores ≤41.3. Patients reporting “often or almost always” for all items had scores ≥57.2. This resulted in patients being grouped into the following 3 pain groupings: 36 patients (30.8%) with no/mild pain, 59 patients (50.4%) with moderate pain, and 22 patients (18.8%) with severe pain. There were significant differences in the proportion of patients who reported they had to take pain medications at home in the past week among those with no/mild, moderate, and severe pain (patients needing pain medications: no/mild, 5.6%; moderate, 31.6%; and severe, 28.6%; P = .0113). Pairwise comparisons indicated significant differences between the mild and the moderate group (P = .0087). However, there were no significant differences in pain medication use between no/ mild and severe (P = .0626) or moderate and severe (P = .7985).
Discussion
Our study uses 2 approaches to add clinical meaning to the PROMIS pain interference and pain behavior domain scores. The 2 approaches resulted in similar score thresholds. Our results show that children with SCD have no/mild pain if the PROMIS T-scores are ≤48 for pain interference and ≤41 for pain behavior. A pain interference T-score >64 and a pain behavior T-score >57 indicate severe pain. The PROMIS T-score thresholds provide value in clinic settings to help identify children with SCD who have varying degrees of pain and can be used as end points in therapeutic trials.
Children with SCD often have pain, which helps characterize the severity of their disease. The experience of pain is subjective in nature and can be difficult to describe and quantify. Having the objective measure of PROs to aid in our understanding of how pain is experienced by children with SCD is hence invaluable. PROs that are clinically useful can provide a better understanding of the disease so that appropriate treatment strategies can be prescribed. Also, the domains of pain interference and pain behavior can serve as potential outcomes used to gain insight about the effectiveness of a particular treatment strategy in reducing pain. The PROMIS measures of pain interference and pain behavior3,15 are highly relevant to children with SCD, and our work provides thresholds to better understand the impact of pain they are experiencing.
The interpretation of PROMIS T-scores is highly dependent on the centering sample or normative population. The T-scores have a mean score of 50, which is the average T score of the domain in the normative population. The pain interference domain was determined based on a sample of the general population, while the pain behavior domain was determined based on a clinical sample of children with painful chronic conditions3,15 . This implies a T-score of 50 on the pain interference domain reflects the average pain interference score for the general population. A T-score of 50 on the pain behavior domain is the average pain behavior score among children with painful chronic conditions. The thresholds identified for the pain interference and pain behavior domains align with the respective normative populations used. On average, children in the clinical sample would be more likely to experience pain, and thus, a much lower than average T-score identifies children with no/mild pain (41 for pain behavior vs 48 for pain interference), and a slightly higher than average score is indicative of severe pain (57 for pain behavior vs 64 on pain interference).
Some guidance exists to understand the meaning of the pain interference T-scores. PROMIS Health Measures provides a heat map for a range of pain interference T-scores suggesting that a SD of 0.5 and 1.5 are reasonable thresholds to identify mild and severe pain, respectively.13 These thresholds are based on the percentage of participants who would fit into each category16,17 and can differ for children with a given condition. The threshold we determined for severe pain for children with SCD overlaps with the thresholds suggested for the general population. However, the threshold determined for no/mild pain was lower than that suggested for the general population. The pain behavior domain was developed more recently and, to the best of our knowledge, ours is the first study to add clinical meaning to its T-score.
Previously, item-level responses have been used to add clinical meaning to PedsQL SCD scores.18 We use similar approaches to add clinical meaning to the PROMIS pain interference and pain behavior domain scores. The 2 approaches we used can enhance the meaning of scores in complementary ways. The dichotomization of item responses directly provides information on the proportion of patients with pain for a specified score. This can also help identify specific items that the patient finds difficult, which will allow the clinical team to understand pain and address any specific needs pertaining to that particular item. Alternatively, the T-score range-based thresholds can help identify if the patient is in severe pain or if a treatment is effective enough to reduce pain that has perceivable implications for the patient. The thresholds determined based on dichotomization and T-score range methods might lead to loss in power for statistical analyses. Therefore, continuous T-scores might be preferred for statistical analysis. However, understanding the thresholds and proportion of patients that are impaired for specific T-score ranges is essential to add clinical meaning to the scores. These methods have also been recommended by other researchers.19
There are some limitations to our study. First, our study is based on a convenience sample of SCD patients. However, the distribution of race, ethnicity, sex, and disease type for children in our study is similar to other published population‐level studies. Secondly, we use short forms to add clinical meaning to PROMIS scores. It would be interesting to evaluate our thresholds for the entire item bank of the respective domains. However, PROMIS measures are based on item response theory, and the scores are interpretable irrespective of the items used. Also, other qualitative methods such as bookmarking can further enhance the interpretations.20 Our study is restricted to children in the clinic setting. Therefore, we have relatively a small number of patients with severe pain. This could limit our ability to find statistically significant differences in pain medication use between children with severe pain and other groups. Finally, we do not know the range of PROMIS scores for children during an active vaso-occlusive crisis in the acute care setting.
In conclusion, our study identifies PROMIS T-score thresholds for the pain interference and pain behavior domains that allow patients to be characterized as having no/mild, moderate, or severe pain. These data provide clinical meaning for PROMIS T-scores for these 2 domains and will facilitate future clinical use of these domains in a clinic setting.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number 1U19AR069519-01.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: A.S. designed the research, conducted analyses, and wrote the manuscript; and J.A.P. designed the research, provided oversight for the analyses, wrote the manuscript, and obtained funding.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ashima Singh, Medical College of Wisconsin, 8701 W Watertown Plank Rd, Suite 3050, Milwaukee, WI 53226; e-mail: ashimasingh@mcw.edu.