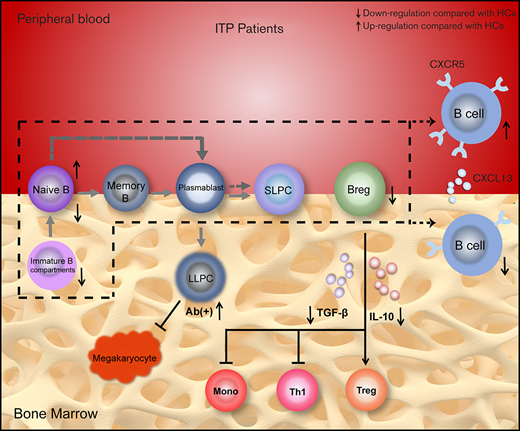
Key Points
The disrupted B-cell subsets and compromised immunosuppressive function of regulatory B cells are found in the BM of patients with ITP.
The abnormally expressed cytokines and their receptors on B cells contribute to the imbalance of BM B-cell subpopulations in ITP.
Abstract
Primary immune thrombocytopenia (ITP) is an autoantibody-mediated hemorrhagic disorder in which B cells play an essential role. Previous studies have focused on peripheral blood (PB), but B cells in bone marrow (BM) have not been well characterized. We aimed to explore the profile of B-cell subsets and their cytokine environments in the BM of patients with ITP to further clarify the pathogenesis of the disease. B-cell subpopulations and their cytokine/chemokine receptors were detected by using flow cytometry. Plasma concentrations of cytokines/chemokines were measured by using enzyme-linked immunosorbent assay. Messenger RNA levels of B cell–related transcription factors were determined by using quantitative polymerase chain reaction. Regulatory B cell (Breg) function was assessed by quantifying their inhibitory effects on monocytes and T cells in vitro. Decreased proportions of total B cells, naive B cells, and defective Bregs were observed in patients with ITP compared with healthy controls (HCs), whereas an elevated frequency of long-lived plasma cells was found in BM of autoantibody-positive patients. No statistical difference was observed in plasmablasts or in short-lived plasma cells between patients with ITP and HCs. The immunosuppressive capacity of BM Bregs from patients with ITP was considerably weaker than HCs. An in vivo study using an active ITP murine model revealed that Breg transfusion could significantly alleviate thrombocytopenia. Moreover, overactivation of CXCL13-CXCR5 and BAFF/APRIL systems were found in ITP patient BM. Taken together, B-cell subsets in BM were skewed toward a proinflammatory profile in patients with ITP, suggesting the involvement of dysregulated BM B cells in the development of the disease.
Introduction
Primary immune thrombocytopenia (ITP) is a common autoimmune hemorrhagic disorder characterized by decreased platelet counts and increased risk of bleeding.1 Abnormalities of humoral and cellular immunity are involved in the pathogenesis of ITP.2,3 Aside from autoantibody-mediated platelet phagocytosis, direct lysis of platelets by cytotoxic T lymphocytes, and impaired thrombopoiesis,4,5 disrupted T-cell subsets such as follicular T helper (Th) cell elevation, enhanced Th1 polarization, and Th17/regulatory T-cell (Treg) dysregulation also hold key positions in the development of ITP.6 Diagnosis of ITP relies on exclusion of other causes of thrombocytopenia,7 and not all patients respond to existing treatments.8 Generally, ITP is a heterogeneous disease, the pathogenesis of which requires further exploration to improve diagnosis and management.
B cells are a subtype of lymphocytes that produce antibodies and generate immunologic memory. Over the last decades, the significance of B cells in autoimmunity has become increasingly recognized. On the one hand, B cells play pathogenic roles by producing autoantibodies and presenting autoantigens; on the other hand, B cells implement anti-inflammatory effects through their immunosuppressive subpopulations.9,10 ITP is a classical model of an organ-specific autoimmune disorder in which autoreactive B cells take center stage in disease development.11 Unbalanced naive and memory B cells, as well as decreased regulatory B cells (Bregs), have been found in the peripheral blood (PB) of patients with ITP.12,13 Bone marrow (BM) is the site for differentiation of various hematopoietic cells, but its immune microenvironment remains unclear. Imbalance of T-cell subsets has been observed in the BM of patients with ITP.14,15 Function of BM mesenchymal stem cells (BM-MSCs) from patients with ITP has also been reported to be defective.16,17 However, few studies have profiled BM B cells in ITP.
In the present study, different B-cell subsets and B cell–related cytokines/chemokines and their receptors were measured in the PB and BM of patients with ITP and healthy controls (HCs). We found that total B cells, naive B cells, and Bregs were significantly decreased in ITP patient BM, whereas an elevated frequency of long-lived plasma cells (LLPCs) was found in autoantibody-positive patients. We also observed that the immunosuppressive capacity of BM Bregs from patients with ITP was compromised, and transfusion of BM-derived Bregs into an active ITP murine model could ameliorate thrombocytopenia. Moreover, enhanced interaction of CXCL13 with CXCR5 and overactivation of the B cell–activating factor/a proliferation-inducing ligand (BAFF/APRIL) system were found in ITP. These abnormalities provide new insights into the immune dysregulation in ITP.
Materials and methods
Patients and HCs
A total of 25 newly diagnosed treatment-naive patients with ITP (9 male subjects and 16 female subjects; 19-71 years of age; median age, 45 years) were enrolled between February 2018 and December 2019 at the Department of Hematology, Qilu Hospital, Shandong University, Jinan, China. All patients met the diagnostic criteria,18 and their BM was routinely examined according to Chinese ITP guidelines.19 Patients’ platelet counts ranged from 1 to 38 × 109/L, with a median count of 15 × 109/L. The main features of the enrolled patients are shown in Table 1. The HC group consisted of 20 healthy volunteers (10 male subjects and 10 female subjects; 18-52 years of age; median age, 40 years) who were related donors for hematopoietic stem cell transplantation. Several examinations, such as BM morphology, complete blood count, and kidney and liver function, were conducted before donation. Platelet counts of HCs ranged from 127 to 294 × 109/L, with a median count of 222 × 109/L.7
Clinical characteristics of enrolled patients with ITP
| Patient no. . | Sex/age (y) . | PLT (×109/L) . | Symptoms . | Anti-GP autoantibody . |
|---|---|---|---|---|
| 1 | Female/21 | 29 | PT + GH | (-) |
| 2 | Female/45 | 38 | GH + ME | GPIIb/IIIa(+) GPIb/IX(+) |
| 3 | Female/27 | 5 | ME | GPIIb/IIIa(+) GPIb/IX(+) |
| 4 | Female/34 | 1 | EC + GH | (-) |
| 5 | Female/60 | 11 | (-) | GPIIb/IIIa(+) GPIb/IX(+) |
| 6 | Female/19 | 32 | EP + GH | (-) |
| 7 | Male/33 | 6 | PT + GIH | (-) |
| 8 | Female/68 | 15 | PT + EC | GPIIb/IIIa(+) GPIb/IX(+) |
| 9 | Male/58 | 8 | EC + GH | (-) |
| 10 | Female/57 | 15 | PT + GH | (-) |
| 11 | Female/42 | 11 | (-) | GPIIb/IIIa(+) |
| 12 | Female/43 | 20 | PT + GH + CH | (-) |
| 13 | Male/55 | 33 | (-) | (-) |
| 14 | Female/57 | 15 | EC | GPIb/IX(+) |
| 15 | Male/23 | 35 | (-) | GPIb/IX(+) |
| 16 | Female/53 | 5 | EC | (-) |
| 17 | Male/52 | 13 | (-) | GPIb/IX(+) |
| 18 | Female/67 | 10 | PT+GH+ME | (-) |
| 19 | Male/64 | 13 | GH | GPIb/IX(+) |
| 20 | Male/58 | 35 | (-) | GPIb/IX(+) |
| 21 | Female/31 | 1 | PT+ME | (-) |
| 22 | Male/32 | 11 | PT | GPIIb/IIIa(+) |
| 23 | Male/40 | 19 | (-) | (-) |
| 24 | Female/31 | 24 | (-) | GPIIb/IIIa(+) |
| 25 | Female/71 | 21 | CH | GPIIb/IIIa(+) |
| Patient no. . | Sex/age (y) . | PLT (×109/L) . | Symptoms . | Anti-GP autoantibody . |
|---|---|---|---|---|
| 1 | Female/21 | 29 | PT + GH | (-) |
| 2 | Female/45 | 38 | GH + ME | GPIIb/IIIa(+) GPIb/IX(+) |
| 3 | Female/27 | 5 | ME | GPIIb/IIIa(+) GPIb/IX(+) |
| 4 | Female/34 | 1 | EC + GH | (-) |
| 5 | Female/60 | 11 | (-) | GPIIb/IIIa(+) GPIb/IX(+) |
| 6 | Female/19 | 32 | EP + GH | (-) |
| 7 | Male/33 | 6 | PT + GIH | (-) |
| 8 | Female/68 | 15 | PT + EC | GPIIb/IIIa(+) GPIb/IX(+) |
| 9 | Male/58 | 8 | EC + GH | (-) |
| 10 | Female/57 | 15 | PT + GH | (-) |
| 11 | Female/42 | 11 | (-) | GPIIb/IIIa(+) |
| 12 | Female/43 | 20 | PT + GH + CH | (-) |
| 13 | Male/55 | 33 | (-) | (-) |
| 14 | Female/57 | 15 | EC | GPIb/IX(+) |
| 15 | Male/23 | 35 | (-) | GPIb/IX(+) |
| 16 | Female/53 | 5 | EC | (-) |
| 17 | Male/52 | 13 | (-) | GPIb/IX(+) |
| 18 | Female/67 | 10 | PT+GH+ME | (-) |
| 19 | Male/64 | 13 | GH | GPIb/IX(+) |
| 20 | Male/58 | 35 | (-) | GPIb/IX(+) |
| 21 | Female/31 | 1 | PT+ME | (-) |
| 22 | Male/32 | 11 | PT | GPIIb/IIIa(+) |
| 23 | Male/40 | 19 | (-) | (-) |
| 24 | Female/31 | 24 | (-) | GPIIb/IIIa(+) |
| 25 | Female/71 | 21 | CH | GPIIb/IIIa(+) |
(-), no bleeding symptom; CH, cerebral hemorrhage; EC, ecchymoses; EP, epistaxis; GH, gingival hemorrhage; GIH, gastrointestinal hemorrhage; GP, glycoprotein; ME, menorrhagia; PT, petechiae.
BM aspirates of the posterior superior iliac spine were obtained under aseptic conditions by experienced physicians. BM aspirate smears were examined simultaneously. To minimize PB dilution, ∼1 mL of BM blood was first aspirated for B-cell subset quantification, and then 4 to 5 mL was further acquired for cell isolation and culture in the functional assays.
This study was approved by the Medical Ethical Committee of Qilu Hospital, Shandong University. Informed consent was obtained from each participant before being included in the study in accordance with the Declaration of Helsinki.
Supplemental methods
The reagents and protocols about flow cytometry, quantitative polymerase chain reaction, enzyme-linked immunosorbent assay, modified monoclonal antibody-specific immobilization of platelet antigen, and in vitro and in vivo functional assays of Bregs are described in detail in the supplemental Methods. The primer sequences are listed in Table 2.
Primers and conditions for quantitative polymerase chain reaction
| Gene . | Sequence (5'-3') . | Annealing T (°C) . | Product (bp) . |
|---|---|---|---|
| GAPDH | F: GCACCGTCAAGGCTGAGAAC | 60 | 138 |
| R: TGGTGAAGACGCCAGTGGA | |||
| CXCR5 | F: ATCGCCGTGGACCGCTACC | 60 | 84 |
| R: ACAGGTGATGTGGATGGAGAGGAG | |||
| BAFF-R | F: CTGTCTCCGGGAATCTCTGATG | 60 | 70 |
| R: GGGTGGTTCCTGGGTCTTCC | |||
| BCMA | F: CATGCTTGCATACCTTGTCAACTTCG | 60 | 120 |
| R: GGTCCAGAGAATCGCATTCGTTCC | |||
| TACI | F: AGCAAGGCAAGTTCTATGACCATCTC | 60 | 157 |
| R: ACTTCTCCACTCCGCTGTCTCC | |||
| XBP1 | F: CCTGGTTGCTGAAGAGGAGG | 60 | 145 |
| R: CCATGGGGAGATGTTCTGGAG | |||
| Pax5 | F: GCCTAGCGTCAGTTCCATCAACAG | 60 | 123 |
| R: ACCGAGGACACCTGCGTCAC | |||
| IRF4 | F: GAGCAATGACTTTGAGGAACTG | 60 | 205 |
| R: CATCATGTAGTTGTGAACCTGC | |||
| PRDM1 | F: CCCTCTGAAGAAACAGAATG | 60 | 241 |
| R: GCTTGTGCTGCTAAATCTCT |
| Gene . | Sequence (5'-3') . | Annealing T (°C) . | Product (bp) . |
|---|---|---|---|
| GAPDH | F: GCACCGTCAAGGCTGAGAAC | 60 | 138 |
| R: TGGTGAAGACGCCAGTGGA | |||
| CXCR5 | F: ATCGCCGTGGACCGCTACC | 60 | 84 |
| R: ACAGGTGATGTGGATGGAGAGGAG | |||
| BAFF-R | F: CTGTCTCCGGGAATCTCTGATG | 60 | 70 |
| R: GGGTGGTTCCTGGGTCTTCC | |||
| BCMA | F: CATGCTTGCATACCTTGTCAACTTCG | 60 | 120 |
| R: GGTCCAGAGAATCGCATTCGTTCC | |||
| TACI | F: AGCAAGGCAAGTTCTATGACCATCTC | 60 | 157 |
| R: ACTTCTCCACTCCGCTGTCTCC | |||
| XBP1 | F: CCTGGTTGCTGAAGAGGAGG | 60 | 145 |
| R: CCATGGGGAGATGTTCTGGAG | |||
| Pax5 | F: GCCTAGCGTCAGTTCCATCAACAG | 60 | 123 |
| R: ACCGAGGACACCTGCGTCAC | |||
| IRF4 | F: GAGCAATGACTTTGAGGAACTG | 60 | 205 |
| R: CATCATGTAGTTGTGAACCTGC | |||
| PRDM1 | F: CCCTCTGAAGAAACAGAATG | 60 | 241 |
| R: GCTTGTGCTGCTAAATCTCT |
Statistical analysis
Results are presented as mean ± standard error of mean, or median and range, depending on the type of data distribution. Normally distributed data are described with mean ± standard error of the mean (line with error bars), and data that were not normally distributed are presented with median (one line) and range. All tests were performed by using SPSS 22.0 (IBM SPSS Statistics, IBM Corporation). Pictures were drawn by using GraphPad Prism 8.3 (GraphPad Software). Statistical significance between patients with ITP and HCs was determined by an independent-sample Student t test or Mann-Whitney U test. Comparisons between PB and BM from the same case were made by using the paired samples Student t test or Wilcoxon matched-pairs signed-rank test. P values <.05 were considered statistically significant.
Results
Decreased percentage of total B cells and disrupted balance of naive/memory B cells in the BM of patients with ITP
After removing adherent cells and fragments, forward scatter and side scatter were used to define the lymphocyte population (Figure 1A). As shown in Figure 1B, the frequency of CD19+ B cells in BM from patients with ITP was significantly lower than that from HCs. By contrast, the number of B cells in PB from patients with ITP was remarkably higher compared with HCs (Figure 1C). Therefore, there might be an abnormal chemotaxis of B cells between BM and PB in patients with ITP. Notably, we found no difference in B cells between BM and PB in the ITP group (Figure 1D), whereas the percentage of B cells in lymphocytes in BM was significantly higher than that in PB from HCs (Figure 1E). The percentage of BM CD19+CD127+ immature B-cell compartments among CD19+ cells was similar between patients with ITP and HCs (Figure 1F), but within total lymphocytes, the percentage of immature B-cell compartments was significantly reduced compared with HCs (Figure 1G).
Total B cells, immature B cells, naive B cells, and memory B cells of patients with ITP and HCs. (A) Elimination of adherent cells and fragments, and gate settings for lymphocytes from BM and PB. (B, C) patients with ITP had fewer CD19+ B cells in BM (7.81 ± 0.84% vs 15.26 ± 1.84%; P < .001) but more in PB (6.94 ± 0.63% vs 4.83 ± 0.41%; P = .013) compared with HCs. (D, E) No statistical difference was found in the proportion of CD19+ B cells between BM and PB in patients with ITP (P = .893), whereas the proportion of BM CD19+ B cells was significantly higher than its PB counterparts in HCs (15.26 ± 1.84% vs 4.83 ± 0.41%; P = .002). (F, G) No difference was found in CD19+CD127+ immature B-cell compartments in BM CD19+ cells between patients with ITP and HCs (4.91 ± 0.94% vs 4.01 ± 0.58%; P = .504), whereas a lower frequency of CD19+CD127+ B cells in lymphocytes was observed in patients with ITP (0.22 ± 0.07% vs 0.53 ± 0.12%; P = .029). (H) Density plots of CD27 and CD38 double staining from CD19+ cells in flow cytometry. CD19+CD27– cells (Q1 + Q3) were naive B cells, and CD19+CD27+CD38–/low cells (Q4) were memory B cells. (I, J) The percentage of naive B cells was significantly lower in BM B cells from patients with ITP compared with HCs (64.04 ± 3.04% vs 77.33 ± 2.33%; P = .011), whereas no statistical difference was found in PB naive B cells between patients with ITP and HCs (median [range], 69.36% [47.66%-93.53%] vs 67.69% [49.45%-85.50%]; P = .817). (K, L) No statistical difference was observed in naive B cells between BM and PB from patients with ITP (median [range], 69.82% [12.78%-89.30%] vs 69.36% [47.66%-93.53%]; P = .731), whereas more naive B cells existed in BM B cells than in PB from HCs (78.10 ± 2.38% vs 64.53 ± 3.24%; P < .001). (M, N) The frequency of memory B cells was higher in BM B cells of patients with ITP than that of HCs (26.23 ± 3.20% vs 12.61 ± 2.57%; P = .002), but no difference was observed in PB memory B cells between patients with ITP and HCs (25.17 ± 2.98% vs 22.84 ± 4.42%; P = .654). (O, P) No statistical difference was found in percentage of memory B cells in CD19+ cells between BM and PB in patients with ITP (26.23 ± 3.20% vs 25.17 ± 2.98%; P = .813). HCs had remarkably decreased frequency of memory B cells in BM compared with PB (12.61 ± 2.57% vs 22.84 ± 4.42%; P = .021). *P < .05; **P < .01; ***P < .001. APC, allophycocyanin; FITC, fluorescein isothiocyanate; FSC-A, forward scatter pulse area; SSC-A, side scatter pulse area.
Total B cells, immature B cells, naive B cells, and memory B cells of patients with ITP and HCs. (A) Elimination of adherent cells and fragments, and gate settings for lymphocytes from BM and PB. (B, C) patients with ITP had fewer CD19+ B cells in BM (7.81 ± 0.84% vs 15.26 ± 1.84%; P < .001) but more in PB (6.94 ± 0.63% vs 4.83 ± 0.41%; P = .013) compared with HCs. (D, E) No statistical difference was found in the proportion of CD19+ B cells between BM and PB in patients with ITP (P = .893), whereas the proportion of BM CD19+ B cells was significantly higher than its PB counterparts in HCs (15.26 ± 1.84% vs 4.83 ± 0.41%; P = .002). (F, G) No difference was found in CD19+CD127+ immature B-cell compartments in BM CD19+ cells between patients with ITP and HCs (4.91 ± 0.94% vs 4.01 ± 0.58%; P = .504), whereas a lower frequency of CD19+CD127+ B cells in lymphocytes was observed in patients with ITP (0.22 ± 0.07% vs 0.53 ± 0.12%; P = .029). (H) Density plots of CD27 and CD38 double staining from CD19+ cells in flow cytometry. CD19+CD27– cells (Q1 + Q3) were naive B cells, and CD19+CD27+CD38–/low cells (Q4) were memory B cells. (I, J) The percentage of naive B cells was significantly lower in BM B cells from patients with ITP compared with HCs (64.04 ± 3.04% vs 77.33 ± 2.33%; P = .011), whereas no statistical difference was found in PB naive B cells between patients with ITP and HCs (median [range], 69.36% [47.66%-93.53%] vs 67.69% [49.45%-85.50%]; P = .817). (K, L) No statistical difference was observed in naive B cells between BM and PB from patients with ITP (median [range], 69.82% [12.78%-89.30%] vs 69.36% [47.66%-93.53%]; P = .731), whereas more naive B cells existed in BM B cells than in PB from HCs (78.10 ± 2.38% vs 64.53 ± 3.24%; P < .001). (M, N) The frequency of memory B cells was higher in BM B cells of patients with ITP than that of HCs (26.23 ± 3.20% vs 12.61 ± 2.57%; P = .002), but no difference was observed in PB memory B cells between patients with ITP and HCs (25.17 ± 2.98% vs 22.84 ± 4.42%; P = .654). (O, P) No statistical difference was found in percentage of memory B cells in CD19+ cells between BM and PB in patients with ITP (26.23 ± 3.20% vs 25.17 ± 2.98%; P = .813). HCs had remarkably decreased frequency of memory B cells in BM compared with PB (12.61 ± 2.57% vs 22.84 ± 4.42%; P = .021). *P < .05; **P < .01; ***P < .001. APC, allophycocyanin; FITC, fluorescein isothiocyanate; FSC-A, forward scatter pulse area; SSC-A, side scatter pulse area.
The percentages of CD19+CD27– naive B cells and CD19+CD27+CD38–/low memory B cells in CD19+ B cells were also analyzed (Figure 1H). Lower BM naive B cells in patients with ITP were found compared with HCs (Figure 1I), but no statistical difference in PB naive B cells was found between patients with ITP and HCs (Figure 1J). In the ITP group, we found no pronounced difference between BM and PB naive B cells (Figure 1K); however, higher naive B cells were observed in the BM than in the PB from HCs (Figure 1L). The frequencies of BM memory B cells in CD19+ cells from patients with ITP were higher compared with HCs (Figure 1M), but no difference was found in PB (Figure 1N). No statistical significance in memory B cells was found between the BM and PB in patients with ITP (Figure 1O), whereas fewer memory B cells were observed in BM B cells than that in PB B cells from HCs (Figure 1P). Percentages of different B-cell subpopulations within lymphocytes were also analyzed, and the trends are shown in Table 3.
Comparison of percentages of B-cell subsets in lymphocytes between patients with ITP and HCs
| B-cell subset . | BM (%) . | PB (%) . | ||||
|---|---|---|---|---|---|---|
| Patients with ITP . | HCs . | P . | Patients with ITP . | HCs . | P . | |
| Immature B | 0.22 ± 0.07 | 0.53 ± 0.12 | .029 | |||
| Naive B | 4.66 ± 0.66 | 12.52 ± 1.75 | <.001 | 4.95 ± 0.61 | 3.16 ± 0.38 | .027 |
| Memory B | 1.52 ± 0.25 | 1.51 ± 0.24 | .992 | 1.28 ± 0.22 | 1.14 ± 0.27 | .693 |
| Plasmablast | 0.06 ± 0.03 | 0.07 ± 0.03 | .782 | 0.06 ± 0.01 | 0.05 ± 0.02 | .617 |
| SLPC | 0.79 ± 0.26 | 1.03 ± 0.26 | .543 | 0.018 ± 0.005 | 0.016 ± 0.008 | .843 |
| Breg | 0.58 ± 0.15 | 2.84 ± 0.34 | <.001 | 0.05 ± 0.01 | 0.18 ± 0.04 | .004 |
| B-cell subset . | BM (%) . | PB (%) . | ||||
|---|---|---|---|---|---|---|
| Patients with ITP . | HCs . | P . | Patients with ITP . | HCs . | P . | |
| Immature B | 0.22 ± 0.07 | 0.53 ± 0.12 | .029 | |||
| Naive B | 4.66 ± 0.66 | 12.52 ± 1.75 | <.001 | 4.95 ± 0.61 | 3.16 ± 0.38 | .027 |
| Memory B | 1.52 ± 0.25 | 1.51 ± 0.24 | .992 | 1.28 ± 0.22 | 1.14 ± 0.27 | .693 |
| Plasmablast | 0.06 ± 0.03 | 0.07 ± 0.03 | .782 | 0.06 ± 0.01 | 0.05 ± 0.02 | .617 |
| SLPC | 0.79 ± 0.26 | 1.03 ± 0.26 | .543 | 0.018 ± 0.005 | 0.016 ± 0.008 | .843 |
| Breg | 0.58 ± 0.15 | 2.84 ± 0.34 | <.001 | 0.05 ± 0.01 | 0.18 ± 0.04 | .004 |
BM, bone marrow; HCs, healthy controls; ITP, immune thrombocytopenia; PB, peripheral blood; SLPC, short-lived plasma cell.
Plasmablasts and plasma cells in BM and PB
We identified CD19+CD27+CD38hiCD138+Ki67+ as plasmablasts and CD19+CD27+CD38hiCD138+Ki67– as short-lived plasma cells (SLPCs) (Figure 2A). No statistical difference was found in the percentage of BM or PB plasmablasts among CD19+ cells between patients with ITP and HCs (Figure 2B-E). Moreover, there was no significant difference in the proportion of BM or PB SLPCs in B cells between patients with ITP and HCs (Figure 2F-G). Much higher frequency of SLPCs existed in BM B cells, from both patients with ITP and HCs, than in PB (Figure 2H-I). Percentages of plasmablasts and SLPCs in lymphocytes were also calculated, and no difference was observed (Table 3). The proportions of plasmablasts and SLPCs were also analyzed between patients with positive antiglycoprotein autoantibodies and HCs, but there was still no statistical difference (data not shown).
Plasmablasts, SLPCs, and LLPCs in patients with ITP and HCs. (A) CD19+CD27+CD38hi cells were gated for analysis of CD19+CD27+CD38hiKi67+CD138+ plasmablasts (Q2) and CD19+CD27+CD38hiKi67–CD138+ SLPCs (Q1). (B-E) No statistical difference in frequency of BM or PB plasmablasts in CD19+ cells was found between patients with ITP and HCs. Moreover, no significant difference in percentage of plasmablasts in CD19+ cells was observed between BM and PB from patients with ITP or HCs. (F, G) There was no remarkable difference in percentage of SLPCs in CD19+ cells between patients with ITP and HCs. (H, I) patients with ITP (median [range], 4.32% [1.32%-34.93%] vs 0.34% [0.06%-1.07%]; P < .001) and HCs (median [range], 10.70% [0.72%-20.39%] vs 0.10% [0.01%-0.93%]; P = .016) have more SLPCs in BM B cells than in PB B cells. (J) CD19–CD38hiCD138+ LLPCs were analyzed from autoantibody-negative or antibody-positive patients and HCs, respectively. (K) No statistical difference was found between all patients with ITP and HCs (median [range], 0.16% [0.01%-1.38%] vs 0.10% [0.02%-0.42%]; P = .273). The proportion of BM LLPCs from autoantibody-positive patients was significantly higher than that from HCs (median [range], 0.27% [0.13%-1.38%] vs 0.10% [0.02%-0.42%]; P = .021) and autoantibody-negative patients (median [range], 0.27% [0.13%-1.38%] vs 0.08% [0.01%-0.15%]; P < .001), whereas no significant difference was found between autoantibody-negative patients and HCs (P = .485). *P < .05; **P < .01; ***P < .001. APC, allophycocyanin; PE, phycoerythrin; SSC, side scatter.
Plasmablasts, SLPCs, and LLPCs in patients with ITP and HCs. (A) CD19+CD27+CD38hi cells were gated for analysis of CD19+CD27+CD38hiKi67+CD138+ plasmablasts (Q2) and CD19+CD27+CD38hiKi67–CD138+ SLPCs (Q1). (B-E) No statistical difference in frequency of BM or PB plasmablasts in CD19+ cells was found between patients with ITP and HCs. Moreover, no significant difference in percentage of plasmablasts in CD19+ cells was observed between BM and PB from patients with ITP or HCs. (F, G) There was no remarkable difference in percentage of SLPCs in CD19+ cells between patients with ITP and HCs. (H, I) patients with ITP (median [range], 4.32% [1.32%-34.93%] vs 0.34% [0.06%-1.07%]; P < .001) and HCs (median [range], 10.70% [0.72%-20.39%] vs 0.10% [0.01%-0.93%]; P = .016) have more SLPCs in BM B cells than in PB B cells. (J) CD19–CD38hiCD138+ LLPCs were analyzed from autoantibody-negative or antibody-positive patients and HCs, respectively. (K) No statistical difference was found between all patients with ITP and HCs (median [range], 0.16% [0.01%-1.38%] vs 0.10% [0.02%-0.42%]; P = .273). The proportion of BM LLPCs from autoantibody-positive patients was significantly higher than that from HCs (median [range], 0.27% [0.13%-1.38%] vs 0.10% [0.02%-0.42%]; P = .021) and autoantibody-negative patients (median [range], 0.27% [0.13%-1.38%] vs 0.08% [0.01%-0.15%]; P < .001), whereas no significant difference was found between autoantibody-negative patients and HCs (P = .485). *P < .05; **P < .01; ***P < .001. APC, allophycocyanin; PE, phycoerythrin; SSC, side scatter.
CD19–CD38hiCD138+ LLPCs have been reported to be involved in the occurrence of several autoimmune diseases.20-22 We analyzed the proportion of LLPCs in BM mononuclear cells and found no statistical difference (Figure 2J-K). Interestingly, the percentage of LLPCs in BM from autoantibody-positive patients was significantly higher than that from HCs and autoantibody-negative patients (Figure 2K). By contrast, we found no difference between autoantibody-negative patients and HCs.
Decreased BM Bregs and their effector cytokines in patients with ITP
Compared with HCs, patients with ITP had a significantly smaller percentage of CD19+CD24hiCD38hi Bregs in B cells both in BM (Figure 3A-B) and PB (Figure 3A, C). The tendency of Breg frequency between BM and PB in patients with ITP and HCs was similar, which showed considerably elevated BM Bregs compared with their PB counterparts (Figure 3D-E). The percentages of BM and PB Bregs within lymphocytes were also decreased remarkably compared with HCs (Table 3). It is still controversial whether Foxp3-expressing B cells exist. Our results showed that CD19+Foxp3+ cells were detectable at a low level in a small proportion of patients and HCs, but they could not be found in most samples (data not shown).
Decreased number and impaired ability of BM Bregs to secrete inhibitory cytokines in patients with ITP. (A) Representative density plots of BM and PB CD19+CD24hiCD38hi Bregs in patients with ITP and HCs. (B, C) The percentages of BM (median [range], 5.50% [0.03%-16.77%] vs 18.67% [4.58%-36.72%]; P < .001) and PB (median [range], 0.31% [0.02%-2.27%] vs 3.05% [0.77%-15.19%]; P < .001) Bregs in CD19+ cells were significantly lower in patients with ITP compared with their respective counterparts in HCs. (D, E) Both patients with ITP and HCs had more Bregs in BM B cells than in PB B cells (patients with ITP: median [range], 5.50% [0.03%-16.77%] vs 0.31% [0.02%-2.27%], P < .001; HCs: median [range], 18.67% [4.58%-36.72%] vs 3.05% [0.77%-15.19%], P = .001). (F, G) Quantification of IL-10+ or TGF-β+ B cells in BM CD19+ cells from patients with ITP and HCs, respectively. (H, I) BM B cells from patients with ITP produced less IL-10 (2.35 ± 0.51% vs 7.15 ± 0.86%; P < .001) and TGF-β (median [range], 1.32% [0.39%-4.11%] vs 4.10% [1.34%-5.91%]; P = .028) compared with HCs after stimulation with CPG-ODN. *P < .05 , **P < .01; ***P < .001. APC, allophycocyanin; CPG-ODN, CPG oligonucleotide; FITC, fluorescein isothiocyanate; PE, phycoerythrin; SSC, side scatter.
Decreased number and impaired ability of BM Bregs to secrete inhibitory cytokines in patients with ITP. (A) Representative density plots of BM and PB CD19+CD24hiCD38hi Bregs in patients with ITP and HCs. (B, C) The percentages of BM (median [range], 5.50% [0.03%-16.77%] vs 18.67% [4.58%-36.72%]; P < .001) and PB (median [range], 0.31% [0.02%-2.27%] vs 3.05% [0.77%-15.19%]; P < .001) Bregs in CD19+ cells were significantly lower in patients with ITP compared with their respective counterparts in HCs. (D, E) Both patients with ITP and HCs had more Bregs in BM B cells than in PB B cells (patients with ITP: median [range], 5.50% [0.03%-16.77%] vs 0.31% [0.02%-2.27%], P < .001; HCs: median [range], 18.67% [4.58%-36.72%] vs 3.05% [0.77%-15.19%], P = .001). (F, G) Quantification of IL-10+ or TGF-β+ B cells in BM CD19+ cells from patients with ITP and HCs, respectively. (H, I) BM B cells from patients with ITP produced less IL-10 (2.35 ± 0.51% vs 7.15 ± 0.86%; P < .001) and TGF-β (median [range], 1.32% [0.39%-4.11%] vs 4.10% [1.34%-5.91%]; P = .028) compared with HCs after stimulation with CPG-ODN. *P < .05 , **P < .01; ***P < .001. APC, allophycocyanin; CPG-ODN, CPG oligonucleotide; FITC, fluorescein isothiocyanate; PE, phycoerythrin; SSC, side scatter.
Interleukin-10 (IL-10) and transforming growth factor β (TGF-β) are important effector cytokines through which Bregs exert immunosuppressive functions. We stimulated the in vitro cultured BM-derived mononuclear cells (BMMNCs) with Toll-like receptor 9 agonists and measured expression of intracellular IL-10 and TGF-β in B cells (Figure 3F-G). The percentage of BM IL-10+ B cells (Figure 3H) and TGF-β+ B cells (Figure 3I) in patients with ITP were both significantly lower than that in HCs.
Functional evaluation of BM Bregs in vitro and in vivo
Because bona fide Bregs were difficult to obtain due to the lack of specific markers, we tested the in vitro suppressive ability of BM B cells activated with CD40 and Toll-like receptor 9 agonists on monocyte’s tumor necrosis factor-α (TNF-α) secretion as a reflection of the function of Bregs. As shown in Figure 4A to C, the frequency of TNF-α+ monocytes cocultured with activated BM B cells was significantly lower than that cultured alone, both in patients with ITP and in HCs. Notably, the inhibition of monocyte’s TNF-α secretion by BM B cells in patients with ITP was greatly reduced compared with that in HCs (Figure 4D).
Compromised immunoregulatory capacity of BM Bregs from patients with ITP. (A) Quantification of CD14+TNF-α+ monocytes cultured alone or with activated BM B cells that contained Bregs from patients with ITP and HCs, respectively. (B, C) Percentage of TNF-α+ monocytes cocultured with BM B cells was significantly lower than that cultured alone, both in patients with ITP (21.24 ± 4.90% vs 36.61 ± 7.82%; P = .001) and in HCs (15.70 ± 5.44% vs 41.52 ± 11.90%; P = .014). (D) BM B cells from patients with ITP exhibited decreased capacity to suppress monocyte TNF-α production compared with HCs (39.95 ± 5.45% vs 61.55 ± 6.89%; P = .029). The percentage of inhibition was calculated as [1 – (percentage of TNF-α+ monocytes cocultured with B cells/percentage of TNF-α+ monocytes cultured alone)] × 100%. (E) IFN-γ secreted by CD4+ T cells cultured alone or with BM B cells from patients with ITP and HCs, respectively. (F, G) BM B cells from the ITP (16.31 ± 4.89% vs 14.46 ± 4.09%; P = .283) and HC (26.22 ± 3.47% vs 15.85 ± 2.31%; P < .001) groups both inhibited IFN-γ secretion by CD4+ T cells, but statistical significance was not reached in the ITP group. (H) Inhibition of CD4+IFN-γ+ T cells by BM B cells from patients with ITP was weaker than that from HCs (median [range], 22.78% [6.84%-35.80%] vs 42.10% [30.16%-48.72%]; P = .009). Percentage of inhibition was calculated as [1 – (percentage of CD3+IFN-γ+ T cells cocultured with B cells/percentage of CD3+IFN-γ+ T cells cultured alone)] × 100%. (I) CD4+ T cells cultured alone or with BM B cells for 72 hours, and frequencies of CD4+CD25+Foxp3+ Tregs were determined by using flow cytometry. (J, K) BM B cells from patients with ITP and HCs both enhanced the percentage of Tregs in CD4+ T cells (patients with ITP, 4.04 ± 1.31% vs 15.09 ± 3.40% [P = .007]; HCs, 1.32 ± 0.38% vs 21.93 ± 1.89% [P < .001]). (L) BM B cells from patients with ITP had weaker ability to promote Treg differentiation compared with HCs (median [range], 3.74 [1.58-14.55] vs 20.70 [8.82-25.82]; P = .009). Promotion of Tregs was calculated as Tregs % cocultured with B cells/Tregs % cultured alone. (M) Platelet counts of active ITP mice transfused with BM Bregs from immunized CD61-KO mice were higher than that of control group mice on days 14, 21, and 28 after splenocyte transfusion. *P < .05; **P < .01; ***P < .001. APC, allophycocyanin; PE, phycoerythrin; SSC, side scatter.
Compromised immunoregulatory capacity of BM Bregs from patients with ITP. (A) Quantification of CD14+TNF-α+ monocytes cultured alone or with activated BM B cells that contained Bregs from patients with ITP and HCs, respectively. (B, C) Percentage of TNF-α+ monocytes cocultured with BM B cells was significantly lower than that cultured alone, both in patients with ITP (21.24 ± 4.90% vs 36.61 ± 7.82%; P = .001) and in HCs (15.70 ± 5.44% vs 41.52 ± 11.90%; P = .014). (D) BM B cells from patients with ITP exhibited decreased capacity to suppress monocyte TNF-α production compared with HCs (39.95 ± 5.45% vs 61.55 ± 6.89%; P = .029). The percentage of inhibition was calculated as [1 – (percentage of TNF-α+ monocytes cocultured with B cells/percentage of TNF-α+ monocytes cultured alone)] × 100%. (E) IFN-γ secreted by CD4+ T cells cultured alone or with BM B cells from patients with ITP and HCs, respectively. (F, G) BM B cells from the ITP (16.31 ± 4.89% vs 14.46 ± 4.09%; P = .283) and HC (26.22 ± 3.47% vs 15.85 ± 2.31%; P < .001) groups both inhibited IFN-γ secretion by CD4+ T cells, but statistical significance was not reached in the ITP group. (H) Inhibition of CD4+IFN-γ+ T cells by BM B cells from patients with ITP was weaker than that from HCs (median [range], 22.78% [6.84%-35.80%] vs 42.10% [30.16%-48.72%]; P = .009). Percentage of inhibition was calculated as [1 – (percentage of CD3+IFN-γ+ T cells cocultured with B cells/percentage of CD3+IFN-γ+ T cells cultured alone)] × 100%. (I) CD4+ T cells cultured alone or with BM B cells for 72 hours, and frequencies of CD4+CD25+Foxp3+ Tregs were determined by using flow cytometry. (J, K) BM B cells from patients with ITP and HCs both enhanced the percentage of Tregs in CD4+ T cells (patients with ITP, 4.04 ± 1.31% vs 15.09 ± 3.40% [P = .007]; HCs, 1.32 ± 0.38% vs 21.93 ± 1.89% [P < .001]). (L) BM B cells from patients with ITP had weaker ability to promote Treg differentiation compared with HCs (median [range], 3.74 [1.58-14.55] vs 20.70 [8.82-25.82]; P = .009). Promotion of Tregs was calculated as Tregs % cocultured with B cells/Tregs % cultured alone. (M) Platelet counts of active ITP mice transfused with BM Bregs from immunized CD61-KO mice were higher than that of control group mice on days 14, 21, and 28 after splenocyte transfusion. *P < .05; **P < .01; ***P < .001. APC, allophycocyanin; PE, phycoerythrin; SSC, side scatter.
We further examined the inhibitory capacity of BM B cells on CD4+ T cells. BM B cells in patients with ITP (Figure 4E-F) and HCs (Figure 4E, G) both inhibited interferon-γ (IFN-γ) secretion by CD4+ T cells, but statistical significance was not reached in the ITP group. BM B cells from patients with ITP had less inhibition compared with those from HCs (Figure 4H). With regard to Treg induction, BM B cells from patients with ITP and HCs both enhanced the percentage of Tregs in CD4+ T cells (Figure 4I-K). Notably, Treg promotion by BM B cells from patients with ITP was significantly lower than those from HCs (Figure 4L).
In addition, the therapeutic effect of BM-derived Bregs was evaluated in a previously described active ITP murine model.23,24 As shown in Figure 4M, ITP mice treated with BM-derived Bregs from immunized CD61-KO mice had significantly higher platelet counts than control ITP mice on days 14, 21, and 28 after splenocyte transfusion.
Abnormal B-lineage transcription factors in patients with ITP
Transcription factors involved in differentiation and development of B cells were determined by using quantitative polymerase chain reaction. As shown in Figure 5A, the level of paired box 5 (Pax5) messenger RNA (mRNA) in BMMNCs from patients with ITP was remarkably lower than that from HCs, whereas no significant difference was observed in X-box–binding protein 1 (XBP-1) or interferon regulatory factor 4 (IRF4). In PBMCs, the Pax5 mRNA level from patients with ITP was more than sixfold lower than that from HCs (Figure 5B). The mRNA levels of XBP1 and IRF4 were also significantly reduced in PBMCs from patients with ITP compared with HCs. No significant difference was observed in positive regulatory domain I–binding factor 1 between patients with ITP and HCs (data not shown).
Aberrant expression of B-lineage transcription factors and abnormal B cell–related cytokines/chemokines in patients with ITP. (A) Patients with ITP had decreased mRNA levels of Pax5 in BMMNCs compared with HCs (0.0049 ± 0.0012 vs 0.0100 ± 0.0017; P = .022). No statistical difference was found in mRNA expression of XBP1 or IRF4 in BMMNCs between patients with ITP and HCs (all P > .05). (B) mRNA levels of Pax5 (0.0017 ± 0.0005 vs 0.0108 ± 0.0016; P < .001), XBP1 (0.0201 ± 0.0033 vs 0.0631 ± 0.0093; P < .001), and IRF4 (0.0037 ± 0.0007 vs 0.0182 ± 0.0037; P < .001) in PBMCs from patients with ITP were much lower compared with HCs. (C, D) Plasma concentrations of BM and PB CXCL13 in patients with ITP were much higher than in HCs (BM: median [range], 94.08 pg/mL [34.46-423.50 pg/mL] vs 55.33 pg/mL [13.31-97.54 pg/mL], P = .003; PB: median [range], 83.21 pg/mL [53.12-291.96 pg/mL] vs 59.27 pg/mL [27.73-116.58 pg/mL], P = .029). (E, F) No difference was found in plasma CXCL13 between BM and PB from the ITP or HC group (all P > .05). (G, H) Plasma levels of BAFF in BM and PB of patients with ITP were higher compared with that in HCs (BM: median [range], 1338.0 pg/mL [989.6-3419.6 pg/mL] vs 1123.0 pg/mL [459.6-1499.6 pg/mL], P = .011; PB: median [range], 1388.0 pg/mL [993.6-3451.6 pg/mL] vs 1022.0 pg/mL [559.6-1255.6 pg/mL], P < .001). (I, J) No divergence was found in BAFF between BM and PB from patients with ITP or HCs (all P > .05). (K, L) Higher plasma concentration of APRIL was observed in BM from patients with ITP than in HCs (median [range], 3.79 ng/mL [1.12-9.34 ng/mL] vs 2.34 ng/mL [1.56-3.79 ng/mL]; P = .005), whereas no difference was found in PB between patients with ITP and HCs (median [range], 1.49 ng/mL [0.47-4.53 ng/mL] vs 1.96 ng/mL [1.42-4.36 ng/mL]; P = .230). (M, N) Plasma APRIL was lower in PB than in BM in patients with ITP (median [range], 3.79 ng/mL [1.12-9.34 ng/mL] vs 1.49 ng/mL [0.47-4.53 ng/mL]; P < .001); no such tendency was found in HCs (median [range], 2.52 ng/mL [1.35-3.18 ng/mL] vs 1.96 ng/mL [1.42-4.53 ng/mL]; P = .770). *P < .05; **P < .01; ***P < .001.
Aberrant expression of B-lineage transcription factors and abnormal B cell–related cytokines/chemokines in patients with ITP. (A) Patients with ITP had decreased mRNA levels of Pax5 in BMMNCs compared with HCs (0.0049 ± 0.0012 vs 0.0100 ± 0.0017; P = .022). No statistical difference was found in mRNA expression of XBP1 or IRF4 in BMMNCs between patients with ITP and HCs (all P > .05). (B) mRNA levels of Pax5 (0.0017 ± 0.0005 vs 0.0108 ± 0.0016; P < .001), XBP1 (0.0201 ± 0.0033 vs 0.0631 ± 0.0093; P < .001), and IRF4 (0.0037 ± 0.0007 vs 0.0182 ± 0.0037; P < .001) in PBMCs from patients with ITP were much lower compared with HCs. (C, D) Plasma concentrations of BM and PB CXCL13 in patients with ITP were much higher than in HCs (BM: median [range], 94.08 pg/mL [34.46-423.50 pg/mL] vs 55.33 pg/mL [13.31-97.54 pg/mL], P = .003; PB: median [range], 83.21 pg/mL [53.12-291.96 pg/mL] vs 59.27 pg/mL [27.73-116.58 pg/mL], P = .029). (E, F) No difference was found in plasma CXCL13 between BM and PB from the ITP or HC group (all P > .05). (G, H) Plasma levels of BAFF in BM and PB of patients with ITP were higher compared with that in HCs (BM: median [range], 1338.0 pg/mL [989.6-3419.6 pg/mL] vs 1123.0 pg/mL [459.6-1499.6 pg/mL], P = .011; PB: median [range], 1388.0 pg/mL [993.6-3451.6 pg/mL] vs 1022.0 pg/mL [559.6-1255.6 pg/mL], P < .001). (I, J) No divergence was found in BAFF between BM and PB from patients with ITP or HCs (all P > .05). (K, L) Higher plasma concentration of APRIL was observed in BM from patients with ITP than in HCs (median [range], 3.79 ng/mL [1.12-9.34 ng/mL] vs 2.34 ng/mL [1.56-3.79 ng/mL]; P = .005), whereas no difference was found in PB between patients with ITP and HCs (median [range], 1.49 ng/mL [0.47-4.53 ng/mL] vs 1.96 ng/mL [1.42-4.36 ng/mL]; P = .230). (M, N) Plasma APRIL was lower in PB than in BM in patients with ITP (median [range], 3.79 ng/mL [1.12-9.34 ng/mL] vs 1.49 ng/mL [0.47-4.53 ng/mL]; P < .001); no such tendency was found in HCs (median [range], 2.52 ng/mL [1.35-3.18 ng/mL] vs 1.96 ng/mL [1.42-4.53 ng/mL]; P = .770). *P < .05; **P < .01; ***P < .001.
Aberrant B cell–related cytokines/chemokines in patients with ITP
CXCL13, BAFF, and APRIL are the 3 cytokines/chemokines closely related to B-cell maturation and survival. The levels of BM and PB CXCL13 from patients with ITP were significantly higher than their counterparts from HCs (Figure 5C-D). In addition, BAFF concentrations in BM and PB from patients with ITP were also significantly higher than those from HCs (Figure 5G-H). However, increased APRIL in patients with ITP was only found in the BM but not the PB compared with HCs (Figure 5K-L).
Levels of these 3 cytokines were also compared between BM and PB. In the ITP group, we found that the level of APRIL in BM was significantly higher than in PB (Figure 5M), whereas no statistical difference in APRIL level between BM and PB in HCs was observed (Figure 5N). We found no statistical difference in CXCL13 or BAFF levels between BM and PB in patients with ITP or HCs (5E, F, I, and J). Those results indicate that B cells in patients with ITP have abnormal developmental and chemotactic environments, which might contribute to their dysregulation.
Abnormal expression of chemokine/cytokine receptors on B cells in patients with ITP
CXCL13 and CXCR5 are a chemokine and receptor pair that is crucial for B-cell trafficking, activation, and germinal center formation.25 BAFF/APRIL and their receptors compose an important system indispensable for B-cell differentiation, maturation, immunoglobulin class switching, and antibody production.26 BAFF binds to 3 different receptors: BAFF receptor (BAFF-R), B-cell maturation antigen (BCMA), and transmembrane activator and CAML interactor (TACI), while APRIL only binds to BCMA and TACI. Surface levels of CXCR5, BAFFR, BCMA, and TACI on B cells were determined by using flow cytometry. CXCR5 on BM B cells from patients with ITP was significantly higher than that from HCs (Figure 6A-B). A significant increase in B-cell CXCR5 was also observed in PB from patients with ITP compared with HCs (Figure 6C). Moreover, surface levels of CXCR5 on B cells in BM were much lower than their matched counterparts in PB from both patients with ITP (Figure 6D) and HCs (Figure 6E). Similarly, BAFF-R levels were also elevated in BM from patients with ITP compared with HCs (Figure 6F-H). In addition, BM BAFF-R levels from both patients with ITP and HCs were considerably lower compared with their PB counterparts (Figure 6I-J). The levels of BCMA on B cells were generally low; we only found a statistical increase in BM BCMA level from patients with ITP compared with HCs (Figure 6K-N). As for the difference between BM and PB, a significantly decreased level of BCMA on B cells was detected in BM compared with in PB from HCs (Figure 6O). No statistical difference was found in B-cell TACI in BM or PB between patients with ITP and HCs (Figure 6P-S).
CXCR5, BAFF-R, BCMA, and TACI expression in BM and PB B cells of patients with ITP and HCs. (A) Representative density plots of CXCR5 on CD19+ cells in BM and PB of patients with ITP and HCs. (B, C) BM (69.25 ± 4.27% vs 26.28 ± 2.64%; P < .001) and PB (median [range], 90.78% [80.86%-96.06%] vs 85.13% [56.76%-91.61%]; P = .046) B cells from patients with ITP had elevated levels of CXCR5 compared with those from HCs. (D, E) Surface CXCR5 level on B cells in PB was significantly higher than in BM, both from patients with ITP (median [range], 72.36% [57.54%-92.65%] vs 90.37% [59.93%-96.06%]; P = .006) and HCs (30.12 ± 3.82% vs 76.86 ± 4.86%; P = .003). (F) Representative density plots of BAFF-R on CD19+ cells in the BM and PB of patients with ITP and HCs. (G, H) BM and PB B cells from patients with ITP had elevated levels of BAFF-R compared with HCs (BM: median [range], 84.10% [60.30%-97.54%] vs 37.08% [16.56%-52.58%], P < .001; PB: median [range], 93.97% [87.20%-97.76%] vs 90.97% [76.97%-95.09%], P = .016). (I, J) BAFF-R expression on B cells was remarkably higher in PB than in BM, both from patients with ITP and from HCs (patients with ITP: median [range], 85.75% [60.30%-97.54%] vs 93.83% [87.20%-97.38%], P = .002; HCs: median [range], 37.76% [16.56%-58.27%] vs 89.00% [76.97%-95.09%], P = .031). (K) Representative density plots of BCMA on CD19+ cells in BM and PB of patients with ITP and HCs. (L, M) BM B cells from patients with ITP exhibited elevated BCMA levels compared with HCs (median [range], 6.04% [0.77%-22.76%] vs 3.77% [1.24%-6.55%]; P = .018), whereas there was no statistical difference in PB B-cell BCMA levels between patients with ITP and HCs. (N, O) No statistical difference was found in B-cell BCMA levels between BM and PB in patients with ITP (P = 0.454), whereas the level of B-cell BCMA in PB was considerably higher than in BM in HCs (2.91 ± 0.52% vs 4.47 ± 0.67%; P = .009). (P-S) No statistical difference was found in BM or PB B-cell TACI levels between patients with ITP and HCs. Furthermore, no statistical difference in B-cell TACI level between BM and PB was observed in patients with ITP or in HCs (all P > .05). *P < .05; **P < .01; ***P < .001. PE, phycoerythrin; SSC, side scatter.
CXCR5, BAFF-R, BCMA, and TACI expression in BM and PB B cells of patients with ITP and HCs. (A) Representative density plots of CXCR5 on CD19+ cells in BM and PB of patients with ITP and HCs. (B, C) BM (69.25 ± 4.27% vs 26.28 ± 2.64%; P < .001) and PB (median [range], 90.78% [80.86%-96.06%] vs 85.13% [56.76%-91.61%]; P = .046) B cells from patients with ITP had elevated levels of CXCR5 compared with those from HCs. (D, E) Surface CXCR5 level on B cells in PB was significantly higher than in BM, both from patients with ITP (median [range], 72.36% [57.54%-92.65%] vs 90.37% [59.93%-96.06%]; P = .006) and HCs (30.12 ± 3.82% vs 76.86 ± 4.86%; P = .003). (F) Representative density plots of BAFF-R on CD19+ cells in the BM and PB of patients with ITP and HCs. (G, H) BM and PB B cells from patients with ITP had elevated levels of BAFF-R compared with HCs (BM: median [range], 84.10% [60.30%-97.54%] vs 37.08% [16.56%-52.58%], P < .001; PB: median [range], 93.97% [87.20%-97.76%] vs 90.97% [76.97%-95.09%], P = .016). (I, J) BAFF-R expression on B cells was remarkably higher in PB than in BM, both from patients with ITP and from HCs (patients with ITP: median [range], 85.75% [60.30%-97.54%] vs 93.83% [87.20%-97.38%], P = .002; HCs: median [range], 37.76% [16.56%-58.27%] vs 89.00% [76.97%-95.09%], P = .031). (K) Representative density plots of BCMA on CD19+ cells in BM and PB of patients with ITP and HCs. (L, M) BM B cells from patients with ITP exhibited elevated BCMA levels compared with HCs (median [range], 6.04% [0.77%-22.76%] vs 3.77% [1.24%-6.55%]; P = .018), whereas there was no statistical difference in PB B-cell BCMA levels between patients with ITP and HCs. (N, O) No statistical difference was found in B-cell BCMA levels between BM and PB in patients with ITP (P = 0.454), whereas the level of B-cell BCMA in PB was considerably higher than in BM in HCs (2.91 ± 0.52% vs 4.47 ± 0.67%; P = .009). (P-S) No statistical difference was found in BM or PB B-cell TACI levels between patients with ITP and HCs. Furthermore, no statistical difference in B-cell TACI level between BM and PB was observed in patients with ITP or in HCs (all P > .05). *P < .05; **P < .01; ***P < .001. PE, phycoerythrin; SSC, side scatter.
In line with the changing trends in surface receptors at the protein level, mRNA levels of B-cell CXCR5 and BAFF-R from patients with ITP were significantly higher than those in HCs, both in BM and PB (Figure 7A-D). Moreover, BCMA and TACI in BM B cells from patients with ITP were also remarkably elevated compared with HCs (Figure 7E, G). We found no statistical difference in BCMA or TACI in PB B cells between patients with ITP and HCs (Figure 7F, H).
mRNA levels of CXCR5, BAFF-R, BCMA, and TACI of B cells. (A, B) The mRNA level of B-cell CXCR5 from patients with ITP was also higher than that from HCs, both in BM (0.070 ± 0.021 vs 0.016 ± 0.007; P = .034) and PB (median [range], 0.071 [0.015 to 0.338] vs 0.035 [0.017-0.089]; P = .017). (C, D) Increased mRNA levels of BAFF-R in BM (0.066 ± 0.020 vs 0.011 ± 0.003; P = .024) and PB (median [range], 0.090 [0.010-0.443] vs 0.030 [0.012-0.115]; P = .023) B cells from patients with ITP was found compared with HCs. (E, F) Elevated mRNA levels of BCMA were observed in BM B cells of patients with ITP compared with HCs (0.049 ± 0.011 vs 0.012 ± 0.002; P = .011), whereas no statistical difference was found in PB B cells between patients with ITP and HCs. (G, H) TACI mRNA levels of BM B cells in patients with ITP were also higher than those in HCs (0.064 ± 0.018 vs 0.016 ± 0.004; P = .034); no statistical difference was reached in PB B cells between patients with ITP and HCs. *P < .05; **P < .01; ***P < .001.
mRNA levels of CXCR5, BAFF-R, BCMA, and TACI of B cells. (A, B) The mRNA level of B-cell CXCR5 from patients with ITP was also higher than that from HCs, both in BM (0.070 ± 0.021 vs 0.016 ± 0.007; P = .034) and PB (median [range], 0.071 [0.015 to 0.338] vs 0.035 [0.017-0.089]; P = .017). (C, D) Increased mRNA levels of BAFF-R in BM (0.066 ± 0.020 vs 0.011 ± 0.003; P = .024) and PB (median [range], 0.090 [0.010-0.443] vs 0.030 [0.012-0.115]; P = .023) B cells from patients with ITP was found compared with HCs. (E, F) Elevated mRNA levels of BCMA were observed in BM B cells of patients with ITP compared with HCs (0.049 ± 0.011 vs 0.012 ± 0.002; P = .011), whereas no statistical difference was found in PB B cells between patients with ITP and HCs. (G, H) TACI mRNA levels of BM B cells in patients with ITP were also higher than those in HCs (0.064 ± 0.018 vs 0.016 ± 0.004; P = .034); no statistical difference was reached in PB B cells between patients with ITP and HCs. *P < .05; **P < .01; ***P < .001.
Discussion
Abnormalities of peripheral B cells in ITP have been well characterized, but little is known about the profile of BM B cells of the disease. In the present study, we showed for the first time that levels of total B cells, naive B cells, and Bregs were significantly lower in the BM of patients with ITP, and the immunosuppressive function of BM B cells in patients with ITP was greatly reduced. Moreover, the CXCL13-CXCR5 axis and BAFF/APRIL system were also found to be abnormally expressed and distributed in patients with ITP. Those results suggest disrupted immune tolerance of B cells and aberrant BM chemotactic microenvironments in patients with ITP.
Although there is undoubtedly an imbalance in B-cell subsets in patients with ITP, the specific changes of each subpopulation remain unclarified. Fang et al27 and Giordano et al28 reported some controversies about B-cell subsets in pediatric patients with ITP. With regard to adult patients, Lyu et al13 reported increased total B cells and memory B cells, with reduced naive B cells in the PB of active patients with ITP. In the present study, significantly reduced total B cells, immature B-cell compartments, and naive B cells were discovered in the BM of patients with ITP, whereas the proportions of memory B cells, plasmablasts, and SLPCs showed no statistical difference between patients with ITP and HCs. B cells are derived from pluripotent hematopoietic stem cells through a series of developmental stages and selection steps in the BM. After successful generation of the functional B-cell receptor, immature B cells develop into naive B cells and are released into PB. Receptor editing, clonal deletion, and clonal anergy are the main mechanisms for the maintenance of early B-cell tolerance, and defects in receptor editing would lead to increased clonal deletion.29 Our unpublished data have shown insufficient receptor editing in naive B cells of patients with ITP, which might elevate the elimination of immature B-cell compartments by clonal deletion. Therefore, the reduction in BM immature B-cell compartments and naive B cells could be largely attributed to the decreased production of these early B-cell subsets.
Li et al30 showed that BM CD38hiCD138+ cells from patients with chronic ITP were higher than those from HCs. Here, we found that frequency of LLPCs from autoantibody-positive patients was considerably higher than that from HCs and autoantibody-negative patients. Recently, Shrestha et al31 showed that the detection rate of autoantibodies in BM was higher than in PB. Therefore, autoantibodies might be at higher titers in BM than in PB, and BM could be a site where autoantibodies were produced. Because LLPCs predominantly reside in BM, the higher detection rate of autoantibodies in BM might be due to the in situ production of autoantibodies by LLPCs. LLPCs could be produced not only by migration of plasmablasts to BM but originating from reactivation of memory B cells in situ,32 which occurs both in spleen and BM.33 Consequently, BM memory B cells and plasmablasts could be a potential source of autoreactive B cells. Platelets are mainly produced by megakaryocytes in the BM, and autoantibodies could bind newly released platelets or megakaryocytes, leading to increased platelet destruction and decreased platelet production. Furthermore, it has been reported that excessive LLPCs could inhibit the production of pro– and pre–B cells,34 which might account for the decrease in total B cells in the BM of autoantibody-positive patients.
Pax5 is expressed throughout the whole life of B cells and controls the commitment of B-lineage cells.35 Downregulation of Pax5 and upregulation of XBP-1 and IRF4 are involved in the process of antibody-secreting cell differentiation.36 The mRNA levels of these 3 transcription factors were remarkably reduced in PBMCs of patients with ITP; among them, Pax5 decreased the most, suggesting that defects in B-cell generation and a tendency toward differentiation into antibody-secreting cells might be involved in the pathophysiology of ITP. As for the discrepancy of those transcription factors between BM and PB, the varied expression of those transcription factors in different B-cell developmental stages could be the main reason.
In addition to antibody secretion and antigen presentation, B cells contain a regulatory subset, known as Bregs, which are capable of suppressing immune responses.37 In ITP, a reduced number of Bregs in PB have been reported, and the abnormalities could be corrected by dexamethasone or thrombopoietic agents.12 In our study, we show for the first time that the number of BM CD19+CD24hiCD38hi Bregs was also decreased. Abnormalities of lymphocyte trafficking play important roles in the immune tolerance disruption in ITP. Aslam et al38 found that Bregs were increased in the spleen of patients with ITP, which might be due to the increased splenic sequestration. Similarly, enhanced retention of Tregs in thymus contributed to the Treg reduction in PB in an active murine model of ITP.39 In addition, chemotaxis of CD3+ T cells toward BM was enhanced, as evidenced by elevated expression of T cell–homing receptors.40 Therefore, Breg reduction in BM might be also related with elevated splenic sequestration. Insufficient production might also contribute to the Breg reduction in the BM of patients with ITP. It has been reported that disease-specific environmental stimuli could lead to the aberrant in situ induction of Bregs.41 In addition to the insufficient quantity, BM Bregs from patients with ITP exhibited functional defects. Decreased IL-10 and TGF-β production, reduced inhibitory capacity on monocyte TNF-α expression and Th1 differentiation, and defective Treg induction were found in the present study. TGF-β, a large proportion of which is derived from platelets, is reportedly significantly decreased in ITP.42 It has been confirmed that TGF-β is essential for Treg induction43 ; it may also affect the differentiation or function of Bregs in ITP, a topic that warrants additional exploration. Furthermore, functionally defective BM-MSCs have been verified in ITP for their compromised ability to induce CD8+ Tregs and regulatory DCs.44,45 Taking into account the capability of BM-MSCs to promote Breg differentiation in experimental autoimmune encephalomyelitis,46 defects could also contribute to Breg impairent in ITP.
The CXCL13-CXCR5 axis is crucial in driving B cells into secondary lymphoid tissues.47 We found a higher concentration of plasma CXCL13 and an elevated level of B-cell CXCR5 in both BM and PB from patients with ITP. Although no statistical difference in CXCL13 was observed between BM and PB in patients with ITP, the level of B-cell CXCR5 in PB was increased compared with that in BM, which might explain the reduction in total BM B cells but an increase in PB B cells of patients with ITP. BAFF/APRIL and their receptors compose an important system indispensable for the differentiation and maturation of B cells.48 Our results showed that patients with ITP had elevated levels of BAFF in BM and PB and an increased concentration of APRIL in BM. In contrast to previous reports,49,50 increased BAFF-R on B cells in both BM and PB, and elevated BCMA on B cells in BM, from patients with ITP was found in our study. In the last decade, the efficacy and safety of the BAFF/APRIL antagonists belimumab and atacicept in the treatment of autoimmune diseases have been gradually verified.51 In view of the hyperfunction of the BAFF/APRIL system in patients with ITP, these monoclonal antibodies might be effective in refractory cases.
In conclusion, BM B cells in patients with ITP were in a state of immune deviation characterized by decreased total B cells, naive B cells, and defective Bregs. The aberrant profile of BM B cells coincided with elevated levels of B cell–related cytokines/chemokines and abnormal distribution of their receptors. Therefore, the abnormalities of BM B cells and their chemotactic environments might provide new therapeutic targets for the management of ITP.
Acknowledgments
The authors thank Alexandra H. Marshall (Marshall Medical Communications) for providing editorial assistance during preparation of the manuscript.
This work was supported by the Cultivation Project of Qilu Sanitation and Health Leading Talents (2019), the National Natural Science Foundation of China (No. 81570103), and the Clinical Science and Technology Innovation Program of Jinan Science and Technology Project (No. 201704085).
Authorship
Contribution: Material preparation, data collection, and analysis were performed by T.Y., H.W., Y.Z. P.H., Y.Y., Y.H., S.L., X.N., X.J., J.P., and M.H.; the first draft of the manuscript was written by T.Y. and X.L.; and all authors commented on previous versions of the manuscript; All authors contributed to the study conception and design, and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xin-guang Liu, Department of Hematology, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China; e-mail: Liuxingrant@163.com; and Yu Hou, Shandong Provincial Key Laboratory of Immunohematology, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China; e-mail: houyu2009@sina.com.
References
Author notes
Requests for original data may be submitted to Xin-guang Liu (e-mail: Liuxingrant@163.com).
The full-text version of this article contains a data supplement.

![Total B cells, immature B cells, naive B cells, and memory B cells of patients with ITP and HCs. (A) Elimination of adherent cells and fragments, and gate settings for lymphocytes from BM and PB. (B, C) patients with ITP had fewer CD19+ B cells in BM (7.81 ± 0.84% vs 15.26 ± 1.84%; P < .001) but more in PB (6.94 ± 0.63% vs 4.83 ± 0.41%; P = .013) compared with HCs. (D, E) No statistical difference was found in the proportion of CD19+ B cells between BM and PB in patients with ITP (P = .893), whereas the proportion of BM CD19+ B cells was significantly higher than its PB counterparts in HCs (15.26 ± 1.84% vs 4.83 ± 0.41%; P = .002). (F, G) No difference was found in CD19+CD127+ immature B-cell compartments in BM CD19+ cells between patients with ITP and HCs (4.91 ± 0.94% vs 4.01 ± 0.58%; P = .504), whereas a lower frequency of CD19+CD127+ B cells in lymphocytes was observed in patients with ITP (0.22 ± 0.07% vs 0.53 ± 0.12%; P = .029). (H) Density plots of CD27 and CD38 double staining from CD19+ cells in flow cytometry. CD19+CD27– cells (Q1 + Q3) were naive B cells, and CD19+CD27+CD38–/low cells (Q4) were memory B cells. (I, J) The percentage of naive B cells was significantly lower in BM B cells from patients with ITP compared with HCs (64.04 ± 3.04% vs 77.33 ± 2.33%; P = .011), whereas no statistical difference was found in PB naive B cells between patients with ITP and HCs (median [range], 69.36% [47.66%-93.53%] vs 67.69% [49.45%-85.50%]; P = .817). (K, L) No statistical difference was observed in naive B cells between BM and PB from patients with ITP (median [range], 69.82% [12.78%-89.30%] vs 69.36% [47.66%-93.53%]; P = .731), whereas more naive B cells existed in BM B cells than in PB from HCs (78.10 ± 2.38% vs 64.53 ± 3.24%; P < .001). (M, N) The frequency of memory B cells was higher in BM B cells of patients with ITP than that of HCs (26.23 ± 3.20% vs 12.61 ± 2.57%; P = .002), but no difference was observed in PB memory B cells between patients with ITP and HCs (25.17 ± 2.98% vs 22.84 ± 4.42%; P = .654). (O, P) No statistical difference was found in percentage of memory B cells in CD19+ cells between BM and PB in patients with ITP (26.23 ± 3.20% vs 25.17 ± 2.98%; P = .813). HCs had remarkably decreased frequency of memory B cells in BM compared with PB (12.61 ± 2.57% vs 22.84 ± 4.42%; P = .021). *P < .05; **P < .01; ***P < .001. APC, allophycocyanin; FITC, fluorescein isothiocyanate; FSC-A, forward scatter pulse area; SSC-A, side scatter pulse area.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/5/20/10.1182_bloodadvances.2020003860/2/m_advancesadv2020003860f1.png?Expires=1769119535&Signature=AXEgbiDd4A-6fi4m78HP4QbMLtYwNOoTklEP~1MpprMDUgQpBvaLmGg2yJiVSC6B1eCD94tNjFz-tG28Hxj8IabwXwLGnyfyRoD1ZQPwsFntAQtX0SP0l63~xUiwtT2mc3QXw8X~4MVQ2Y8Ws5IssCdVzF-GjLBY~DSJuwI1lFhHzUgxIvDuE3Za4u2Pt0-UquP0C-4Oi13BPgiVrHUW5trkrc~cy7GivBBV5ngK0Yr6TORCerHDosRlMUX0RxOAMW~fA-yeZUgA2e13uLIiPZQfxsgwB6edTUUBcRO1hJf8wB643TLjGkwqo9pDGYK-V9koxFLo2XzStv4NKx0CCA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Plasmablasts, SLPCs, and LLPCs in patients with ITP and HCs. (A) CD19+CD27+CD38hi cells were gated for analysis of CD19+CD27+CD38hiKi67+CD138+ plasmablasts (Q2) and CD19+CD27+CD38hiKi67–CD138+ SLPCs (Q1). (B-E) No statistical difference in frequency of BM or PB plasmablasts in CD19+ cells was found between patients with ITP and HCs. Moreover, no significant difference in percentage of plasmablasts in CD19+ cells was observed between BM and PB from patients with ITP or HCs. (F, G) There was no remarkable difference in percentage of SLPCs in CD19+ cells between patients with ITP and HCs. (H, I) patients with ITP (median [range], 4.32% [1.32%-34.93%] vs 0.34% [0.06%-1.07%]; P < .001) and HCs (median [range], 10.70% [0.72%-20.39%] vs 0.10% [0.01%-0.93%]; P = .016) have more SLPCs in BM B cells than in PB B cells. (J) CD19–CD38hiCD138+ LLPCs were analyzed from autoantibody-negative or antibody-positive patients and HCs, respectively. (K) No statistical difference was found between all patients with ITP and HCs (median [range], 0.16% [0.01%-1.38%] vs 0.10% [0.02%-0.42%]; P = .273). The proportion of BM LLPCs from autoantibody-positive patients was significantly higher than that from HCs (median [range], 0.27% [0.13%-1.38%] vs 0.10% [0.02%-0.42%]; P = .021) and autoantibody-negative patients (median [range], 0.27% [0.13%-1.38%] vs 0.08% [0.01%-0.15%]; P < .001), whereas no significant difference was found between autoantibody-negative patients and HCs (P = .485). *P < .05; **P < .01; ***P < .001. APC, allophycocyanin; PE, phycoerythrin; SSC, side scatter.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/5/20/10.1182_bloodadvances.2020003860/2/m_advancesadv2020003860f2.png?Expires=1769119535&Signature=5BC3nPIYzHuCeqYjzJJqSn3ZVK3bWqrtPsWm4KotmXsXaszcvPvWI9VyIWC74X6mAjYuYrNOk3CqaMY8wHYNjnCFflwSxZrjFuzDNgP6yux3oJCHTUmV9Gl7EDDcsWno6IGDMWmTkjy--hn0iwYoJtmBS8pfUPACbF-LXcz1dRkw47QQbYFakoLJ9PTPuupISPB-g1ce31BpIWhEoMjIzbeAr3rf7uENlSRTvduMB0FbCN6plavn9ofAeke6wYreaQwqnksbY~ZkHD~XmKlOfhBkaiGyhijsM20B0P8wiwHaOmmDUlYJXAJu-q7ng-vfi9-~EA9EWspEZZbYCAMdtg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Decreased number and impaired ability of BM Bregs to secrete inhibitory cytokines in patients with ITP. (A) Representative density plots of BM and PB CD19+CD24hiCD38hi Bregs in patients with ITP and HCs. (B, C) The percentages of BM (median [range], 5.50% [0.03%-16.77%] vs 18.67% [4.58%-36.72%]; P < .001) and PB (median [range], 0.31% [0.02%-2.27%] vs 3.05% [0.77%-15.19%]; P < .001) Bregs in CD19+ cells were significantly lower in patients with ITP compared with their respective counterparts in HCs. (D, E) Both patients with ITP and HCs had more Bregs in BM B cells than in PB B cells (patients with ITP: median [range], 5.50% [0.03%-16.77%] vs 0.31% [0.02%-2.27%], P < .001; HCs: median [range], 18.67% [4.58%-36.72%] vs 3.05% [0.77%-15.19%], P = .001). (F, G) Quantification of IL-10+ or TGF-β+ B cells in BM CD19+ cells from patients with ITP and HCs, respectively. (H, I) BM B cells from patients with ITP produced less IL-10 (2.35 ± 0.51% vs 7.15 ± 0.86%; P < .001) and TGF-β (median [range], 1.32% [0.39%-4.11%] vs 4.10% [1.34%-5.91%]; P = .028) compared with HCs after stimulation with CPG-ODN. *P < .05 , **P < .01; ***P < .001. APC, allophycocyanin; CPG-ODN, CPG oligonucleotide; FITC, fluorescein isothiocyanate; PE, phycoerythrin; SSC, side scatter.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/5/20/10.1182_bloodadvances.2020003860/2/m_advancesadv2020003860f3.png?Expires=1769119535&Signature=ugU8ep2lb7~cGNUbGa1E9XmX-5Kg4RKfuFofVCiqxs2s3jigF9C9-1QQmqIx7Ww31omsuC3dieEtgEZB8INKDNTzJp82YvFNTilsFrnPtt8T2xbq-tsLz9zd5OZk-X0qHXUHN393aUTK048st4RAAJcxgV19UY8vkPTpOj2RDPaNOVDMuGrIlMEoqwqj6k1f3y9QthPCb9xsY8wbuBz0XC58cKlVd~1zcH1y2gTJSq~XexfVtQMxe3g6~eleiE~E70vgdXXFNG395jYiIs~nQaWbKG7aEzNIW4ESp~VB2MoAiILiF3RFQiADu5tRnN0jcJvgCgnQZ4dwLeVLu~a8jA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Compromised immunoregulatory capacity of BM Bregs from patients with ITP. (A) Quantification of CD14+TNF-α+ monocytes cultured alone or with activated BM B cells that contained Bregs from patients with ITP and HCs, respectively. (B, C) Percentage of TNF-α+ monocytes cocultured with BM B cells was significantly lower than that cultured alone, both in patients with ITP (21.24 ± 4.90% vs 36.61 ± 7.82%; P = .001) and in HCs (15.70 ± 5.44% vs 41.52 ± 11.90%; P = .014). (D) BM B cells from patients with ITP exhibited decreased capacity to suppress monocyte TNF-α production compared with HCs (39.95 ± 5.45% vs 61.55 ± 6.89%; P = .029). The percentage of inhibition was calculated as [1 – (percentage of TNF-α+ monocytes cocultured with B cells/percentage of TNF-α+ monocytes cultured alone)] × 100%. (E) IFN-γ secreted by CD4+ T cells cultured alone or with BM B cells from patients with ITP and HCs, respectively. (F, G) BM B cells from the ITP (16.31 ± 4.89% vs 14.46 ± 4.09%; P = .283) and HC (26.22 ± 3.47% vs 15.85 ± 2.31%; P < .001) groups both inhibited IFN-γ secretion by CD4+ T cells, but statistical significance was not reached in the ITP group. (H) Inhibition of CD4+IFN-γ+ T cells by BM B cells from patients with ITP was weaker than that from HCs (median [range], 22.78% [6.84%-35.80%] vs 42.10% [30.16%-48.72%]; P = .009). Percentage of inhibition was calculated as [1 – (percentage of CD3+IFN-γ+ T cells cocultured with B cells/percentage of CD3+IFN-γ+ T cells cultured alone)] × 100%. (I) CD4+ T cells cultured alone or with BM B cells for 72 hours, and frequencies of CD4+CD25+Foxp3+ Tregs were determined by using flow cytometry. (J, K) BM B cells from patients with ITP and HCs both enhanced the percentage of Tregs in CD4+ T cells (patients with ITP, 4.04 ± 1.31% vs 15.09 ± 3.40% [P = .007]; HCs, 1.32 ± 0.38% vs 21.93 ± 1.89% [P < .001]). (L) BM B cells from patients with ITP had weaker ability to promote Treg differentiation compared with HCs (median [range], 3.74 [1.58-14.55] vs 20.70 [8.82-25.82]; P = .009). Promotion of Tregs was calculated as Tregs % cocultured with B cells/Tregs % cultured alone. (M) Platelet counts of active ITP mice transfused with BM Bregs from immunized CD61-KO mice were higher than that of control group mice on days 14, 21, and 28 after splenocyte transfusion. *P < .05; **P < .01; ***P < .001. APC, allophycocyanin; PE, phycoerythrin; SSC, side scatter.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/5/20/10.1182_bloodadvances.2020003860/2/m_advancesadv2020003860f4.png?Expires=1769119535&Signature=BH7DtLz285GuZOUhQee59vE4qvX2skKyb~bv6r7098G4dBnTMdpdSOARvQ1mRZcMbBZZZPah-e7j93tuDBwtdIFJClQKFOYoP64ahdwzEjwh-LWjHNeONDlaZdModIrDsLzmYPDv0~r1F7dVX5qqyMQDWBM8La8izwuFKo5q86JdV~v5tth~uQi9ECej32iGDtS-x~~D2tXRX4664-ev8G6sjLkcXCo0HEXrPLrrSWbo~~iUPrPoTP-qMqjIcLcQ~D8bflJA4bKjX8tinpDtQmiSGy8~BivURAZkZESboko7dCJ4Z~eieyg~jAzag2TuqweKuG7Kpsf9hq8etzUggQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Aberrant expression of B-lineage transcription factors and abnormal B cell–related cytokines/chemokines in patients with ITP. (A) Patients with ITP had decreased mRNA levels of Pax5 in BMMNCs compared with HCs (0.0049 ± 0.0012 vs 0.0100 ± 0.0017; P = .022). No statistical difference was found in mRNA expression of XBP1 or IRF4 in BMMNCs between patients with ITP and HCs (all P > .05). (B) mRNA levels of Pax5 (0.0017 ± 0.0005 vs 0.0108 ± 0.0016; P < .001), XBP1 (0.0201 ± 0.0033 vs 0.0631 ± 0.0093; P < .001), and IRF4 (0.0037 ± 0.0007 vs 0.0182 ± 0.0037; P < .001) in PBMCs from patients with ITP were much lower compared with HCs. (C, D) Plasma concentrations of BM and PB CXCL13 in patients with ITP were much higher than in HCs (BM: median [range], 94.08 pg/mL [34.46-423.50 pg/mL] vs 55.33 pg/mL [13.31-97.54 pg/mL], P = .003; PB: median [range], 83.21 pg/mL [53.12-291.96 pg/mL] vs 59.27 pg/mL [27.73-116.58 pg/mL], P = .029). (E, F) No difference was found in plasma CXCL13 between BM and PB from the ITP or HC group (all P > .05). (G, H) Plasma levels of BAFF in BM and PB of patients with ITP were higher compared with that in HCs (BM: median [range], 1338.0 pg/mL [989.6-3419.6 pg/mL] vs 1123.0 pg/mL [459.6-1499.6 pg/mL], P = .011; PB: median [range], 1388.0 pg/mL [993.6-3451.6 pg/mL] vs 1022.0 pg/mL [559.6-1255.6 pg/mL], P < .001). (I, J) No divergence was found in BAFF between BM and PB from patients with ITP or HCs (all P > .05). (K, L) Higher plasma concentration of APRIL was observed in BM from patients with ITP than in HCs (median [range], 3.79 ng/mL [1.12-9.34 ng/mL] vs 2.34 ng/mL [1.56-3.79 ng/mL]; P = .005), whereas no difference was found in PB between patients with ITP and HCs (median [range], 1.49 ng/mL [0.47-4.53 ng/mL] vs 1.96 ng/mL [1.42-4.36 ng/mL]; P = .230). (M, N) Plasma APRIL was lower in PB than in BM in patients with ITP (median [range], 3.79 ng/mL [1.12-9.34 ng/mL] vs 1.49 ng/mL [0.47-4.53 ng/mL]; P < .001); no such tendency was found in HCs (median [range], 2.52 ng/mL [1.35-3.18 ng/mL] vs 1.96 ng/mL [1.42-4.53 ng/mL]; P = .770). *P < .05; **P < .01; ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/5/20/10.1182_bloodadvances.2020003860/2/m_advancesadv2020003860f5.png?Expires=1769119535&Signature=kfx7mtUPIC~yMAFv3mUUtm7xVVvh1nD-5MK9Oi11d-gAIMUMn5t54d8pNVzf3jrj2H-SdkikmMSyUzKMNRaxfe13hy4Ckw7kK6eHg10gijsjr3QEIydosnuLMnxN6m8z1p-QSo-1vGqksDaMnU5JdySHQ-hheqWwwVjSsmcJTzZWB5uuS25JEY3mglOOtfMQFKzGKx0VOl9IZcmBu5O-EQ6PWAk7cDA-SvdxZ51uTPsSD8xQIK5-V0Dv-TZ8BkgKRcLC2bzMJT0acqI5wd3aLnsMBos32VpxUBiO8uMHSAtMGre9GvrNM2XLH6WvqDf90J9vkuGb2oJRXzvevV-f2Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![CXCR5, BAFF-R, BCMA, and TACI expression in BM and PB B cells of patients with ITP and HCs. (A) Representative density plots of CXCR5 on CD19+ cells in BM and PB of patients with ITP and HCs. (B, C) BM (69.25 ± 4.27% vs 26.28 ± 2.64%; P < .001) and PB (median [range], 90.78% [80.86%-96.06%] vs 85.13% [56.76%-91.61%]; P = .046) B cells from patients with ITP had elevated levels of CXCR5 compared with those from HCs. (D, E) Surface CXCR5 level on B cells in PB was significantly higher than in BM, both from patients with ITP (median [range], 72.36% [57.54%-92.65%] vs 90.37% [59.93%-96.06%]; P = .006) and HCs (30.12 ± 3.82% vs 76.86 ± 4.86%; P = .003). (F) Representative density plots of BAFF-R on CD19+ cells in the BM and PB of patients with ITP and HCs. (G, H) BM and PB B cells from patients with ITP had elevated levels of BAFF-R compared with HCs (BM: median [range], 84.10% [60.30%-97.54%] vs 37.08% [16.56%-52.58%], P < .001; PB: median [range], 93.97% [87.20%-97.76%] vs 90.97% [76.97%-95.09%], P = .016). (I, J) BAFF-R expression on B cells was remarkably higher in PB than in BM, both from patients with ITP and from HCs (patients with ITP: median [range], 85.75% [60.30%-97.54%] vs 93.83% [87.20%-97.38%], P = .002; HCs: median [range], 37.76% [16.56%-58.27%] vs 89.00% [76.97%-95.09%], P = .031). (K) Representative density plots of BCMA on CD19+ cells in BM and PB of patients with ITP and HCs. (L, M) BM B cells from patients with ITP exhibited elevated BCMA levels compared with HCs (median [range], 6.04% [0.77%-22.76%] vs 3.77% [1.24%-6.55%]; P = .018), whereas there was no statistical difference in PB B-cell BCMA levels between patients with ITP and HCs. (N, O) No statistical difference was found in B-cell BCMA levels between BM and PB in patients with ITP (P = 0.454), whereas the level of B-cell BCMA in PB was considerably higher than in BM in HCs (2.91 ± 0.52% vs 4.47 ± 0.67%; P = .009). (P-S) No statistical difference was found in BM or PB B-cell TACI levels between patients with ITP and HCs. Furthermore, no statistical difference in B-cell TACI level between BM and PB was observed in patients with ITP or in HCs (all P > .05). *P < .05; **P < .01; ***P < .001. PE, phycoerythrin; SSC, side scatter.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/5/20/10.1182_bloodadvances.2020003860/2/m_advancesadv2020003860f6.png?Expires=1769119535&Signature=tmKMGw3KPppRPOjDiwy45iNjFIc-wIGXdRY~mjPOS4zNRh7-C0Ui4iE7fJ9q593CWhdY8rnyOAmWC4b8LGFxGzUz3PxIzbk4AQ1-g6A2ooaEhHPkZcnLWvCGiSjjSuXoS~6s9QzoKqNnUTOJD8EcPhLvNQW0ugtQsbkqaPe-hAmAQsRTu3lmSn~BP9-ZirvSMeRLLLH327zS0nzVOZViv9LLkgtCdsglrZbyUeaX1~SxWqKV83yRBI~tMl2NNcL69mZH~btjC0XCPVB6TYLGkSG5LM6sx5YcELzGHnIdmx2CAaOTSwZdVrBZXE1AkIQK9thrd4Myt-OYKW-V4ZowYQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![mRNA levels of CXCR5, BAFF-R, BCMA, and TACI of B cells. (A, B) The mRNA level of B-cell CXCR5 from patients with ITP was also higher than that from HCs, both in BM (0.070 ± 0.021 vs 0.016 ± 0.007; P = .034) and PB (median [range], 0.071 [0.015 to 0.338] vs 0.035 [0.017-0.089]; P = .017). (C, D) Increased mRNA levels of BAFF-R in BM (0.066 ± 0.020 vs 0.011 ± 0.003; P = .024) and PB (median [range], 0.090 [0.010-0.443] vs 0.030 [0.012-0.115]; P = .023) B cells from patients with ITP was found compared with HCs. (E, F) Elevated mRNA levels of BCMA were observed in BM B cells of patients with ITP compared with HCs (0.049 ± 0.011 vs 0.012 ± 0.002; P = .011), whereas no statistical difference was found in PB B cells between patients with ITP and HCs. (G, H) TACI mRNA levels of BM B cells in patients with ITP were also higher than those in HCs (0.064 ± 0.018 vs 0.016 ± 0.004; P = .034); no statistical difference was reached in PB B cells between patients with ITP and HCs. *P < .05; **P < .01; ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/5/20/10.1182_bloodadvances.2020003860/2/m_advancesadv2020003860f7.png?Expires=1769119535&Signature=ZzlF8xyC7L3JJ5pEjGrWGVB0iJvptvajsUdI8~gtQe6OKqaOWvqJRUbzCF4-7QH0WMxDBuTL5aqMZxzhB3hNcuomjx1hHOKrSLEkmxHuJoxCc8pMpO7eDg-brp34~Yb~szE-sTpPvQuQQqqHDy-WYgN9zudXzyFc4UaUw3PG7zHMNFFbnVIlQ3pobhZKbmBC9omQ1xr9hTRJuNx1IHxGgPrudypzMzTSA-9x7Z-tdsZH9DlqwraIyBZ3geRQmF8uarN-Cs-9CVySSxx2I47vLJWS9m7Klut~9EtYiscSLdn2dyEtFOoFUsrDJ9ndddpnan~EKc1eN5DLCIf6ih-E9w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)