Key Points
The Spanish AHA Registry updates the management of AHA in Spain and provides findings relevant to clinical practice.
Mortality was commonly related to antithrombotic therapy and/or infection in the first months of immunosuppressive therapy.
Abstract
The Spanish Acquired Hemophilia A (AHA) Registry is intended to update the status of AHA in Spain. One hundred and fifty-four patients were included and retrospectively followed for a median of 12 months. Patients were predominantly male (56.3%), with median age at diagnosis of 74 years. AHA was more frequently idiopathic (44.1%) and autoimmune disorder-associated (31.7%). Thirty-four percent of patients were on antithrombotic therapy at diagnosis. Hemostatic treatment was used in 70% of patients. Recombinant activated factor VII was more frequently infused (60.3% vs 20.6% activated prothrombin complex concentrate). Only 1 patient did not achieve control of hemorrhage. Complete remission (CR) was achieved by 84.2% of cases after immunosuppressive therapy. Steroids alone were less efficient than the other strategies (68.2% vs 87.2%, P = .049), whereas no differences existed among these (steroids/cyclophosphamide, 88.5%, vs steroids/calcineurin inhibitors, 81.2%, vs rituximab-based regimens, 87.5%). Female sex and high inhibitor levels influenced CR negatively. Thirty-six deaths (23.8%) were reported. Main causes of death were infection (15 patients, 9.9%) and hemorrhage (5 patients, 3.3%). All hemorrhage-related and half the infection-related deaths occurred within 2 months of diagnosis. Prior antithrombotic therapy was inversely associated with survival, irrespective of age. Median age of nonsurvivors was significantly higher (79 vs 73 years in survivors). Patients dying of infection were older than the other nonsurvivors (85 vs 78 years). In summary, fatal infection in the first months is common in our series. Antithrombotic therapy is associated with mortality. Particular care should be taken to avoid misdiagnosis.
Introduction
Acquired hemophilia A (AHA) is a rare autoimmune disorder caused by autoantibodies against factor VIII (FVIII) that prevent its interaction with factor IX (FIX) or phospholipids, or accelerate its clearance. The incidence in the population is about 1.5 case per million persons/year,1 with a biphasic distribution involving specially the elderly but also female at the postpartum period.2–5 AHA shows a high mortality rate, which ranges between 9% and 33%.3,6–9 Although roughly half of all cases are idiopathic, AHA is often associated with underlying diseases and conditions such as autoimmune disorders, neoplasia, the puerperal period, or drug intake. Bleeding manifestations may occur at varied localizations, spontaneously or induced by trauma or invasive procedures, and differ greatly in severity, being sometimes life-threatening. Using bypassing agents, such as recombinant activated factor VII (rFVIIa) or activated prothrombin complex concentrate (aPCC) to stop bleeding is not the only required action. Immunosuppressive therapy (IST) should be administered immediately after diagnosis to eliminate the inhibitor and restore normal FVIII levels. Steroid-based regimens are currently the preferred first line of treatment.1
AHA is not well-known among clinicians unfamiliar with hemostatic disorders. Lack of awareness may preclude early diagnosis, thus exposing patients to an unacceptably high bleeding risk. Furthermore, the guidelines on which drug/drugs should be used in the first line of IST rely on registry findings and authors’ experience rather than on comparative studies.1,10,11 Therefore, any valuable knowledge regarding clinical experience in managing this disorder should be helpful. The rarity of the condition prompts the design of registries to compile as much information as possible concerning baseline status, diagnosis, treatment, and follow-up. These databases provide information to continuously update guidelines on disease management procedures, the last version of which has recently become available.11 We present a national registry intended to recruit and follow-up Spanish patients diagnosed with AHA.
Methods
Patients
This registry, the AHA Spanish Registry (AHASR), retrospectively collected data regarding patients diagnosed with AHA in 36 Spanish hospitals from May 2014 to September 2020. The AHASR is located in the Spanish Society of Thrombosis and Haemostasis website (www.seth.es). Institutional review boards of all participating hospitals explicitly approved participation. The study was conducted in accordance with the Declaration of Helsinki.
Data collection
Electronic registration forms captured age, sex, underlying diseases, drug intake history, circulating factor VIII activity (FVIII:C), FVIII inhibitor titer and bleeding location at diagnosis, exposure to bypassing agents, FVIII preparations or other blood products, comorbidities, and first-line IST. Data collection was coordinated by 1 physician in each participating center, and information was transferred to a contract research organization. In the event that missing data were detected, they were requested from the original source. FVIII 1-stage clotting assay was available in all hospitals and FVIII:C chromogenic substrate assay in 25 hospitals. Inhibitor titers were determined by the Bethesda assay. Complete remission (CR) was considered when FVIII:C activity levels recovered above 50 IU/dL, the inhibitor turned <0.6 BU/mL, and no AHA-related clinical manifestations were observed in absence of IST.12 Recruited patients were followed to assess remission, including time to CR when achieved, onset of adverse events, and survival.
Statistical methods
Descriptive statistical analyses were performed to describe AHASR patients. Medians and interquartile ranges (IQRs), and absolute and relative frequencies were used for quantitative and qualitative variables respectively. Comparisons between groups of patients were performed by the Mann-Whitney U test or the Fisher’s exact test. Correlation was assessed by the Spearman’s ρ test. Predictive value of selected clinical variables on CR and survival was determined using a multivariate logistic regression analysis. Kaplan-Meier curves and log-rank tests were used to compare cumulative survival between groups of patients.
Results
One hundred and fifty-four patients diagnosed with AHA constituted the cohort of AHASR patients by September 2020. Appropriate information was retrospectively collected by 36 Spanish hospitals (supplemental Table 1).
Features of the patients of the AHASR cohort
The main characteristics of AHASR patients are outlined in Table 1. Patients were predominantly elderly, with 108 (70.1%) and 44 (28.6%) with ages above 65 and 80 years, respectively. The follow-up period varied greatly, although it was longer than 1 year in 70 (45.4%) subjects. FVIII:C was determined by both coagulative and chromogenic assays in 62 (40.3%) patients, and only by the coagulative or chromogenic method in 87 (56.5%) and 5 (3.2%) patients, respectively. Thirty-eight patients presented with FVIII:C levels <1 IU/dL, and only 54, one-third of the cohort, had inhibitor titers ≤10 BU/mL. Eighty-nine patients had at least 1 underlying clinical disease or condition at the time of diagnosis. Among these, disorders with a relevant autoimmune component were the most common presentations. AHA was idiopathic in 64 subjects.
Main features of the AHASR cohort patients
| Variable . | n/N (%)* . |
|---|---|
| Age at DX (y), median (IQR) | 74 (64-83) |
| Male | 85/151 (56.3) |
| Follow-up (mo)†, median (IQR) | |
| Whole cohort (n = 154) | 12 (2-33) |
| Patients alive at study end (n = 36) | 15 (4-36) |
| FVIII:C at Dx, median (IQR) | 1.9 (0.6-4.7) |
| 6-40 IU/dL | 27/135 (20.0) |
| 1-5 IU/dL | 70/135 (51.8) |
| <1 IU/dL | 38/135 (28.1) |
| Inhibitor at Dx, median (IQR) | 16.00 (5.50-42.40) |
| 0-10 BU/mL | 54/145 (37.2) |
| >10-100 BU/mL | 76/145 (52.4) |
| 101-1000 BU/mL | 11/145 (7.6) |
| >1000 BU/mL | 4/145 (2.8) |
| Bleeding at Dx‡ | 135/149 (90.6) |
| Mucocutaneous | 91/132 (68.9) |
| Muscular | 41/132 (31.1) |
| Urinary | 22/132 (16.7) |
| Digestive | 16/132 (12.1) |
| Retroperitoneal | 13/132 (9.8) |
| Thoracic | 13/132 (9.8) |
| Other§ | 16/132 (12.1) |
| Required bypassing treatment | 67/95 (70.5) |
| rFVIIa | 38/63 (60.3) |
| aPCC | 13/63 (20.6) |
| Both | 12/63 (19.0) |
| Underlying disease/condition║ | |
| Idiopathic | 64/145 (44.1) |
| Autoimmune disorder¶ | 46/145 (31.7) |
| Type 1 diabetes | 5/46 (10.9) |
| Rheumatoid arthritis | 4/46 (8.7) |
| Rheumatic polymyalgia | 5/46 (10.9) |
| Other/nonspecified** | 21/46 (45.6) |
| Neoplasia | 15/145 (10.3) |
| Colon | 4/15 (26.7) |
| Prostate | 3/15 (20.0) |
| Other†† | 7/15 (46.7) |
| Postpartum | 9/145 (6.2) |
| Other | 19/145 (13.1) |
| Received IST | 142/147 (96.6) |
| Achieved CR | 112/133 (84.2) |
| Relapsed after CR | 8/112 (7.1) |
| Time to relapse (d), median (IQR) | 178 (125-248) |
| Variable . | n/N (%)* . |
|---|---|
| Age at DX (y), median (IQR) | 74 (64-83) |
| Male | 85/151 (56.3) |
| Follow-up (mo)†, median (IQR) | |
| Whole cohort (n = 154) | 12 (2-33) |
| Patients alive at study end (n = 36) | 15 (4-36) |
| FVIII:C at Dx, median (IQR) | 1.9 (0.6-4.7) |
| 6-40 IU/dL | 27/135 (20.0) |
| 1-5 IU/dL | 70/135 (51.8) |
| <1 IU/dL | 38/135 (28.1) |
| Inhibitor at Dx, median (IQR) | 16.00 (5.50-42.40) |
| 0-10 BU/mL | 54/145 (37.2) |
| >10-100 BU/mL | 76/145 (52.4) |
| 101-1000 BU/mL | 11/145 (7.6) |
| >1000 BU/mL | 4/145 (2.8) |
| Bleeding at Dx‡ | 135/149 (90.6) |
| Mucocutaneous | 91/132 (68.9) |
| Muscular | 41/132 (31.1) |
| Urinary | 22/132 (16.7) |
| Digestive | 16/132 (12.1) |
| Retroperitoneal | 13/132 (9.8) |
| Thoracic | 13/132 (9.8) |
| Other§ | 16/132 (12.1) |
| Required bypassing treatment | 67/95 (70.5) |
| rFVIIa | 38/63 (60.3) |
| aPCC | 13/63 (20.6) |
| Both | 12/63 (19.0) |
| Underlying disease/condition║ | |
| Idiopathic | 64/145 (44.1) |
| Autoimmune disorder¶ | 46/145 (31.7) |
| Type 1 diabetes | 5/46 (10.9) |
| Rheumatoid arthritis | 4/46 (8.7) |
| Rheumatic polymyalgia | 5/46 (10.9) |
| Other/nonspecified** | 21/46 (45.6) |
| Neoplasia | 15/145 (10.3) |
| Colon | 4/15 (26.7) |
| Prostate | 3/15 (20.0) |
| Other†† | 7/15 (46.7) |
| Postpartum | 9/145 (6.2) |
| Other | 19/145 (13.1) |
| Received IST | 142/147 (96.6) |
| Achieved CR | 112/133 (84.2) |
| Relapsed after CR | 8/112 (7.1) |
| Time to relapse (d), median (IQR) | 178 (125-248) |
One hundred and fifty-four patients were recruited for the AHASR until September 2020. Data corresponding to the moment of the AHA diagnosis are depicted. Missing data were not considered.
Dx, diagnosis; LAC, lupus anticoagulant.
Unless otherwise specified.
For patients who were alive, the time between the date of diagnosis and the date of the last contact (by any means) was considered to calculate the follow-up period. For those who died, the time between diagnosis and exitus was calculated.
Fifty-six patients had bleeding in more than 1 site.
Postpartum bleeding (3), retropharyngeal (2), hemarthros (3), hemopericardium (1), gynecological bleeding not related to pregnancy/postpartum (4), breast bleeding (1), carotid hematoma subsequent to lymph node biopsy (1), postpuncture upper limb hematoma (1).
Seven patients had more than one underlying disease/condition.
Twelve patients had LAC.
Pemphigus (2), bowel inflammatory disease (1), psoriatic arthritis (1), anti-nuclear antibodies (2), nonspecified (15).
Bladder (2), kidney (1), multiple myeloma (1), chronic lymphocytic leukemia (1), lung (1), nonspecified (2).
Bleeding symptoms at admittance and hemostatic treatment
Bleeds at hospital attendance were predominantly of mucocutaneous origin, although muscle bleeding was often reported, and, to a lesser extent, urinary, digestive, retroperitoneal, and thoracic bleeds were documented (Table 1). Bleeds were predominantly spontaneous. There were 3 cases of postpartum bleeding and 2 bleeds subsequent to iatrogenic intervention. The median (IQR) time elapsed between the first bleeding signs and AHA diagnosis was 17.0 (4.2-43.2) days. Hemostatic therapy was initiated in bleeding patients with indication as soon as the diagnosis of AHA was established, according to the guidelines recommended by the Spanish Society of Thrombosis and Haemostasis.13 Bypassing treatments were applied when bleedings were graded ≥2 according to the World Health Organization scale (moderate to severe bleeding).14 This occurred in 70% of cases. The extent of FVIII:C deficiency did not influence the requirement of hemostatic support, whereas patients with inhibitor levels >100 BU/mL seemed to require bypass treatments more frequently than those whose levels were <10 BU/mL (supplemental Table 2). rFVIIa was largely preferred over aPCC, and 12 patients were sequentially treated with both (Table 1). The choice of bypassing agent was irrespective of the level of FVIII:C activity or inhibitor rate. rFVIIa- and aPCC-treated patients had FVIII:C of 1.5 (0.4-3.1) IU/dL vs 1.0 (0.1-2.0) IU/dL (median, IQR), respectively, P = .197, and had inhibitor levels of 15.00 (6.36-44.00) BU/mL vs 21.00 (15.00-44.00) BU/mL respectively, P = .188. Median (IQR) duration of therapies was 7 (3-13) days for rFVIIa and 5.5 days (3-21) for aPCC, with median (IQR) average dose and number of doses per bleed of 90 (90-100) μg/kg and 15 (5-48) for rFVIIa, and 50 (45-100) FU/kg and 15 (3-42) for aPCC. Only 1 patient was reported to fail in controlling hemorrhage after bypass treatment, although her level of circulating inhibitors was 7000 BU/mL. She presented with bleeds in multiple sites, showed refractoriness to first-line IST, and finally died of uncontrolled hemorrhage 2 months after being diagnosed.
One-third of patients were on anticoagulant or antiplatelet treatment at a time coincident with, or close to, the onset of the bleeding episode that led to the diagnosis of AHA (Table 2). Twenty-nine of them (ie, >90%) corresponded to patients older than 65 years, and 81% were men. More than two-thirds of patients of this cohort were using antiplatelet drugs. The most common reason they were following antithrombotic therapy was a personal history of arterial ischemic events, especially coronary heart disease, which was reported in the records of 61.1% of these patients. Atrial fibrillation was documented in one-third of the whole group of patients on antithrombotic therapy. In this subgroup of patients, bleeding location did not differ significantly from that reported for the entire AHASR cohort, with predominance of muscular and, especially, mucocutaneous tissue (31.2% and 65.6%, respectively). Requirement of bypass treatment to control bleeding was similar to that assessed in those patients who were not receiving antithrombotic therapy (77.4% vs 66.7%, respectively), and rFVIIa was also generally preferred (52.4%, 33.3%, and 14.3% were treated with rFVIIa, aPCC, or both, respectively). Once FVIII:C levels recovered >50%, primary or secondary thromboprophylaxis was restarted if criteria for anticoagulant or antiplatelet therapy were still met. Two patients that had achieved CR had thromboembolic complications subsequent to FVIII:C recovery: 1 acute myocardial infarction in a patient with previous history of arterial thrombosis who was on double antiplatelet therapy at the moment of the episode and 1 pulmonary thromboembolic event in a patient who had not resumed anticoagulant therapy after CR achievement. Both of them recovered after appropriate management. In the first case, double anticoagulant and antiplatelet therapy was established; in the second, permanent full-dose anticoagulant therapy was restored.
Features of the AHASR patients who were on anticoagulant/antiplatelet therapy at the time of diagnosis
| Variable . | n/N (%)* . |
|---|---|
| On ACG/APT therapy 1 mo before Dx | |
| Whole cohort | 32/93 (34.4) |
| >65 y | 29/72 (40.3) |
| ≤65 y | 3/21 (14.3) |
| Age at Dx (y), median (IQR) | 79 (73-86) |
| Male | 25/31 (80.7) |
| On ACGs† | 12/32 (37.5) |
| VKAs | 10/11 (90.9) |
| DOACs | 1/11 (9.1) |
| On APTs† | 22/32 (68.7) |
| Rationale for therapy‡ | |
| Previous arterial Ischemic event§ | 19/32 (59.4) |
| Previous venous thromboembolism | 3/32 (9.4) |
| Atrial fibrillation | 9/32 (28.1) |
| Primary prevention for CV risk | 3/32 (9.4) |
| Other║ | 4/32 (12.5) |
| Bleeding location at Dx¶ | |
| Mucocutaneous | 21/32 (65.6) |
| Muscular | 10/32 (31.2) |
| Urinary | 7/32 (21.9) |
| Digestive | 5/32 (15.6) |
| Retroperitoneal | 3/32 (9.4) |
| Thoracic | 3/32 (9.4) |
| Other# | 4/32 (12.5) |
| Required bypassing treatment at Dx | 24/31 (77.4) |
| rFVIIa | 11/21 (52.4) |
| aPCC | 7/21 (33.3) |
| Both | 3/21 (14.3) |
| Thromboembolic events after FVIII recovery | 2/32 (6.2) |
| Survival at the end of follow-up | 17/31 (54.8) |
| Variable . | n/N (%)* . |
|---|---|
| On ACG/APT therapy 1 mo before Dx | |
| Whole cohort | 32/93 (34.4) |
| >65 y | 29/72 (40.3) |
| ≤65 y | 3/21 (14.3) |
| Age at Dx (y), median (IQR) | 79 (73-86) |
| Male | 25/31 (80.7) |
| On ACGs† | 12/32 (37.5) |
| VKAs | 10/11 (90.9) |
| DOACs | 1/11 (9.1) |
| On APTs† | 22/32 (68.7) |
| Rationale for therapy‡ | |
| Previous arterial Ischemic event§ | 19/32 (59.4) |
| Previous venous thromboembolism | 3/32 (9.4) |
| Atrial fibrillation | 9/32 (28.1) |
| Primary prevention for CV risk | 3/32 (9.4) |
| Other║ | 4/32 (12.5) |
| Bleeding location at Dx¶ | |
| Mucocutaneous | 21/32 (65.6) |
| Muscular | 10/32 (31.2) |
| Urinary | 7/32 (21.9) |
| Digestive | 5/32 (15.6) |
| Retroperitoneal | 3/32 (9.4) |
| Thoracic | 3/32 (9.4) |
| Other# | 4/32 (12.5) |
| Required bypassing treatment at Dx | 24/31 (77.4) |
| rFVIIa | 11/21 (52.4) |
| aPCC | 7/21 (33.3) |
| Both | 3/21 (14.3) |
| Thromboembolic events after FVIII recovery | 2/32 (6.2) |
| Survival at the end of follow-up | 17/31 (54.8) |
Missing data were not considered.
ACGs, anticoagulants; APTs, antiplatelets; CV, cardiovascular; DOACs, direct oral anticoagulants; Dx, diagnosis; VKAs, vitamin K antagonists.
Unless otherwise specified.
Two patients were on ACG and APT therapy.
Six patients presented with more than 1 cause to indicate antithrombotic therapy.
Coronary (11), cerebrovascular (4), peripheral (5), mesenteric (1).
Aortic valve replacement (1), dilated cardiomyopathy (2), nonspecified (1).
Sixteen patients had bleedings in more than 1 site.
Retropharyngeal (1), nonspecified (3).
Drug combinations and outcomes of the IST strategies
One hundred and forty-two of 147 patients (96.6%) were administered IST shortly after AHA diagnosis (supplemental Table 3). Steroids were the most used immunosuppressive agents, especially in combination with cyclophosphamide (n = 65). Rituximab, in monotherapy or combined with other immunosuppressive agents, including steroids, was the first choice in 23.2% of IST-treated patients. Twenty-two of the 33 rituximab-treated subjects (66.7%) started therapy in 2017 or later. One of the 5 patients who did not receive inhibitor eradication had FVIII:C levels <1 IU/dL and died of fulminant acute hepatitis 2 days after being diagnosed with AHA before beginning immunosuppression. The remaining 4 patients were younger than those in the IST-treated subjects (44 [36-61] years vs 74 [65-84] years, median [IQR], P = .014), and there were 2 women in the postpartum period among them.
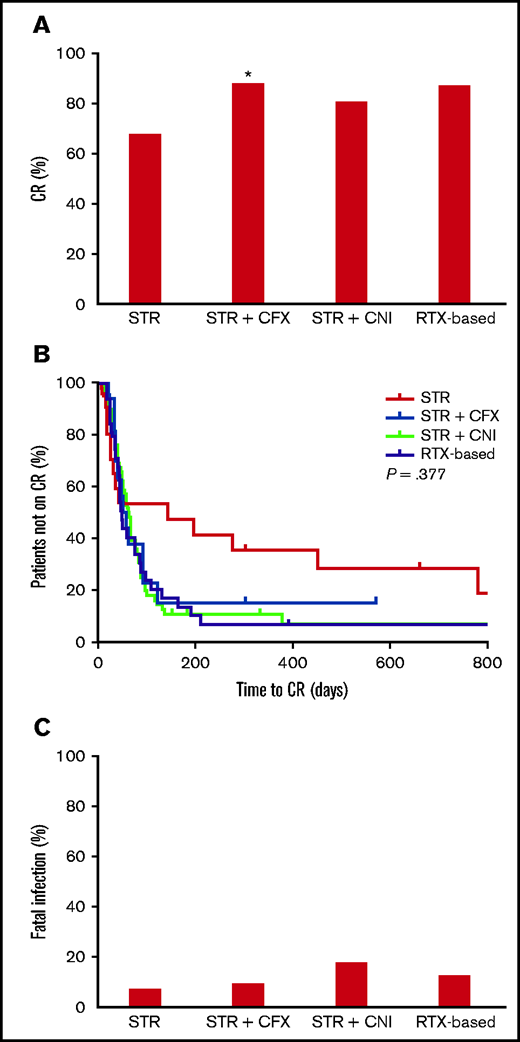
CR was achieved by 112 of 133 (84.2%) patients. Steroids, when given alone, were less effective than the other IST options (68.2% vs 87.2%, P = .049). No differences existed among the latter (steroids/cyclophosphamide, 88.5%, vs steroids/calcineurin inhibitors, 81.2%, vs rituximab-based regimens, 87.5%) (Table 3; Figure 1A). Rituximab-based strategies also achieved CR rates along the lines of those observed with steroids and cyclophosphamide. There were no significant differences among treatments in the time required to achieve CR, which generally oscillated between 40 and 50 days (Table 3; Figure 1B). Relapse rate was 7.1% for a median (IQR) follow-up of 13 (2-34) months since CR.
Efficacy of inhibitor eradication according to selected clinical variables and first-line immunosuppressant treatment
| Variable . | CR n/N (%) . | P . | Days until CR Median (IQR) . | P . |
|---|---|---|---|---|
| Age, y | ||||
| ≤65 | 28/34 (82.3) | .789 | 55 (26-89) | .662 |
| >65 | 82/97 (84.5) | 45 (28-83) | ||
| Sex | ||||
| Female | 44/57 (77.2) | .049 | 41 (29-83) | .798 |
| Male | 67/74 (90.5) | 46 (29-86) | ||
| FVIII:C | ||||
| <1 IU/dL | 26/33 (78.8) | .260 | 47 (31-84) | .420 |
| ≥1 IU/dL | 76/87 (87.4) | 42 (23-86) | ||
| Inhibitor | ||||
| ≤20 BU/mL | 79/83 (95.2) | .005 | 38 (22-67) | .001 |
| >20 BU/mL | 41/52 (78.8) | 62 (41-90) | ||
| Underlying disease | ||||
| Idiopathic | 46/54 (85.2) | 1.000 | 49 (31-74) | .759 |
| Others | 65/77 (84.4) | 45 (28-93) | ||
| Autoimmune | 32/40 (80.0) | .429 | 43 (30-77) | .722 |
| Others | 79/91 (86.8) | 49 (26-87) | ||
| Neoplasia | 15/15 (100) | .125 | 41 (28-89) | .932 |
| Others | 96/116 (82.8) | 45 (29-85) | ||
| Postpartum | 6/6 (100) | .590 | 65 (22-318) | .680 |
| Others | 105/125 (84.0) | 45 (30-83) | ||
| First-line IST choice | ||||
| Steroids | 15/22 (68.2) | .049 | 30 (15-214) | .503 |
| Other IST choices | 95/109 (87.2) | 47 (31-82) | ||
| Steroids + CFX | 54/61 (88.5) | .235 | 53 (31-82) | .491 |
| Other IST choices | 56/70 (80.0) | 41 (25-89) | ||
| Steroids + CNI | 13/16 (81.2) | .721 | 45 (30-73) | .815 |
| Other IST choices | 97/115 (84.3) | 45 (28-84) | ||
| Rituximab-based regimens | 28/32 (87.5) | .782 | 42 (26-84) | .929 |
| Other IST choices | 82/99 (82.8) | 49 (29-85) |
| Variable . | CR n/N (%) . | P . | Days until CR Median (IQR) . | P . |
|---|---|---|---|---|
| Age, y | ||||
| ≤65 | 28/34 (82.3) | .789 | 55 (26-89) | .662 |
| >65 | 82/97 (84.5) | 45 (28-83) | ||
| Sex | ||||
| Female | 44/57 (77.2) | .049 | 41 (29-83) | .798 |
| Male | 67/74 (90.5) | 46 (29-86) | ||
| FVIII:C | ||||
| <1 IU/dL | 26/33 (78.8) | .260 | 47 (31-84) | .420 |
| ≥1 IU/dL | 76/87 (87.4) | 42 (23-86) | ||
| Inhibitor | ||||
| ≤20 BU/mL | 79/83 (95.2) | .005 | 38 (22-67) | .001 |
| >20 BU/mL | 41/52 (78.8) | 62 (41-90) | ||
| Underlying disease | ||||
| Idiopathic | 46/54 (85.2) | 1.000 | 49 (31-74) | .759 |
| Others | 65/77 (84.4) | 45 (28-93) | ||
| Autoimmune | 32/40 (80.0) | .429 | 43 (30-77) | .722 |
| Others | 79/91 (86.8) | 49 (26-87) | ||
| Neoplasia | 15/15 (100) | .125 | 41 (28-89) | .932 |
| Others | 96/116 (82.8) | 45 (29-85) | ||
| Postpartum | 6/6 (100) | .590 | 65 (22-318) | .680 |
| Others | 105/125 (84.0) | 45 (30-83) | ||
| First-line IST choice | ||||
| Steroids | 15/22 (68.2) | .049 | 30 (15-214) | .503 |
| Other IST choices | 95/109 (87.2) | 47 (31-82) | ||
| Steroids + CFX | 54/61 (88.5) | .235 | 53 (31-82) | .491 |
| Other IST choices | 56/70 (80.0) | 41 (25-89) | ||
| Steroids + CNI | 13/16 (81.2) | .721 | 45 (30-73) | .815 |
| Other IST choices | 97/115 (84.3) | 45 (28-84) | ||
| Rituximab-based regimens | 28/32 (87.5) | .782 | 42 (26-84) | .929 |
| Other IST choices | 82/99 (82.8) | 49 (29-85) |
CR was considered when FVIII:C was ≥50%, the inhibitor was undetectable and no clinical manifestations related with AH were observed. Missing data were not considered. Some patients had more than 1 underlying disease/condition. Fisher’s exact or Mann-Whitney tests were used.
CFX, cyclophosphamide; CNI, calcineurin inhibitors.
Outcomes according to immunosuppressive therapy. *<0.05 vs STR (Fisher’s exact test). Missing data were not considered. In the Kaplan-Meier curves of panel B, tick marks indicate patients whose data were censored by the time of last follow-up date. CFX, cyclophosphamide; CNI, calcineurin inhibitors; RTX, rituximab; STR, steroids.
Outcomes according to immunosuppressive therapy. *<0.05 vs STR (Fisher’s exact test). Missing data were not considered. In the Kaplan-Meier curves of panel B, tick marks indicate patients whose data were censored by the time of last follow-up date. CFX, cyclophosphamide; CNI, calcineurin inhibitors; RTX, rituximab; STR, steroids.
Among baseline predictors of the IST success, age was not a determining factor. Patients whose ages were older than age 65 years showed similar CR rates and took a similar number of days to achieve CR to those found with younger patients (Table 3). Similar findings were obtained when using either 75 or 80 years as cutoff age for comparison purposes (not shown). However, IST was more efficient in male than in female patients (90.5% vs 77.2%, P = .049) (Table 3). The association of sex with CR rate was at the limit of statistical significance when applying multivariate regression analysis (supplemental Table 4). All women who developed inhibitor in the postpartum period achieved CR. Thus, the rate of IST efficacy in the rest of them was 75.0% (ie, slightly lower than that of the whole women group). Baseline FVIII:C activity levels did not influence the outcome of IST because patients with pronounced deficiency only showed a slight, nonsignificant decrease in CR rate when compared with those with moderate and mild FVIII:C levels. However, baseline concentrations of inhibitor were associated with IST outcome. Patients whose circulating levels were >20 BU/mL showed a significantly lower rate of CR and required a significantly longer time to achieve it (Table 3). Finally, all 15 neoplastic patients with cancer had CR, whereas this was achieved by 97 of 118 (82.2%) patients without cancer.
Fatal adverse events and influence of clinical variables on survival
Survival information was available from 151 patients. Thirty-six deaths (23.8%) were reported by the participating hospitals during the period between diagnosis and September 2020. The main causes were complications derived from infectious processes, followed by severe hemorrhage (Table 4). Fifteen patients (ie, almost 10% of the AHASR cohort) died of infection-related disorders, with deaths occurring within the first 2 months from the IST start in 7 (Table 4; supplemental Figure 1). Five of the 8 remaining patients died within the first 7 months of AHA diagnosis, and death took place at least 20 months after the end of IST in the other 3 cases. Reported deaths from uncontrolled bleeding always took place within the first 2 months of diagnosis (Table 4; supplemental Figure 1). Only 1 of these patients showed FVIII:C activity levels <1 IU/dL, whereas inhibitor was markedly increased in this and another subject (300 and 7000 BU/mL, respectively), with levels being <10 BU/mL in the 3 remaining cases. Dates of deaths by other causes, which a priori were not directly related to either AHA or IST complications, varied greatly, ranging between a few days and more than 5 years after AHA diagnosis was made (Table 4).
Fatal outcomes of AHASR patients during the study period
| . | Exitus . | Months to death . | |
|---|---|---|---|
| Causes . | Events n/N (%) . | ≤2 mo after Dx§ n/N (%) . | Median (IQR) . |
| All-cause | 36/151 (23.8) | 16/36 (44.4) | 2.7 (1.0-19.0) |
| Infection-related* | 15/36 (41.7) | 7/15 (46.7) | 2.5 (1.5-7.0) |
| Hemorrhage-related† | 5/36 (13.9) | 4/4 (100) | 1.0 (0.4-1.7) |
| Other causes‡ | 16/36 (44.4) | 5/15 (33.3) | 8.0 (1.0-38.0) |
| . | Exitus . | Months to death . | |
|---|---|---|---|
| Causes . | Events n/N (%) . | ≤2 mo after Dx§ n/N (%) . | Median (IQR) . |
| All-cause | 36/151 (23.8) | 16/36 (44.4) | 2.7 (1.0-19.0) |
| Infection-related* | 15/36 (41.7) | 7/15 (46.7) | 2.5 (1.5-7.0) |
| Hemorrhage-related† | 5/36 (13.9) | 4/4 (100) | 1.0 (0.4-1.7) |
| Other causes‡ | 16/36 (44.4) | 5/15 (33.3) | 8.0 (1.0-38.0) |
Missing data were not considered.
Dx, diagnosis.
Sepsis (5, 2 of which caused by pneumonia), pneumonia (4, 1 of which caused by SARS-CoV-2), urinary (1), nonspecified (5).
Pulmonary and cerebral (1), nonspecified (4).
Respiratory failure subsequent to heart failure (3), neoplasia (1), abdominal ischemia (1), fulminant acute hepatitis of unknown origin (1), nonspecified (10).
Calculations were performed considering those patients who died in the first 2 months of follow-up with respect to the whole group of patients who had died by the study end.
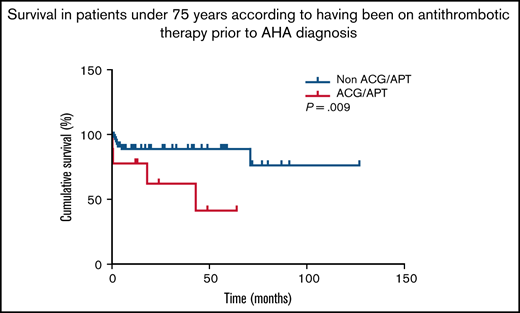
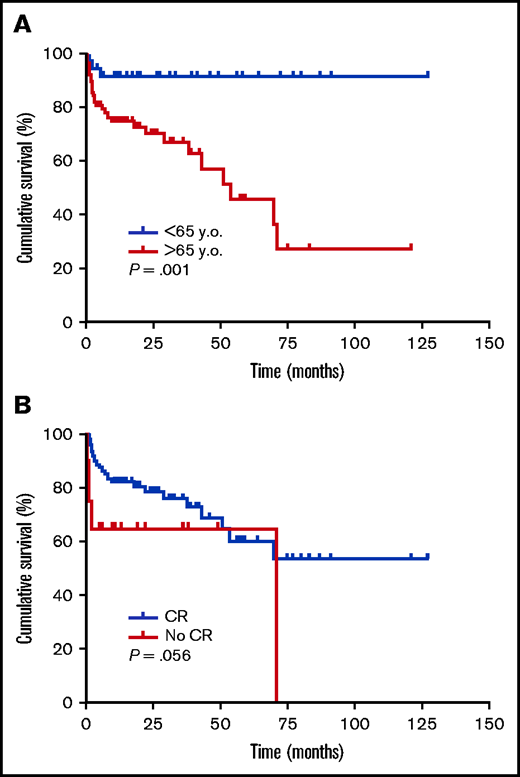
Patients who died were significantly older (79 [73-87] vs 73 [62-80] years, median [IQR], P < .001). Age was independently and inversely associated with survival (Table 5; supplemental Table 5), and was also able to discriminate between survivors and nonsurvivors (Figure 2A). Antithrombotic therapy before AHA diagnosis was inversely associated with survival (Table 5). Interestingly, this association remained significant for the subpopulation of AHA patients <75 years old, according to Fisher’s exact test (4 exitus of 9 patients [44.4%] on antithrombotic therapy vs 7 of 68 [10.3%] not on antithrombotic therapy, P = .020) and log-rank test (supplemental Figure 2). FVIII:C, inhibitors, and choice of IST strategy were not able to predict survival in our series (Table 5; supplemental Table 5). CR was reached by 84.2% of patients in the whole cohort. Rates were 87.5% in survivors vs 73.5% in nonsurvivors. There was an independent direct association between this variable and survival odds (supplemental Table 5; Figure 2B). There were no significant differences in the days required to reach CR between future survivors and nonsurvivors (Table 5).
Baseline features and eradication strategies in the AHASR patients stratified according to the occurrence of all-cause fatalities
| Variable . | All-cause exitus . | P . | |
|---|---|---|---|
| Yes (N = 36) | No (N = 115) | ||
| Age at Dx (y), median (IQR) | 79 (73-87) | 73 (62-80) | <.001 |
| Sex (male), n/N (%) | 22/35 (62.9) | 61/113 (54.0) | .437 |
| Follow-up (months)*, median (IQR) | 2.7 (1.0-19.0) | 15.0 (4.0-36.0) | .001 |
| FVIII:C (%) at Dx, median (IQR) | 2.0 (0.3-5.2) | 1.9 (0.9-4.7) | .899 |
| FVIII:C (%) <1%, n/N (%) | 10/33 (30.3) | 27/99 (27.3) | .449 |
| Inhibitor at Dx, median (IQR) | 16.50 (7.37-49.20) | 16.00 (4.14-42.40) | .607 |
| Antithrombotic therapy†, n/N (%) | 14/36 (38.9) | 17/115 (14.8) | .003 |
| Underlying disease‡ | |||
| Idiopathic, n/N (%) | 11/35 (31.4) | 51/111 (45.9) | .170 |
| Autoimmune, n/N (%) | 12/35 (34.3) | 29/111 (26.1) | .391 |
| Neoplasia, n/N (%) | 5/35 (14.3) | 10/111 (9.0) | .355 |
| Postpartum, n/N (%) | 0/35 (0) | 9/111 (8.1) | .115 |
| Other, n/N (%) | 8/35 (22.9) | 15/111 (13.5) | .192 |
| Immunosuppressive therapy | |||
| Steroids, n/N (%) | 7/36 (19.4) | 20/108 (18.5) | 1.000 |
| Steroids + CFX, n/N (%) | 14/36 (38.9) | 49/108 (45.4) | .563 |
| Steroids + CNI, n/N (%) | 6/36 (16.7) | 11/108 (10.2) | .370 |
| Rituximab-based regimens, n/N (%) | 8/36 (22.2) | 24/108 (22.2) | 1.000 |
| No eradication treatment, n/N (%) | 1/36 (2.8) | 4/108 (3.7) | 1.000 |
| CR, n/N (%) | 25/34 (73.5) | 84/96 (87.5) | .100 |
| Days until CR, median (IQR) | 38 (24-59) | 49 (30-87) | .163 |
| Variable . | All-cause exitus . | P . | |
|---|---|---|---|
| Yes (N = 36) | No (N = 115) | ||
| Age at Dx (y), median (IQR) | 79 (73-87) | 73 (62-80) | <.001 |
| Sex (male), n/N (%) | 22/35 (62.9) | 61/113 (54.0) | .437 |
| Follow-up (months)*, median (IQR) | 2.7 (1.0-19.0) | 15.0 (4.0-36.0) | .001 |
| FVIII:C (%) at Dx, median (IQR) | 2.0 (0.3-5.2) | 1.9 (0.9-4.7) | .899 |
| FVIII:C (%) <1%, n/N (%) | 10/33 (30.3) | 27/99 (27.3) | .449 |
| Inhibitor at Dx, median (IQR) | 16.50 (7.37-49.20) | 16.00 (4.14-42.40) | .607 |
| Antithrombotic therapy†, n/N (%) | 14/36 (38.9) | 17/115 (14.8) | .003 |
| Underlying disease‡ | |||
| Idiopathic, n/N (%) | 11/35 (31.4) | 51/111 (45.9) | .170 |
| Autoimmune, n/N (%) | 12/35 (34.3) | 29/111 (26.1) | .391 |
| Neoplasia, n/N (%) | 5/35 (14.3) | 10/111 (9.0) | .355 |
| Postpartum, n/N (%) | 0/35 (0) | 9/111 (8.1) | .115 |
| Other, n/N (%) | 8/35 (22.9) | 15/111 (13.5) | .192 |
| Immunosuppressive therapy | |||
| Steroids, n/N (%) | 7/36 (19.4) | 20/108 (18.5) | 1.000 |
| Steroids + CFX, n/N (%) | 14/36 (38.9) | 49/108 (45.4) | .563 |
| Steroids + CNI, n/N (%) | 6/36 (16.7) | 11/108 (10.2) | .370 |
| Rituximab-based regimens, n/N (%) | 8/36 (22.2) | 24/108 (22.2) | 1.000 |
| No eradication treatment, n/N (%) | 1/36 (2.8) | 4/108 (3.7) | 1.000 |
| CR, n/N (%) | 25/34 (73.5) | 84/96 (87.5) | .100 |
| Days until CR, median (IQR) | 38 (24-59) | 49 (30-87) | .163 |
Mann-Whitney or Fisher’s exact tests were used. Missing data were not considered.
CFX, cyclophosphamide; CNI, calcineurin inhibitors; Dx, diagnosis.
Follow-up criteria were those explained in Table 1.
≤1 mo before diagnosis.
Four patients had more than 1 underlying disease.
Survival according to selected clinical characteristics. Kaplan-Meier survival curves according to age (≥65 vs < 65 y) (A) and achievement of CR (yes vs no) (B), were generated, and compared by log-rank tests. Tick marks indicate patients whose data were censored by the time of last follow-up date.
Survival according to selected clinical characteristics. Kaplan-Meier survival curves according to age (≥65 vs < 65 y) (A) and achievement of CR (yes vs no) (B), were generated, and compared by log-rank tests. Tick marks indicate patients whose data were censored by the time of last follow-up date.
Patients who died of infection were significantly older than the rest of the cohort (85 [74-88] vs 74 [64-81] years, median [IQR], P = .002) (supplemental Table 6), and older than those who died of other causes, albeit nonsignificantly (85 [74-88] vs 78 [70-86], median [IQR], P = .234). Sex, FVIII:C, inhibitors, underlying disease, and IST choice did not influence the risk of fatal infection (Figure 1C; supplemental Table 6).
Discussion
This study introduces the AHASR and provides information to depict the initial scenario of AHA diagnosis and management in Spain. The retrospective nature of data compilation made it difficult to estimate a nationwide incidence of AHA accurately. Spain population is close to 47 million habitants (https://ine.es/dyngs/INEbase/es/operacion.htm?c=Estadistica_C&cid=1254736176951&menu=ultiDatos&idp=1254735572981), and only 39 newly diagnosed patients were incorporated in the AHASR in 2019, when AHA incidence in Western countries is 1.5 cases per million per year2 led us to suspect that AHA is underdiagnosed in Spain. Although some cases may not have been recruited, misdiagnosis or underdiagnoses may have fatal consequences.15 Educating physicians is an urgent duty.
The median age of the AHASR cohort was similar to that described by others.2–4,7,8 A large number of idiopathic AHA cases was documented, and the main underlying diseases were coincident with those previously reported, most commonly autoimmune disorders and neoplastic processes.16 Postpartum women were at a proportion similar to that observed in other registries.7,17,18 One-half of the patients in the cohort had FVIII:C levels between 1 and 5 IU/dL. Although others have reported higher proportions of subjects with FVIII:C <1 IU/dL,9 our values were not different from those of reference series.3,7,8 Inhibitor levels were along the lines of these and others,3,7,8,16,19 with large oscillations and a few patients with amounts >1000 BU/mL.
The number of patients who required bypass treatment was similar to that of reference studies. FVIII:C levels were not predictive of bleeding risk.9,15,20 The bleeding pattern did not differ from that previously reported, with a large prevalence of bleeding of mucocutaneous origin followed by muscular and, to a lesser extent, urinary, digestive, or retroperitoneal bleeds.2,3,20
One remarkable finding, not described before in AHA, was the high proportion of patients on antithrombotic therapy in the days before AHA diagnosis, namely one-third of the whole series. This proportion is comparable to that of the Spanish population of similar age groups (www.anticoagulados.info). The advanced age of these subjects, as well as their underlying comorbidities, underscore their vulnerability and highlight the importance of a prompt diagnosis. Remarkably, antithrombotic therapy before AHA diagnosis was associated with mortality during follow-up, also among those patients <75 years. Attributing the bleeding episode to a hemostatic imbalance caused by the antithrombotic therapy may entail a risk of misdiagnosis.21 Frail patients could be admitted to hospital shortly after having been administered anticoagulants. In these cases, misdiagnosis would prevent early IST, and patients could be exposed to potentially life-threatening bleedings. Delays in diagnosis have been reported. More than 30% of patients of the European Acquired Haemophilia Registry (EACH2) were not promptly diagnosed,3 and the China Acquired Hemophilia Registry (CARE) detected a delay in almost half of patients. The researchers of CARE also detected that younger patients were more likely to be referred for further consultation than older patients.9 Interestingly, antiplatelet therapy has been described to delay AHA diagnosis or lead to misdiagnosis of AHA patients.22 Furthermore, the underlying diseases that prompted antithrombotic therapy highlight the vulnerability of these patients and could contribute to a negative outcome.
Seventy percent of patients required bypassing treatment, in agreement with literature.15 The use of rFVIIa tripled that of aPCC. Both approaches were safe and successful except in 1 case of rFVIIa failure in a subject with extremely high inhibitor levels, FVIII:C <1 IU/dL, and IS refractoriness. The guides claim that both of them are valid, especially if inhibitor levels are >10 BU/mL and episodes are non-life-threatening/limb-threatening. Otherwise, porcine FVIII would be advisable.11,15 The EACH2 Registry found no differences between rFVIIa and aPCC in efficacy or safety.23 Other series have reported successful and safe use of rFVIIa, with no thromboembolic events directly associated with its use.19,24 The FAIR study found that aPCC was efficient and safe in 56 AHA patients, with no thromboembolic episodes in those treated with the agent in monotherapy or with antifibrinolytics.25 The CARE researchers have documented 84% efficacy in bleeding control with prothrombin complex concentrate.9
In our cohort, the combination of steroids and cyclophosphamide was the most common IST choice, mainly in those subjects whose AHA was idiopathic. Nevertheless, in the past few years, the use of rituximab has become increasingly frequent among our patients, with two-thirds of treatments being given from 2017 onward. Although the cohort size precludes reliable statistical analyses, it can be appreciated that rituximab-based regimens were specially used in patients with idiopathic or autoimmune disease-related AHA. IST achieves remission in 60% to 80% of AHA patients after 5 to 6 weeks.15 We observed that 78% of the AHASR patients achieved CR after generally 6 to 7 weeks. Steroids in monotherapy were less efficient than the other IST choices. The efficacy of steroids with cyclophosphamide or calcineurin, and rituximab-based regimens, was >80%. The time required to achieve CR was no longer in those patients treated with rituximab, in disagreement with previous observations.26 Age or underlying disease did not make relevant differences, although all cancer patients achieved CR. IST was more efficient in male than female patients. An explanation may be found in the fact that the proportion of patients with underlying autoimmune disease was higher in women (33% vs 26%). Inhibitor levels influenced CR. In disagreement with others who found FVIII:C, but not inhibitor levels, to predict remission,8 our patients with levels >20 BU/mL showed lower rates of CR and longer periods to achieve it, whereas FVIII:C levels did not predict failure or success of IST. The main reference studies to provide context to our findings were performed with the United Kingdom Haemophilia Centre Doctors’ Organisation database and the EACH2 Registry, with 172 and 501 patients, respectively. The United Kingdom Haemophilia Centre Doctors’ Organisation researchers did not find differences in the efficacy of steroids when these were given alone or in combination,2 but the studies carried out with the EACH2 patients found that the combination of steroids and cyclophosphamide was more efficient than steroids alone and than rituximab-based therapies.26 Nevertheless, the lack of specification of what drugs were used together with rituximab in both the EACH2 Registry and AHASR precludes further analyses of this controversy. The prospective study by the Group of the German, Austrian and Swiss Thrombosis and Hemostasis Society, which enrolled 102 patients, is also worth mentioning, although its findings are not easily comparable to ours because a predefined protocol was used, which consisted of corticosteroids alone followed by, sequentially, cyclophosphamide, and rituximab only in cases in which remission was not achieved using the previous strategy. Following this strategy, CR and partial remission were attained by 61% and 83% of patients, respectively.8 Finally, the CARE researchers reported CR rates similar to ours and also found that steroids and cyclophosphamide or rituximab-based treatments were better than steroids alone.9
Almost 25% of the AHASR patients had died by the end of the study. Their median age was similar to that of the French AHA patients whose death was documented in a study carried out over 9 years.27 Although deaths in AHA patients have been more frequently reported in those with cancer,28 in our cohort deaths in patients with malignancies were only slightly more common than those observed in patients who presented with autoimmune disorders at diagnosis. Aside from the underlying disease, the main cause of death was far more strongly related to complications of infectious processes, as reported.3,27 Patients who died of hemorrhage did so early after diagnosis, thus underscoring the importance of proper management and close monitoring during the first weeks. Remarkably, death was documented in one-third of AHASR patients older than 75 years, and age was independently associated with death in our study, unlike others that found FVIII:C <1 IU/dL, but not age, to be death predictive.8
Infection risk subsequent to exposure to immunosuppressants is one of the weaknesses of IST. Risk may be unacceptably high in a frail population such as that of AHA patients. Indeed, 15 of our patients died of infection. Of note, their ages were older than those of patients who died of other causes, and one-half of them died in the first 2 months after diagnosis (ie, a few weeks after starting IST). Thus, there seems to be no absolute requirement of long exposure to immunosuppressants for AHA-treated patients, especially the oldest ones, to be at risk of life-threatening infection. This means that, although an aggressive IST may minimize exposure to immunosuppressive drugs, the benefit-to-risk ratio must be carefully balanced to choose the more suitable strategy in each particular situation.29 The recently published guidelines of AHA management suggest the use of steroids alone in those patients with FVIII:C ≥1 IU/dL and inhibitor ≤20 BU/mL.11 Predictors of time to achieve inhibitor eradication would be helpful to guide the intensity of IST, but unfortunately these have not been established so far.8 The use of antifungal, antibiotic, or antiviral drugs during IST treatment has long been discussed but there is so far no definitive recommendation concerning this topic. The independent direct association found between CR and survival may argue in favor of the benefit of shorter periods on inhibitor eradication therapy, since, on average, nonresponders/partial responders may spend more time using immunosuppressive treatments. Indeed, CR also reduces the risk of life-threatening bleeding events. Individually tailored therapies, low-intensity immunosuppressants, or new drugs such as emicizumab to overcome FVIII deficiency may positively influence outcomes in the near future.30
Our work has some limitations. The study is retrospective. This design increases the odds of missing either patients or valuable patient information. The limited sample size precludes fully reliable statistical approaches. Misdiagnosis and underdiagnosis are probably precluding the accurate calculation of the nationwide incidence and prevalence of AHA. The follow-up periods are still too short to reliably study relapses and second-line treatments. Nonfatal adverse events directly related to IST were not documented, which hinders calculations that assess the total number of infections suffered by patients exposed to immunosuppressive agents.
In sum, we have presented the first results obtained with the patients in the new AHASR. The high proportion of patients on anticoagulant/antiplatelet therapy may lead to misdiagnosis or delayed diagnosis. Fatal infection in the first 2 months after starting IST is a common presentation in our cohort.
Authorship
Contribution: M.E.M.-C. and P.M.V. designed and performed research, analyzed data, and wrote paper; and the remaining authors included data in the database and all reviewed the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: María-Eva Mingot-Castellano, Hospital Universitario Virgen del Rocio, Avda. Manuel Siurot, 41013 Sevilla, Spain; e-mail: mariae.mingot.sspa@juntadeandalucia.es; and Pascual Marco, Hospital General Universitario de Alicante, 03010 Alicante, Spain; e-mail: marco_pas@gva.es.
References
Author notes
For data sharing, contact the article’s corresponding authors: mariae.mingot.sspa@juntadeandalucia.es and marco_pas@gva.es.
The full-text version of this article contains a data supplement.