Key Points
Shingrix is poorly immunogenic following allogeneic hematopoietic cell transplantation independent of age, CD4, and B-cell recovery.
In hematopoietic cell transplantation recipients with antibody response to the vaccine, varicella zoster virus reactivation risk is not null.
Introduction
Hematopoietic cell transplantation (HCT) recipients are at high risk for varicella zoster virus (VZV) reactivation.1,2 Shingrix (hereafter referred to as the adjuvanted recombinant zoster vaccine [aRZV]), a subunit vaccine containing VZV glycoprotein E (gE) and the AS01B adjuvant system, is a new alternative to the live-attenuated VZV vaccine for immunocompromised individuals, with reported efficacy of 68% among autologous HCT recipients.3 Humoral response to the aRZV occurs in ∼70% to 80% of autologous HCT and patients with hematological malignancies.4,5 Similar rates of humoral response have been reported in other immunocompromised populations including patients with solid tumors, HIV infection, and solid organ transplant.6-8
Postmarketing non-industry–sponsored “real-world” data with the aRZV following autologous HCT is limited, and there are no published data on aRZV efficacy and immunogenicity in allogeneic HCT recipients.
Methods
We conducted a single-center retrospective study of 135 consecutive adult allogeneic and autologous HCT recipients who received the full series (2 doses) of aRZV (supplemental Table 1). The primary end point of this pilot study was immunogenicity defined as either seroconversion in previously seronegative individuals or a fourfold increase from baseline VZV immunoglobulin G (IgG) titers in subjects who were seropositive before vaccination. Samples were processed at a commercial laboratory using the LIAISON VZV IgG assay. This assay uses a partially purified extract of infected cell cultures (VZVROD strain) and is calibrated against World Health Organization International Preparation W1044 (assay range, 10-4000 mIU/mL) with a specificity of 97% and sensitivity of 100%, per the manufacturer. All patients received antiviral prophylaxis with acyclovir 800 mg orally twice daily for 6 to 12 months depending on the type of transplant. The study was conducted consistent with Declaration of Helsinki principles. Mann-Whitney U, Fisher’s exact, or Wilcoxon matched-pairs signed-rank test were used where appropriate. Multivariable logistic regression modeling was developed to identify predictors of humoral immunogenicity. Statistical analyses were performed using GraphPad Prism Software, version 7.03, and SPSS, version 26.
Results and discussion
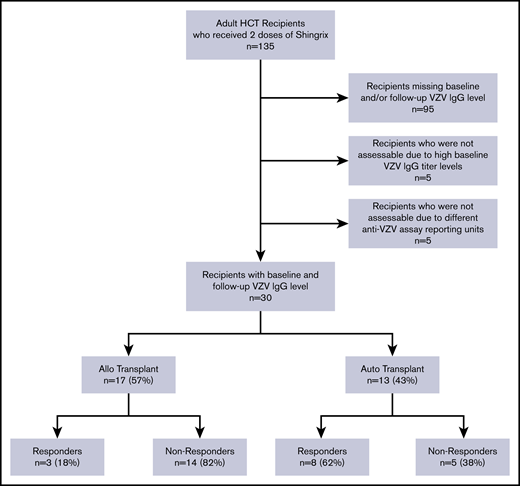
Only 40 patients had serological data available at baseline and after completion of vaccine series and 10 were not assessable (Figure 1). A total of 30 patients (17 allogeneic and 13 autologous) were analyzed. The characteristics of the assessable study subjects are presented in Table 1. The median age of HCT patients was 61 years. The majority of the patients received no immunosuppression or only single-agent immunosuppression at the time of vaccination. The median time from HCT to first dose of aRZV was 8 months for the entire cohort, but it was significantly shorter in autologous vs allogeneic HCT recipients (Table 1). The median time from the second vaccine dose to postvaccination VZV IgG titer assessment was 4 months.
Characteristics of study subjects
| Characteristic . | All patients (N = 30) . | Responders (n = 11) . | Nonresponders (n = 19) . | P* . |
|---|---|---|---|---|
| Age, median (IQR), y | 61 (54-68) | 56 (51-58) | 65 (58-69) | .048 |
| Male sex | 16 (53) | 6 (55) | 10 (53) | >.99 |
| Hispanic or Latino | 12 (40) | 6 (55) | 6 (32) | .27 |
| Race | .58 | |||
| White | 24 (80) | 8 (73) | 16 (84) | |
| Black/African American | 5 (17) | 3 (27) | 2 (11) | |
| Asian | 1 (3) | 0 | 1 (5) | |
| Type of transplant | .02 | |||
| Allogeneic | 17 (57) | 3 (27) | 14 (74) | |
| Autologous | 13 (43) | 8 (73) | 5 (26) | |
| Stem cell source | .62 | |||
| Peripheral blood | 25 (83) | 10 (91) | 15 (79) | |
| Bone marrow | 5 (17) | 1 (9) | 4 (21) | |
| Immunosuppression at vaccine administration | ||||
| Any | 9 (30) | 3 (27) | 6 (32) | >.99 |
| Tacrolimus | 3 (10) | 1 (9) | 2 (11) | >.99 |
| Steroids† | 6 (20) | 1 (9) | 5 (26) | .37 |
| Dasatinib | 1 (3) | 1 (9) | 0 | .37 |
| ≥2 immunosuppressants at the time of vaccination | 3 (10) | 1 (9) | 2 (11) | >.99 |
| IVIG administration within 3 mo before vaccination or VZV IgG titer assessment | 3 (10) | 1 (9) | 2 (11) | >.99 |
| Type of donor‡ | >.99 | |||
| Unrelated | 12 (71) | 2 (67) | 10 (71) | |
| HLA-matched related | 5 (29) | 1 (33) | 4 (29) | |
| (D)onor/(R)ecipient CMV serostatus‡ | .58 | |||
| D-R+ | 5 (29) | 2 (67) | 3 (21) | |
| D+R+ | 8 (47) | 0 | 8 (57) | |
| D+R− | 2 (12) | 1 (33) | 1 (7) | |
| D+R− | 2 (12) | 0 | 2 (14) | |
| Time between transplant and vaccine first dose, median (IQR), mo | 8 (7-12) | 7 (6-10) | 10 (7-14) | .18 |
| Time between vaccine doses, median (IQR), d | 95 (59-138) | 116 (61-156) | 69 (59-128) | .45 |
| Time between completion of vaccine series and VZV IgG titers, median (IQR), mo | 4 (2-6) | 3 (2-6) | 5 (3-7) | .25 |
| CD34+ cells infused (1 × 106), median (IQR) | 4.8 (3.7-8.7) | 5 (3.7-8.2) | 4.6 (3.5-9) | .74 |
| ATG§ | 9 (30) | 2 (18) | 7 (37) | .42 |
| CD4+ T cells/µL, median (IQR)‡|| | 376 (255-442) | 242 (205-319) | 405 (284-475) | .08 |
| B cells/µL, median (IQR)*|| | 105 (33-218) | 101 (32-158) | 109 (25-230) | .69 |
| Conditioning regimen | .002 | |||
| Myeloablative | 15 (50) | 10 (91) | 5 (26) | |
| Reduced Intensity | 15 (50) | 1 (9) | 14 (74) | |
| Charlson Comorbidity Index, median (IQR) | 3 (2-3) | 3 (2-3) | 3 (2-3) | .62 |
| Main diagnosis | ||||
| Leukemia | 11 (37) | 1 (9) | 10 (53) | .02 |
| Lymphoma | 5 (17) | 1 (9) | 4 (21) | .63 |
| MDS/MPN | 2 (7) | 0 | 2 (11) | .52 |
| MM | 10 (33) | 8 (73) | 2 (11) | .001 |
| Other | 2 (6) | 1 (9) | 1 (5) | >.99 |
| Characteristic . | All patients (N = 30) . | Responders (n = 11) . | Nonresponders (n = 19) . | P* . |
|---|---|---|---|---|
| Age, median (IQR), y | 61 (54-68) | 56 (51-58) | 65 (58-69) | .048 |
| Male sex | 16 (53) | 6 (55) | 10 (53) | >.99 |
| Hispanic or Latino | 12 (40) | 6 (55) | 6 (32) | .27 |
| Race | .58 | |||
| White | 24 (80) | 8 (73) | 16 (84) | |
| Black/African American | 5 (17) | 3 (27) | 2 (11) | |
| Asian | 1 (3) | 0 | 1 (5) | |
| Type of transplant | .02 | |||
| Allogeneic | 17 (57) | 3 (27) | 14 (74) | |
| Autologous | 13 (43) | 8 (73) | 5 (26) | |
| Stem cell source | .62 | |||
| Peripheral blood | 25 (83) | 10 (91) | 15 (79) | |
| Bone marrow | 5 (17) | 1 (9) | 4 (21) | |
| Immunosuppression at vaccine administration | ||||
| Any | 9 (30) | 3 (27) | 6 (32) | >.99 |
| Tacrolimus | 3 (10) | 1 (9) | 2 (11) | >.99 |
| Steroids† | 6 (20) | 1 (9) | 5 (26) | .37 |
| Dasatinib | 1 (3) | 1 (9) | 0 | .37 |
| ≥2 immunosuppressants at the time of vaccination | 3 (10) | 1 (9) | 2 (11) | >.99 |
| IVIG administration within 3 mo before vaccination or VZV IgG titer assessment | 3 (10) | 1 (9) | 2 (11) | >.99 |
| Type of donor‡ | >.99 | |||
| Unrelated | 12 (71) | 2 (67) | 10 (71) | |
| HLA-matched related | 5 (29) | 1 (33) | 4 (29) | |
| (D)onor/(R)ecipient CMV serostatus‡ | .58 | |||
| D-R+ | 5 (29) | 2 (67) | 3 (21) | |
| D+R+ | 8 (47) | 0 | 8 (57) | |
| D+R− | 2 (12) | 1 (33) | 1 (7) | |
| D+R− | 2 (12) | 0 | 2 (14) | |
| Time between transplant and vaccine first dose, median (IQR), mo | 8 (7-12) | 7 (6-10) | 10 (7-14) | .18 |
| Time between vaccine doses, median (IQR), d | 95 (59-138) | 116 (61-156) | 69 (59-128) | .45 |
| Time between completion of vaccine series and VZV IgG titers, median (IQR), mo | 4 (2-6) | 3 (2-6) | 5 (3-7) | .25 |
| CD34+ cells infused (1 × 106), median (IQR) | 4.8 (3.7-8.7) | 5 (3.7-8.2) | 4.6 (3.5-9) | .74 |
| ATG§ | 9 (30) | 2 (18) | 7 (37) | .42 |
| CD4+ T cells/µL, median (IQR)‡|| | 376 (255-442) | 242 (205-319) | 405 (284-475) | .08 |
| B cells/µL, median (IQR)*|| | 105 (33-218) | 101 (32-158) | 109 (25-230) | .69 |
| Conditioning regimen | .002 | |||
| Myeloablative | 15 (50) | 10 (91) | 5 (26) | |
| Reduced Intensity | 15 (50) | 1 (9) | 14 (74) | |
| Charlson Comorbidity Index, median (IQR) | 3 (2-3) | 3 (2-3) | 3 (2-3) | .62 |
| Main diagnosis | ||||
| Leukemia | 11 (37) | 1 (9) | 10 (53) | .02 |
| Lymphoma | 5 (17) | 1 (9) | 4 (21) | .63 |
| MDS/MPN | 2 (7) | 0 | 2 (11) | .52 |
| MM | 10 (33) | 8 (73) | 2 (11) | .001 |
| Other | 2 (6) | 1 (9) | 1 (5) | >.99 |
Data are presented as absolute number (percentage), unless specified otherwise. Statistically significant P values (P < .05) are indicated in bold.
ATG, antithymocyte globulin; CMV, cytomegalovirus; IQR, interquartile range; IVIG, intravenous immunoglobulins; MDS/MPN, myelodysplastic syndrome/myeloproliferative neoplasm; MM, multiple myeloma.
P value for comparison between the responders and nonresponders groups by using Mann-Whitney U or Fisher’s exact test.
All the patients on steroids were receiving <0.5 mg/kg prednisone equivalent per day.
Data for 17 allogeneic transplant recipients.
Typical dose of ATG at our center is 4 mg/kg total.
Refers to cell counts in allogeneic HCT recipients before completion of vaccine series. Data missing for 1 patient.
A total of 11 (37%) patients had documented humoral vaccine responses as measured by postvaccination VZV IgG levels (hereafter referred to as responders) including 8/13 (62%) autologous HCT recipients compared with 3/17 (18%) allogeneic HCT recipients (P = .02). Among the 11 responders, the VZV IgG antibody concentration index increased from 415 (135-597) to 3482 (1439-4000; P = .001; supplemental Figure 1).
There were no differences in type of donor, stem cell source, cytomegalovirus serostatus, type of immunosuppression, intravenous immunoglobulin use, T-cell depletion, CD34 cells infused, comorbidity score, time to vaccine administration, time interval between vaccine doses, or time to serological testing between responders (n = 11) and nonresponders (n = 19). However, responders were significantly younger at the time of vaccine administration compared with nonresponders (56 vs 65 years, respectively; P = .048). Autologous transplant (73%) and multiple myeloma (73%) were the more common type of transplant and disease indication among responders whereas the nonresponders group was enriched for patients with acute leukemia (53%) and allogeneic transplant (74%; P ≤ .02 for all comparisons; Table 1).
In a univariate analysis, allogeneic transplantation was significantly associated with a reduced probability of responding to aRZV (odds ratio, 0.13; 95% confidence interval, 0.03-0.72; P = .02). Other variables such as age, CD4+ T-cell count, and time from transplant to vaccination were not associated with vaccine response. Allogeneic transplantation remained the only variable associated with poor vaccine response in a multivariate model adjusted for age (odds ratio, 0.08; 95% confidence interval, 0.01-0.60; P = .01). Underlying diagnosis and type of conditioning regimen were not included in the multivariate analysis as they were both codependent variables with strong correlation with the type of transplant (data not shown).
The optimal time for administration of aRZV following HCT remains to be defined. In the randomized clinical trial of aRVZ in autologous HCT recipients, patients had undergone transplant in the previous 50 to 70 days.3 Because complete reconstitution of the CD4+ T-cell compartment typically takes longer than 6 to 12 months after transplant, particularly among T-cell–depleted patients,9,10 many clinicians delay vaccine administration until there is documented CD4+ count >200 cells/mm3. However, in the present study, CD4+ T-cell count did not seem to influence vaccine response; among allogeneic HCT recipients, there were no differences in CD4+ T- or B-cell counts at the time of vaccination between responders and nonresponders (242 vs 405 for CD4 cells, P = .08, and 101 vs 109 for B cells, P = .69 respectively; Table 1; supplemental Figure 2). A recent study showed no association between CD4+ T-cell counts at 1 year after cord blood transplantation and subsequent risk for herpes zoster.1 Whether delaying vaccination 1 to 2 years after transplant, administering a booster dose or nonstandard formulation (high dose) can overcome low responsiveness to aVZV requires further study.
Our study has a number of limitations. First, we had to exclude many patients primarily on the basis of missing data. Small sample size precluded more comprehensive multivariate analyses. We did, however, account for the effects of age and type of transplant. Second, donor VZV serological status was not available. Of interest, the 3 patients who were seronegative before vaccination seroconverted in response to aRZV; these 3 patients received autologous HCT, excluding the possibility that VZV humoral immunity was conferred by the donor. This observation suggests that aRZV may also be useful for primary VZV immunization. Third, lack of control group; we identified a group of 8 allogeneic HCT recipients who were not vaccinated and had VZV IgG titers available, but only 2 were assessable. Neither of these 2 subjects had an increase in VZV antibody titers posttransplant, suggesting that antibody responses observed in immunized patients reflected vaccine, rather than donor, conferred immunity. Fourth, we did not assess clinical efficacy, which would require a more prolonged (ie, years) follow-up. One of the patients in the responder group had disseminated VZV 3 months after vaccination, suggesting that VZV antibody level might not be an ideal immune correlate of protection, in contrast to subjects with hepatitis B surface antibody concentrations ≥10 mIU/mL who are considered protected against hepatitis B virus infection.11 Finally, we did not use an immunoassay for vaccine-induced epitope-specific antibodies. However, considering that gE is a predominant component of the VZV virion envelope and essential for VZV replication,12 and the lysate preparation contains all the viral proteins, the commercially available VZV IgG test likely captures specific humoral immunity to immunodominant epitopes on the VZV gE glycoprotein as well.
In conclusion, observations derived from this pilot study suggest that the 2-dose aRZV series is less immunogenic in allogeneic compared with autologous HCT recipients, independent of age, CD4, and B-cell recovery; and even among those with antibody response to the vaccine, the risk of VZV reactivation is not null. Until larger studies are available, transplant clinicians should be mindful of the limitations of the aRZV in allogeneic HCT recipients before discontinuation of antiviral prophylaxis.
Send data sharing requests via e-mail to the corresponding author, Jose F. Camargo (jfc31@med.miami.edu).
Acknowledgments
The authors thank all the patients who participated in the study and Humberto Elejalde for assistance with data collection.
This study was supported by National Institutes of Health, National Cancer Institute grant P30CA240139. This study was also supported by the kind support of the Kalish Family Foundation (K.V.K.) and Applebaum Foundation (J.F.C. and K.V.K.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: J.F.C., R.Y.L., and K.V.K. conceived and designed the study; J.F.C., R.Y.L., A.D.A., M.C.A., T.P.W., M.I.M., and K.V.K. acquired the data; J.F.C., R.Y.L., Y.N., and K.V.K. analyzed the data; J.F.C. prepared the first draft of the manuscript; and all authors were involved in the revision of the draft manuscript and have agreed to the final content.
Conflict-of-interest disclosure: K.V.K. has served as an ad hoc consultant to Kite/Gilead, Novartis, Celgene, Atara, Kiadis, Kadmon, and Takeda. M.I.M. has received research funding from Merck. The remaining authors declare no competing financial interests.
Correspondence: Jose F. Camargo, Division of Infectious Diseases, University of Miami Miller School of Medicine, 1120 NW 14th St, Miami, FL 33136; e-mail: jfc31@med.miami.edu.
References
Author notes
The full-text version of this article contains a data supplement.