Key Points
Malglycemia occurs commonly in the pediatric/AYA HSCT population and is independently associated with increased mortality and infection.
Risk factors for malglycemia include adolescent age, allogeneic HSCT, underlying malignancy, post-HSCT steroids, and sirolimus/tacrolimus.
Abstract
Malglycemia (hypoglycemia, hyperglycemia, and/or glycemic variability) in adult hematopoietic stem cell transplant (HSCT) recipients is associated with increased infection, graft-versus-host disease, organ dysfunction, delayed engraftment, and mortality. Malglycemia has not been studied in pediatric HSCT recipients. This study aimed to characterize the incidence and consequences of malglycemia in this population. Medical records for a cohort of 344 patients, age 0 to 30 years, who underwent first HSCT from 2007 to 2016 at Children’s Hospital Colorado were retrospectively reviewed. Glucose data were analyzed in intervals and assessed for potential risk factors and associated outcomes. Malglycemia occurred in 43.9% of patients. Patients with a day 0 to 100 mean glucose of 100 to 124 mg/dL had a 1.76-fold (95% confidence interval [CI], 1.10-2.82; P = .02) increased risk of death and patients with a day 0 to 100 mean glucose ≥ 125 mg/dL had a 7.06-fold (95% CI, 3.84-12.99; P < .0001) increased risk of death compared with patients with a day 0 to 100 mean glucose < 100 mg/dL. For each 10 mg/dL increase in pre-HSCT glucose, there was a 1.11-fold (95% CI, 1.04-1.18; P = .0013) increased risk of post-HSCT infection. These adverse impacts of malglycemia occurred independent of transplant type, graft-versus-host disease, and steroid therapy. Malglycemia in the pediatric HSCT population is independently associated with significantly increased risk of morbidity and mortality. Further research is required to evaluate the utility of glucose control to mitigate these relationships and improve HSCT outcomes. This trial was registered at www.clinicaltrials.gov as #NCT03482154.
Introduction
Pediatric and adolescent/young adult (AYA) patients who undergo hematopoietic stem cell transplant (HSCT) are at risk of complications, such as primary disease recurrence, infection, graft-versus-host disease (GVHD), organ dysfunction, and other causes of morbidity and mortality. Identifying potentially modifiable risk factors for these complications is critical to further improve HSCT outcomes. Malglycemia, defined as hypoglycemia (blood glucose [BG] <70 mg/dL), hyperglycemia (BG ≥126 mg/dL), or glycemic variability (standard deviation [SD] ≥29 mg/dL), is associated with adverse outcomes in various adult and pediatric patient populations, such as those in intensive care units (ICUs).1,2 Studies in adults undergoing HSCT demonstrate associations between malglycemia and adverse clinical outcomes, including infection, length of hospital stay, organ dysfunction, GVHD, delayed hematopoietic recovery, and mortality.1-14 The increased incidence of malglycemia in HSCT patients has been postulated to be related to a combination of factors, including stress hyperglycemia, underlying insulin resistance, decreased insulin secretion, steroid treatment, total parenteral nutrition (TPN), and calcineurin inhibitors.5,7,9,14-17 The underlying pathophysiology of the association between malglycemia and adverse outcomes may relate to known immunologic effects, such as increased inflammation and impaired leukocyte function.1,3,12,15,18,19 Hyperglycemia is associated with increased tumor necrosis factor α and interleukin-6 production, impaired neutrophil chemotaxis and degranulation, increased lymphocyte apoptosis, and immune effects.18-22 These dysregulatory effects may explain the association between abnormal glucose control and infections and acute GVHD.
In contrast to the adult population, there are no published studies evaluating malglycemia in the pediatric/AYA HSCT population. This population differs in numerous ways from its adult counterparts, including higher rates of nonmalignant indications for HSCT, increased use of myeloablative preparative regimens, and reduced predilection for metabolic syndrome.23-25 Hence, the incidence of, and risk factors for, malglycemia, as well as its impacts on clinical outcomes, may differ from adults.
In this study, we aimed to retrospectively establish the incidence of, and risk factors for, malglycemia in the pediatric/AYA HSCT population, and, moreover, evaluate possible associations between malglycemia and HSCT outcomes.
Methods
Patients
An existing HSCT program database was used to identify all pediatric and AYA patients who underwent first HSCT at a single academic children’s hospital from 2007 to 2016. Demographic and clinical data were extracted from this database and electronic health records through 31 March 2017 via research informatics and manual review. Inclusion criteria were age 0 to 26 years at the time of transplant and allogeneic or autologous HSCT recipient at Children’s Hospital Colorado between 1 January 2007 and 31 July 2016. Exclusion criteria were preexisting diabetes mellitus, insulin requirement within the 2 weeks prior to transplant, and inadequate blood glucose data. Of 351 patients identified, 5 patients were excluded because of insulin use in the 2 weeks prior to transplant, and 2 patients were excluded because <20 glucose measurements were available. This study was approved by the University of Colorado Institutional Review Board.
Exposure and outcomes definitions
Malglycemia and its components, glucose mean and SD, served as outcome and exposure variables and were determined by analysis of all laboratory-measured serum glucose values. Whole-blood glucose measurements were usually performed daily in the early morning (between 12 and 3 am) during hospital admission, and typically 1 to 3 daytime measurements were collected each week after discharge. Glycemic variables were analyzed from 14 days pretransplant to 100 days posttransplant, as well as by subintervals: pretransplant (days −14 to 0), days 0 to 30, and days 0 to 100. Malglycemia was defined as hyperglycemia (mean glucose ≥ 126 mg/dL), hypoglycemia (mean glucose ≤ 70 mg/dL), or glycemic variability (SD ≥ 29 mg/dL).2 Glycemic variability, or the fluctuation of glucose values for an individual, was defined by SD for its familiarity among clinicians and its use in the type 1 diabetes literature.26,27 Of note, although malglycemia definitions refer to fasting glucose, glucose values in this study cannot be assumed to be fasting; thus, to reduce misclassification bias by analyzing binary malglycemia and to improve sensitivity, mean glucose values were also evaluated continuously and categorically (<100 mg/dL, 100-124 mg/dL, and ≥125 mg/dL).
Exposure variables included race/ethnicity, age (<12 years or ≥12 years), continuous and categorized (standard World Health Organization definitions) body mass index (BMI) percentile, diagnosis type (nonmalignant or malignant), transplant type (allogeneic or autologous), conditioning regimen (total body irradiation [TBI]–based regimen vs chemotherapy-based regimen), pre- and posttransplant therapies, including steroids (>5 consecutive days), asparaginase, radiotherapy field incorporating the pancreas, TPN, and immunosuppressive agents.
Clinically significant infections in the first 100 days after HSCT were defined by a positive microbiology result or diagnostic/problem list code, which was clinically significant (determined by necessity of treatment, hospitalization or complication) on chart review. Infections were further categorized by serious bacterial infection (SBI) (ie, bacteremia, pneumonia, meningitis, peritonitis), viremia/viruria, invasive fungal infection, or “other” infection (ie, infectious colitis, urinary tract infection, viral respiratory infection). Secondary outcomes included mortality, relapse of primary malignancy (where applicable), diagnosis of severe forms of GVHD (defined for this study as acute grades 3 or 4 or chronic based on known to impact survival in allogeneic recipients), time to engraftment, length of stay for primary HSCT admission, and number of ICU and total hospital days in the first 100 days. To reduce the risk of misclassification bias, the outcomes of infection, recurrence of primary disease, and diagnosis of GVHD were reviewed by a minimum of 1 physician.
Data were censored at the time of any recurrence/progression of primary disease or graft failure.
Statistical analysis
Descriptive statistics reported include median and percentiles (minimum, 25th percentile, 75th percentile, maximum) for continuous variables and counts and percentages for categorical variables. Because of the nature of HSCT care, there was not a notable amount of missing data or loss to follow-up. Associations between categorical exposures and glycemic outcomes were tested using the Mann-Whitney U test for mean glucose and the χ2 test or Fisher's exact test for malglycemia. Associations between continuous predictors and glycemic outcomes were tested using linear and logistic regression. The association between glycemic variables and HSCT-related outcomes was examined using logistic regression (infection subtype outcomes) or linear regression (length of stay) in unadjusted models and multiple regression models, adjusting for potential confounders. For time-to-event outcomes (overall survival [OS], relapse, treatment-related mortality [TRM], any infection, GVHD, and ICU stay), glucose values were censored at the time of the event; the log-rank test was used to compare groups in unadjusted analyses, and Cox proportional hazards models were used for adjusted analyses. For analysis of GVHD and TRM, death due to other causes was treated as a competing risk; GVHD analysis was censored by date of last follow-up. For analysis of time to infection and time to ICU visit, death was also treated as a competing risk, and patients were censored at 100 days if an event had not yet occurred.
Multivariable logistic regression was used to test for effect modification. Steroid use posttransplant and transplant type were tested as effect modifiers of the relationship between infection and mean glucose. For each model, the outcome was infection, and the predictors were the effect modifier, the mean glucose variable, and the interaction between the effect modifier and the mean glucose variable. A significant interaction term was interpreted as evidence of effect modification. For all tests, a significance level of 0.05 was used. SAS v9.4 (SAS Institute Inc., Cary, NC) was used for all analyses.
Results
Patient characteristics
Among the 344 patients included in this analysis, median age at transplant was 8.0 years, and 59.3% were male (Table 1). Of all transplants, 65.4% were allogeneic, and 34.6% were autologous. The primary indication for transplant was malignancy in 75.3% of patients.
Patient characteristics
| Variable . | Data . |
|---|---|
| Age at transplant | |
| Median, (25th, 75th percentile), y | 8.00 (3.00, 15.00)* |
| Preadolescent (<12 y) | 214 (62.21) |
| Adolescent (≥12 y) | 130 (37.79) |
| Race/ethnicity | |
| Non-Hispanic white | 173 (50.29) |
| Hispanic/Latino | 109 (31.69) |
| Multiple races or other race | 28 (8.14) |
| American Indian/Alaska native | 13 (3.78) |
| Asian | 13 (3.78) |
| African American | 6 (1.74) |
| Native Hawaiian or Pacific Islander | 2 (0.58) |
| Sex | |
| Male | 204 (59.30) |
| Female | 140 (40.70) |
| BMI percentile | |
| Normal or underweight (<85th percentile) | 254 (78.15) |
| Overweight (85th-95th percentile) | 45 (13.85) |
| Obese (≥95th percentile) | 26 (8.00) |
| Transplant type | |
| Allogeneic | 225 (65.41) |
| Autologous | 119 (34.59) |
| Specific diagnosis | |
| Leukemia/MDS | 130 (37.04) |
| Solid tumors (non-CNS) | 63 (17.95) |
| CNS tumors | 36 (10.26) |
| Bone marrow failure | 35 (9.97) |
| Lymphoma | 34 (9.69) |
| Primary immunodeficiency/immune dysfunction | 29 (8.26) |
| Hemoglobinopathy | 15 (4.27) |
| Metabolic disorder | 8 (2.28) |
| Other | 1 (0.28) |
| Variable . | Data . |
|---|---|
| Age at transplant | |
| Median, (25th, 75th percentile), y | 8.00 (3.00, 15.00)* |
| Preadolescent (<12 y) | 214 (62.21) |
| Adolescent (≥12 y) | 130 (37.79) |
| Race/ethnicity | |
| Non-Hispanic white | 173 (50.29) |
| Hispanic/Latino | 109 (31.69) |
| Multiple races or other race | 28 (8.14) |
| American Indian/Alaska native | 13 (3.78) |
| Asian | 13 (3.78) |
| African American | 6 (1.74) |
| Native Hawaiian or Pacific Islander | 2 (0.58) |
| Sex | |
| Male | 204 (59.30) |
| Female | 140 (40.70) |
| BMI percentile | |
| Normal or underweight (<85th percentile) | 254 (78.15) |
| Overweight (85th-95th percentile) | 45 (13.85) |
| Obese (≥95th percentile) | 26 (8.00) |
| Transplant type | |
| Allogeneic | 225 (65.41) |
| Autologous | 119 (34.59) |
| Specific diagnosis | |
| Leukemia/MDS | 130 (37.04) |
| Solid tumors (non-CNS) | 63 (17.95) |
| CNS tumors | 36 (10.26) |
| Bone marrow failure | 35 (9.97) |
| Lymphoma | 34 (9.69) |
| Primary immunodeficiency/immune dysfunction | 29 (8.26) |
| Hemoglobinopathy | 15 (4.27) |
| Metabolic disorder | 8 (2.28) |
| Other | 1 (0.28) |
Unless otherwise specified, all data are n (%).
CNS, central nervous system; MDS, myelodysplastic syndrome.
Minimum age 2 months, maximum age 29 years.
Malglycemia
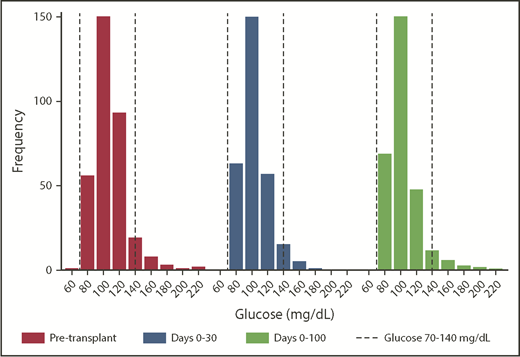
There was a mean of 69.5 glucose measurements per subject (range, 25-693). Malglycemia occurred in 26.0% of patients pretransplant, in 22.3% of patients in days 0 to 30, and in 22.6% of patients in days 0 to 100. Overall, 43.9% of patients had malglycemia during ≥1 period, although only 18 (5.2%) patients were treated with insulin after HSCT. Figure 1 shows the mean glucose distributions. Among patients classified as having malglycemia, the most common reason was glycemic variability (eg, in the pre-HSCT interval, 24.1% of patients had variability, 12.5% had a high mean glucose, and only 0.3% had a low mean glucose). There was an intrapatient association between pre- and posttransplant malglycemia and mean glucose; patients who had pretransplant malglycemia had a 2.48 times increased odds of posttransplant malglycemia (95% confidence [CI], 1.45-4.25; P = .001).
Distribution of patient mean blood glucose values by time interval. Although the medians (of mean) glucose values were in the normal range at each time interval analyzed, the distribution demonstrates skew toward hyperglycemia. The dashed lines represent the normal range of nonfasting glucose, which is 70 to 140 mg/dL.
Distribution of patient mean blood glucose values by time interval. Although the medians (of mean) glucose values were in the normal range at each time interval analyzed, the distribution demonstrates skew toward hyperglycemia. The dashed lines represent the normal range of nonfasting glucose, which is 70 to 140 mg/dL.
In univariate analysis, male sex; adolescent age; prior asparaginase exposure; allogeneic HSCT; TBI-based conditioning; any pre-HSCT radiation to the pancreas (including TBI); post-HSCT radiation; post-HSCT immunosuppression with sirolimus, tacrolimus, mycophenolate mofetil, or cyclosporine; steroids post-HSCT; and TPN post-HSCT were risk factors for increased median glucose over the entire pretransplant to 100 days posttransplant time interval (supplemental Table 1).
In a multivariable model adjusting for transplant type, primary diagnostic category, adolescent age, and other various treatment-associated risk factors (Table 2), adolescent age and underlying malignant diagnosis were associated with pre-HSCT malglycemia, whereas other factors, such as pre-HSCT steroids and asparaginase, were not.
Multivariable analysis of risk factors for malglycemia
| Potential risk factor . | Pretransplant OR (95% CI)† . | Days 0-30 OR (95% CI)† . | Days 0-100 OR (95% CI)† . |
|---|---|---|---|
| Adolescent age | 2.97 (1.72-5.14)** | 1.27 (0.70-2.31) | 1.51 (0.81-2.81) |
| BMI percentile | 1.00 (0.99-1.01) | 1.00 (0.99-1.01) | 1.00 (0.99-1.01) |
| Allogeneic transplant | 1.91 (0.72-5.10) | 3.15 (1.07-9.23)* | |
| Malignant primary diagnosis | 3.38 (1.76-6.48)** | 0.75 (0.32-1.80) | 1.16 (0.49-2.75) |
| Previous radiation | 0.48 (0.04-6.36) | 0.89 (0.11-7.16) | 1.99 (0.24-16.8) |
| Pretransplant steroids | 0.71 (0.40-1.27) | 1.40 (0.73-2.72) | 1.86 (0.94-3.69) |
| Pretransplant asparaginase | 1.99 (0.87-4.53) | 0.83 (0.34-2.03) | 0.84 (0.33-2.14) |
| Pretransplant insulin | 1.12 (0.21-6.10) | 3.35 (0.61-18.5) | 1.71 (0.28-10.3) |
| TBI-based preparative regimen | 4.30 (0.55-33.9) | 0.59 (0.08-4.44) | 0.43 (0.05-3.47) |
| Posttransplant steroids | 3.15 (1.73-5.71)** | 4.32 (2.35-7.93)** | |
| Posttransplant radiation | 1.95 (0.73-5.22) | 2.42 (0.82-7.11) | |
| Posttransplant TPN | 1.32 (0.66-2.66) | 1.41 (0.69-2.87) | |
| Immunosuppressive agent | Day 0-30 OR (95% CI)‡ | Day 0-100 OR (95% CI)‡ | |
| Sirolimus | 2.01 (0.64-6.34) | 6.99 (1.94-25.1)* | |
| Tacrolimus | 2.30 (1.09-4.83)* | 3.15 (1.51-6.57)* | |
| Mycophenolate mofetil | 5.11 (0.45-57.7) | 4.93 (0.43-56.7) | |
| Cyclosporine | 1.04 (0.29-3.81) | 2.74 (0.68-11.0) | |
| Methotrexate | 1.19 (0.21-6.68) | 1.01 (0.17-6.08) | |
| Potential risk factor . | Pretransplant OR (95% CI)† . | Days 0-30 OR (95% CI)† . | Days 0-100 OR (95% CI)† . |
|---|---|---|---|
| Adolescent age | 2.97 (1.72-5.14)** | 1.27 (0.70-2.31) | 1.51 (0.81-2.81) |
| BMI percentile | 1.00 (0.99-1.01) | 1.00 (0.99-1.01) | 1.00 (0.99-1.01) |
| Allogeneic transplant | 1.91 (0.72-5.10) | 3.15 (1.07-9.23)* | |
| Malignant primary diagnosis | 3.38 (1.76-6.48)** | 0.75 (0.32-1.80) | 1.16 (0.49-2.75) |
| Previous radiation | 0.48 (0.04-6.36) | 0.89 (0.11-7.16) | 1.99 (0.24-16.8) |
| Pretransplant steroids | 0.71 (0.40-1.27) | 1.40 (0.73-2.72) | 1.86 (0.94-3.69) |
| Pretransplant asparaginase | 1.99 (0.87-4.53) | 0.83 (0.34-2.03) | 0.84 (0.33-2.14) |
| Pretransplant insulin | 1.12 (0.21-6.10) | 3.35 (0.61-18.5) | 1.71 (0.28-10.3) |
| TBI-based preparative regimen | 4.30 (0.55-33.9) | 0.59 (0.08-4.44) | 0.43 (0.05-3.47) |
| Posttransplant steroids | 3.15 (1.73-5.71)** | 4.32 (2.35-7.93)** | |
| Posttransplant radiation | 1.95 (0.73-5.22) | 2.42 (0.82-7.11) | |
| Posttransplant TPN | 1.32 (0.66-2.66) | 1.41 (0.69-2.87) | |
| Immunosuppressive agent | Day 0-30 OR (95% CI)‡ | Day 0-100 OR (95% CI)‡ | |
| Sirolimus | 2.01 (0.64-6.34) | 6.99 (1.94-25.1)* | |
| Tacrolimus | 2.30 (1.09-4.83)* | 3.15 (1.51-6.57)* | |
| Mycophenolate mofetil | 5.11 (0.45-57.7) | 4.93 (0.43-56.7) | |
| Cyclosporine | 1.04 (0.29-3.81) | 2.74 (0.68-11.0) | |
| Methotrexate | 1.19 (0.21-6.68) | 1.01 (0.17-6.08) | |
P < .05, **P < .0001.
Adjusted for all factors in the column.
Adjusted for all factors in table section and for age category.
Allogeneic HSCT was independently associated with days 0 to 100 malglycemia, and posttransplant steroids were independently associated with days 0 to 30 and days 0 to 100 malglycemia (Table 2). Although adolescent age did not predict malglycemia post-HSCT, after adjusting for other factors, adolescents had higher mean glucose values at all intervals. Other aforementioned factors, including primary diagnostic category, prior asparaginase exposure, conditioning regimen, radiation, and post-HSCT TPN, were no longer associated with post-HSCT malglycemia in the multivariable analysis (Table 2).
Additionally, among patients who underwent allogeneic transplant, GVHD prophylaxis with sirolimus or tacrolimus was associated with increased mean glucose after adjusting for exposure to other immunosuppressive agents (Table 2). Patients who received sirolimus had a mean days 0 to 100 glucose that was 19.0 mg/dL (95% CI, 8.6-29.4; P < .0001) higher than patients who did not receive sirolimus, and patients who received tacrolimus had a mean days 0 to 100 glucose that was 9.2 mg/dL (95% CI, 2.7-15.8; P = .006) higher than patients who did not receive tacrolimus.
OS
The median duration of follow-up was 3.1 years. Death at any time occurred in 100 (29.1%) patients, with 60 dying from primary disease, 36 from complications, and 4 from another/unknown cause. After adjusting for transplant type, occurrence of severe GVHD, and post-HSCT steroids, days 0 to 30 malglycemia was associated with a 1.63-fold (95% CI 1.05-2.54; P = .03) increased risk for death, and days 0 to 100 malglycemia was associated with a 2.81-fold (95% CI, 1.83-4.31; P < .0001) increased risk for death. Pretransplant malglycemia was not significantly associated with risk of death (P = .13).
In addition, increasing post-HSCT mean glucose was associated with increased risk of death after adjusting for transplant type, severe GVHD, and post-HSCT steroids. In the days 0 to 30 and days 0 to 100 intervals, a 10 mg/dL increase in mean glucose was associated with 23.4% (95% CI, 9.3-39.3; P = .0007) and 40.2% (95% CI, 26.0-56.0; P < .0001) increased risk of death, respectively. Increased pretransplant mean glucose was not significantly associated with risk of death after adjusting for covariates. Survival was also analyzed by categorical mean glucose; adjusted hazard ratios are shown in Table 3, and unadjusted survival curves are shown in Figure 2. The relationship between mean glucose and survival was not affected by transplant type or treatment with post-HSCT steroids.
Adjusted hazard ratios for all mortality and TRM by mean glucose category (vs mean glucose <100 mg/dL)
| . | Adjusted HR for death† (95% CI) . | ||
|---|---|---|---|
| n = 100 . | Pre-HSCT glucose . | Days 0-30 glucose . | Days 0-100 glucose . |
| Mean glucose 100-124 mg/dL | 1.83 (1.12-2.97)* | 1.51 (0.96-2.35) | 1.76 (1.10-2.82)* |
| Mean glucose ≥125 mg/dL | 2.55 (1.40-4.65)* | 2.47 (1.28-4.74)* | 7.06 (3.84-12.99)** |
| . | Adjusted HR for death† (95% CI) . | ||
|---|---|---|---|
| n = 100 . | Pre-HSCT glucose . | Days 0-30 glucose . | Days 0-100 glucose . |
| Mean glucose 100-124 mg/dL | 1.83 (1.12-2.97)* | 1.51 (0.96-2.35) | 1.76 (1.10-2.82)* |
| Mean glucose ≥125 mg/dL | 2.55 (1.40-4.65)* | 2.47 (1.28-4.74)* | 7.06 (3.84-12.99)** |
| . | Adjusted HR for TRM† (95% CI) . | ||
|---|---|---|---|
| n = 36 . | Pre-HSCT glucose . | Days 0-30 glucose . | Days 0-100 glucose . |
| Mean glucose 100-124 mg/dL | 1.63 (0.65-4.08) | 1.61 (0.70-3.69) | 2.29 (0.89-5.85) |
| Mean glucose ≥125 mg/dL | 3.04 (1.12-8.26)* | 3.21 (1.22-8.49)* | 12.23 (4.31-34.72)** |
| . | Adjusted HR for TRM† (95% CI) . | ||
|---|---|---|---|
| n = 36 . | Pre-HSCT glucose . | Days 0-30 glucose . | Days 0-100 glucose . |
| Mean glucose 100-124 mg/dL | 1.63 (0.65-4.08) | 1.61 (0.70-3.69) | 2.29 (0.89-5.85) |
| Mean glucose ≥125 mg/dL | 3.04 (1.12-8.26)* | 3.21 (1.22-8.49)* | 12.23 (4.31-34.72)** |
HR, hazard ratio.
P < .05, **P < .0001.
Adjusted for transplant type, posttransplant steroids, and severe (grade 3 or 4 or chronic) GVHD.
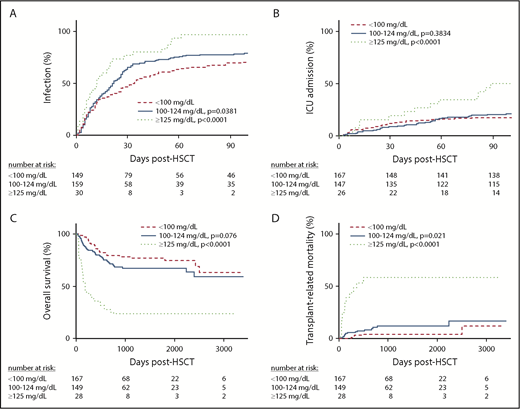
Time to morbidity and mortality by day −14 to +100 mean glucose category. On days −14 to +100, there was a statistically significant difference in time to infection (A; P < .0001), time to ICU hospitalization (B; P = .004), OS (C; P < .0001), and TRM (D; P < .0001) among patients with a mean glucose level of <100 mg/dL, 100 to 124 mg/dL, and ≥125 mg/dL. The number of patients at risk at each time point are shown in the table below each graph.
Time to morbidity and mortality by day −14 to +100 mean glucose category. On days −14 to +100, there was a statistically significant difference in time to infection (A; P < .0001), time to ICU hospitalization (B; P = .004), OS (C; P < .0001), and TRM (D; P < .0001) among patients with a mean glucose level of <100 mg/dL, 100 to 124 mg/dL, and ≥125 mg/dL. The number of patients at risk at each time point are shown in the table below each graph.
TRM
Malglycemia and mean glucose were also associated with TRM (Figure 2). After adjusting for transplant type, development of severe GVHD, and post-HSCT steroids, days 0 to 100 malglycemia was associated with a 5.78-fold (95% CI, 2.77-12.09) increased risk for TRM (P < .0001). Malglycemia in the pretransplant and days 0 to 30 intervals was not significantly associated with TRM. Increased mean glucose posttransplant was associated with TRM after adjusting for transplant type, development of severe GVHD, and post-HSCT steroids. In the days 0 to 30 and days 0 to 100 intervals, a 10 mg/dL increase in mean glucose was associated with a 28.5% (95% CI, 9.2-51.2; P = .003) and a 44.8% (95% CI, 26.3-66.1; P < .0001) increased risk for death, respectively. Pretransplant mean glucose, analyzed continuously, was not significantly associated with TRM after controlling for the same factors (P = .25). TRM was also analyzed by categorical mean glucose (Table 3).
Relapse
Among patients with malignant diagnoses, 68 (26.2%) experienced relapse. Neither malglycemia nor mean glucose, with glucose data censored by the date of relapse, was associated with relapse in unadjusted or adjusted analyses (supplemental Table 2).
Infection
In the 100 days posttransplant, 264 (76.7%) patients had a documented infection; 80 (23.3%) had an SBI, 154 (44.8%) had viremia or viruria, 14 (4.1%) had an invasive fungal infection, and 191 (55.5%) had “other” infections, such as urinary tract, upper respiratory, or gastrointestinal infection.
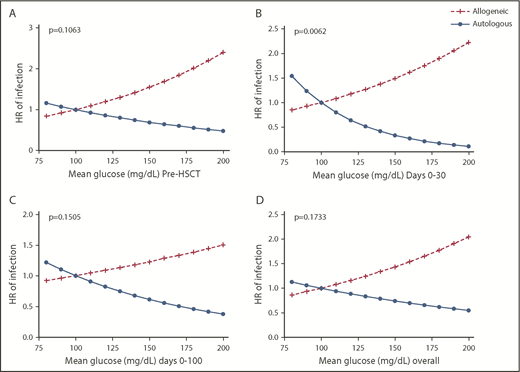
In univariate analysis, mean glucose and malglycemia pre-HSCT and during days −14 to +100 were associated with time to infection (Figure 2). After adjusting for age group, pretransplant radiation, HSCT type, severe GVHD diagnosis, and post-HSCT steroids, time-to-infection analysis showed that, for each 10 mg/dL increase in pre-HSCT glucose, there was a 11.1% (95% CI, 4.2-18.4) increased risk for any infection (P = .0013). After adjusting for the same variables, post-HSCT days 0 to 30 mean glucose was also associated with earlier time to infection, but the effect was modified by HSCT type and only true for allogeneic HSCT patients (P = .006). In allogeneic transplant patients, for each 10 mg/dL increase in days 0 to 30 mean glucose, there was a 1.07-fold increase in the risk for infection (95% CI, 1.00-1.16), unlike in autologous HSCT patients (hazard ratio, 0.78; 95% CI, 0.63-0.97). Figure 3 demonstrates how transplant type affects the association between mean glucose post-HSCT and time to infection. Steroids were not found to modify the association between mean glucose and time to any type of infection.
Hazard ratios for time to infection by mean glucose (relative to a reference value of mean glucose of 100 mg/dL) stratified by HSCT type for each time interval and adjusted for the interaction between HSCT type and glucose. (A) Pre-HSCT mean glucose. (B) Days 0 to 30 mean glucose. (C) Days 0 to 100 mean glucose. (D) Days −14 to +100 mean glucose. The association between mean glucose at any interval and time to infection was modified by HSCT type. Patients who underwent allogeneic HSCT continued to demonstrate that increased mean glucose was associated with shorter time to infection, whereas patients who underwent autologous HSCT demonstrated slightly longer time to infection with increased mean glucose.
Hazard ratios for time to infection by mean glucose (relative to a reference value of mean glucose of 100 mg/dL) stratified by HSCT type for each time interval and adjusted for the interaction between HSCT type and glucose. (A) Pre-HSCT mean glucose. (B) Days 0 to 30 mean glucose. (C) Days 0 to 100 mean glucose. (D) Days −14 to +100 mean glucose. The association between mean glucose at any interval and time to infection was modified by HSCT type. Patients who underwent allogeneic HSCT continued to demonstrate that increased mean glucose was associated with shorter time to infection, whereas patients who underwent autologous HSCT demonstrated slightly longer time to infection with increased mean glucose.
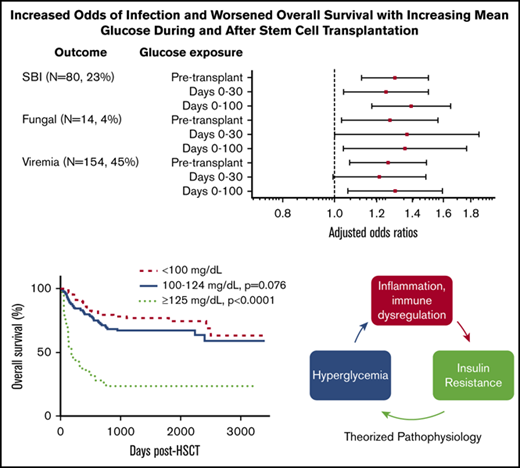
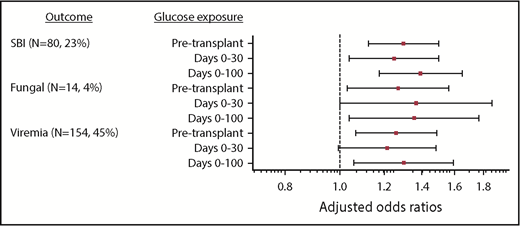
In univariate analysis evaluating infection subtype, there was an association between increased mean glucose at every time interval analyzed and increased odds of SBI, viremia/viruria, and fungal infection (supplemental Table 2). Multivariable logistic regression demonstrated that, with each 10 mg/dL increase in pretransplant mean glucose, there was a 29.8% (95% CI, 12.4-49.9) increase in SBI (P < .0001), a 26.0% (95% CI, 6.8-48.8) increase in viremia/viruria (P = .006), and 27.0% (95% CI, 3.1-56.3) increase in fungal infection (P = .02) (Figure 4). Similar relationships were noted for glucose values from days 0 to 100 (Figure 4). Adjusted odds ratios are presented in supplemental Table 2.
Adjusted odds ratios of infection subtypes for each incremental 10-mg/dL increase in mean glucose. Odds ratios for SBI, viremia/viruria, and fungal infection are adjusted for age group, HSCT type, pretransplant radiation, GVHD, and post-HSCT steroids. After adjusting for age group, pre-HSCT radiation, HSCT type, severe (grade 2, grade 4, or chronic) GVHD diagnosis, and post-HSCT steroids, the risk of SBI and fungal infection increased with increased mean glucose at all time intervals, and the risk of viremia/viruria increased with increased pre-HSCT and days 0 to 100 mean glucose. For example, with each 10-mg/dL increase in pretransplant glucose, there was a 29.8% (95% CI, 12.4-49.9) increase in SBI (P < .0001), a 26.0% (95% CI, 6.8-48.8) increase in viremia/viruria (P = .006), and a 27.0% (95% CI, 3.1-56.3) increase in fungal infection (P = .02). Similar relationships were noted for the days 0 to 100 interval. After adjusting for the aforementioned variables, there was an increased risk for GVHD (grade 3 or 4 or chronic) with increasing days 0 to 100 mean glucose.
Adjusted odds ratios of infection subtypes for each incremental 10-mg/dL increase in mean glucose. Odds ratios for SBI, viremia/viruria, and fungal infection are adjusted for age group, HSCT type, pretransplant radiation, GVHD, and post-HSCT steroids. After adjusting for age group, pre-HSCT radiation, HSCT type, severe (grade 2, grade 4, or chronic) GVHD diagnosis, and post-HSCT steroids, the risk of SBI and fungal infection increased with increased mean glucose at all time intervals, and the risk of viremia/viruria increased with increased pre-HSCT and days 0 to 100 mean glucose. For example, with each 10-mg/dL increase in pretransplant glucose, there was a 29.8% (95% CI, 12.4-49.9) increase in SBI (P < .0001), a 26.0% (95% CI, 6.8-48.8) increase in viremia/viruria (P = .006), and a 27.0% (95% CI, 3.1-56.3) increase in fungal infection (P = .02). Similar relationships were noted for the days 0 to 100 interval. After adjusting for the aforementioned variables, there was an increased risk for GVHD (grade 3 or 4 or chronic) with increasing days 0 to 100 mean glucose.
GVHD
Of 225 patients receiving allogeneic transplants, 50 (22.2%) developed acute GVHD grade 3 or 4 and/or chronic GVHD. Pre-HSCT mean glucose was not associated with time to severe GVHD. However, post-HSCT mean glucose was associated with an increased risk for GVHD in univariate and multivariable analysis. After adjusting for steroids given after HSCT but before diagnosis of severe GVHD, for every 10 mg/dL increase in days 0 to 30 mean glucose, there was a 1.29-fold risk for severe GVHD (95% CI, 1.11-1.50); there was a similar effect with days 0 to 100 mean glucose.
Engraftment
The median time to neutrophil engraftment was 21.0 days (interquartile range [IQR], 17.0-25.0) after allogeneic HSCT and 11.0 days (IQR, 10-12) after autologous HSCT. After adjusting for transplant type and donor source, there was no association between malglycemia or mean glucose and time to engraftment.
Hospital and ICU days
The median number of total hospital days from days 0 to 100 was 37.0 (IQR, 25-57), including days of primary admission and readmissions. After adjusting for transplant type and development of severe GVHD, increased mean glucose and malglycemia during days 0 to 30, days 0 to 100, and days −14 to +100 were associated with more hospitalized days from days 0 to 100 (P < .0001 to .01). For example, patients with malglycemia in the first 100 days post-HSCT were hospitalized for 13.0 more days compared with patients without malglycemia (P < .0001).
Among the entire cohort, 75 (21.8%) patients required an ICU admission during the first 100 days, with a median of 3.6 ICU days (IQR, 1.6-8.7) among these patients. In univariate analysis, post-HSCT and overall malglycemia and mean glucose were associated with time to ICU stay (Figure 2). After adjusting for transplant type and development of severe GVHD, mean glucose ≥125 mg/dL (compared with <100 mg/dL) and malglycemia in the days 0 to 100 and days −14 to +100 intervals were associated with more ICU days in time-to-event analysis. Patients who had a mean glucose ≥125 mg/dL in the first 100 days had a 2.80-fold increased risk for requiring ICU care (95% CI, 1.30-6.03) compared with patients with a mean glucose < 100 mg/dL (P = .008).
Discussion
Patients undergoing HSCT are at high risk for infection, GVHD, relapse, death, and other adverse outcomes. Improvements in post-HSCT morbidity, survival, and quality of life depend on identifying modifiable factors that directly or indirectly influence these outcomes.28 This retrospective cohort study sought to establish the incidence, risk factors, and consequences of malglycemia in the pediatric/AYA HSCT population. This study demonstrates that malglycemia is highly prevalent in this population and is associated with increased morbidity and mortality.
Glycemic variability, the most common form of malglycemia, is easily overlooked by care teams; it warrants special attention in future investigations. In contrast, hypoglycemia was extremely rare; therefore, this study’s analyses of categorical malglycemia primarily represent associations around hyperglycemia and variability. Because of the subtle nature of glycemic variability, and of increasing glucose in association with outcomes, understanding risk factors is critical to aid recognition. Adolescent age and underlying malignancy were independent risk factors for pre-HSCT malglycemia, with the former possibly due to insulin resistance associated with puberty.29 Neither characteristic remained a risk factor for malglycemia after HSCT, possibly due to a leveling effect of supervening posttransplant events, which were controlled for in our analysis. As might be expected, allogeneic HSCT and post-HSCT steroids were independent risk factors for post-HSCT malglycemia, as was treatment with sirolimus or tacrolimus.30 Of interest, underlying diagnosis, increased BMI, cyclosporine use, and previous insulin requirement were not risk factors for malglycemia in the multivariable model. The last 2 factors occurred infrequently, which may explain their lack of significance in this cohort.
In this study, malglycemia and higher mean glucose, before and after transplant, were associated with adverse outcomes, including worsened OS, TRM, and infections. In fact, even small increases in mean glucose (10 mg/dL) were associated with substantially increased mortality and, among allogeneic HSCT patients, infection, independent of factors such as severe GVHD and steroid therapy. This effect was dose dependent and cumulative for each incremental increase in glucose. Further, there was a temporal association demonstrated by time-to-event analyses for survival, TRM, infection, severe GVHD, and ICU stay, as well as in logistic regression analyses between pretransplant mean glucose and posttransplant outcomes, such as infection subtypes. Similar temporality has been demonstrated in adult HSCT studies, with preexisting glucose metabolism challenges, from mild hyperglycemia3,8 to diabetes mellitus,31,32 being associated with adverse outcomes, such as infection, and increased mortality and early post-HSCT glucose issues being associated with worse TRM and OS.33 These temporal relationships, as well as the dose-dependent nature of associations between glucose and morbidity/mortality, are critical in considering causation.
Although it could be suggested that factors such as steroid treatment may confound these data, this study and several others have demonstrated that the adverse outcomes associated with malglycemia occur even when controlling for or excluding patients treated with steroids.1,6 Additionally, adult patients with steroid-associated malglycemia experienced worse OS, TRM, and infection rates compared with patients treated with steroids who did not have malglycemia.3,10 This relationship suggests that steroid therapy exerts at least part of its untoward effects by causing malglycemia, which, in turn, triggers downstream pathology,3,10,34 although a limitation of our study was the lack of available information regarding the dose or duration of steroid therapy, resulting in controlling for steroids in a binary fashion.
The underlying pathophysiology potentially linking hyperglycemia and poor outcomes may be direct or indirect and could promote immune dysregulation and inflammation. These effects are suggested by the striking association between glucose levels during days 0 to 100 and longer-term outcomes, such as TRM, OS, and severe GVHD. Hyperglycemia, particularly when chronic, impairs neutrophil chemotaxis, generation of oxygen radicals, and phagocytosis. It also impairs lymphocytes by increasing apoptosis, suppressing proliferation, and attenuating antibody production and function.1,3,18-22 In addition, hyperglycemia is associated with a proinflammatory state with increased circulating cytokines, including interleukin-6 and tumor necrosis factor α.35 Furthermore, there is reason to believe that improved glucose control can restore immune dysregulation. For example, Yano et al demonstrated in a diabetic mouse model that insulin treatment restored neutrophil function, including phagocytosis, superoxide production, and Staphylococcus aureus killing.36 Between the known adverse effects of malglycemia on the immune system and the temporal and dose-dependent relationships between malglycemia and adverse outcomes in this study and previous adult studies, it is plausible to consider malglycemia a possible causal factor in post-HSCT morbidity and mortality.
Although we found multiple significant associations between malglycemia and HSCT outcomes, our investigations have several limitations. Although this study includes time-to-event analyses censoring glucose values by the date of first post-HSCT infection, severe GVHD diagnosis, and ICU stay to reduce the effect of postevent glucose values, obtaining exact dates for infection subtypes was not feasible. Therefore, in this retrospective study, although temporal relationships were established between glucose and overall infection, severe GVHD, ICU stay, TRM, and OS, it is not possible to clearly describe the temporal relationships between post-HSCT glucose and infection subtypes. In addition, the definition of malglycemia classically relies on the assumption of fasting blood glucose values, whereas we included all glucose data; therefore, malglycemia may be overestimated. However, this does not affect the relationship between mean glucose and outcomes. Outcomes, such as invasive fungal infection and GVHD, relied on correct International Statistical Classification of Diseases and Related Health Problems diagnostic coding and manual review and, therefore, have potential for misclassification bias. Although this is unlikely to bias the data, because classification challenges would be true across all patients, it may have resulted in decreased statistical power due to underreporting. Additionally, this study aims to highlight correlations between glucose and adverse events, and any biological causes are hypothetical and require prospective human and animal model–based studies. To these ends, future work is ongoing.
This study has numerous implications for future research that might influence clinical management of HSCT patients. First, even mild to moderate hyperglycemia and glycemic variability may represent biomarkers for heightened risk of infection and severe GVHD among allogeneic HSCT patients and, in all HSCT patients, may represent biomarkers for increased TRM and reduced OS. Moreover, malglycemia may be causal in these associations. Retrospective and prospective studies are ongoing to better establish risk factors, including evaluating risk associated with acute lymphoblastic leukemia and acute myeloid leukemia, temporality, and causality. The ability to predict which patients are most likely to have malglycemia based on pre-HSCT characteristics might be valuable to incorporate into the pretransplant comorbidity prognostic index.37 Furthermore, investigation of intensive glucose control to mitigate the adverse consequences of malglycemia is merited. We found that pre- and post-HSCT malglycemia have significant associations with undesirable outcomes; therefore, both time periods might be targeted for interventions.
Current adult literature evaluating the benefit of intervention to mitigate the relationship between malglycemia and outcomes is limited to a single small case-control study in 22 adult HSCT patients. In the intensive glucose control group, the incidence of infection was markedly decreased (14% vs 46%; P = .004).4 Outside of HSCT, there have been multiple pediatric and adult studies evaluating the benefit of improved glucose control in non-HSCT patients in the intensive care setting.38-42 These studies were largely disappointing. However, they cannot be extrapolated to the HSCT population for several reasons. The HSCT population is at high risk for immune dysfunction, infection, and other morbidity and mortality for a significantly longer period of time than that of a typical ICU hospitalization. In addition to these differences, 1 of the problems with the ICU studies was the relative frequency of hypoglycemia, which complicated management.43 As diabetes technology progresses, achieving safe, intensive, and predictable glucose control will be increasingly feasible.
In summary, malglycemia is a prevalent and potentially modifiable problem in the pediatric/AYA HSCT population. Based on the present study, control of hyperglycemia and glycemic variability should be investigated as a strategy to improve HSCT outcomes.
The full-text version of this article contains a data supplement.
Acknowledgments
Rajeev Vibhakar provided guidance in the completion of this work; there was no funding for these contributions.
This work was supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grant 2K12DK094712-06 and National Institutes of Health, National Center for Advancing Translational Sciences Colorado Clinical and Translational Sciences Institute grant UL1 TR001082.
The contents are the authors’ sole responsibility and do not necessarily represent official views of the National Institutes of Health. The sponsors of this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Authorship
Contribution: J.S. is the principal investigator and designed the study, performed data collection and analyses, and wrote the manuscript; G.P.F., R.H.G., and A.K.K. oversaw and contributed to the study design and analysis plan, as well as contributed to and edited the manuscript; L.P. and K.C. performed all data analyses and prepared figures; R.P.W. and M.R.V. assisted in editing the manuscript; A.K.L. provided all radiation data; and all authors had access to primary data.
Conflict-of-interest disclosure: G.P.F. has served as a consultant for Abbott Diabetes Care and an advisory board member for Dexcom and conducts research sponsored by Medtronic, Dexcom, Bigfoot Biomedical, Tandem Diabetes Care, Insulet, and Novo Nordisk. M.R.V. has served as a consultant for Fate Therapeutics and B-MoGen Biotechnologies. R.P.W. has received grants and personal fees from Dexcom, Novo Nordisk, MannKind Corporation, and Eli Lilly and Company for unrelated work. The remaining authors declare no competing financial interests.
Correspondence: Jenna Sopfe, Center for Cancer and Blood Disorders, Department of Pediatrics, University of Colorado School of Medicine, 13123 E 16th Ave, B115, Aurora, CO 80045; e-mail: jenna.sopfe@ucdenver.edu.