Key Points
An open ADAMTS13 conformation is a novel biomarker for iTTP and is induced by anti-ADAMTS13 autoantibodies.
The autoantibodies against the CS region play an important role in the appearance of an open ADAMTS13 conformation.
Abstract
Immune-mediated thrombotic thrombocytopenic purpura (iTTP) is caused by an autoantibody-mediated deficiency in ADAMTS13. In healthy individuals, ADAMTS13 has a folded conformation in which the central spacer (S) domain interacts with the C-terminal CUB domains. We recently showed that ADAMTS13 adopts an open conformation in iTTP and that patient immunoglobulin G antibodies (IgGs) can open ADAMTS13. Anti-ADAMTS13 autoantibodies in patients with iTTP are directed against the different ADAMTS13 domains, but almost all patients have autoantibodies binding to the cysteine/spacer (CS) domains. In this study, we investigated whether the autoantibodies against the CS and CUB domains can disrupt the S-CUB interaction of folded ADAMTS13, thereby opening ADAMTS13. To this end, we purified anti-CS and anti-CUB autoantibodies from 13 patients with acute iTTP by affinity chromatography. The successfully purified anti-CS (10/13 patients) and anti-CUB (4/13 patients) autoantibody fractions were tested further in our ADAMTS13 conformation enzyme-linked immunosorbent assay to study whether they could open ADAMTS13. Interestingly, all purified anti-CS fractions (10/10 patients) were able to open ADAMTS13. On the other hand, only half of the purified anti-CUB fractions (2/4 patients) opened ADAMTS13. Our finding highlights that anti-CS autoantibodies that open ADAMTS13 are a common feature of the autoimmune response in iTTP.
Introduction
Immune-mediated thrombotic thrombocytopenic purpura (iTTP) is a rare, but life-threatening, thrombotic microangiopathy caused by an autoantibody-induced deficiency of the enzyme ADAMTS13.1,2 Because ADAMTS13 is required to cleave its substrate von Willebrand factor (VWF) into smaller multimers, inhibition and/or clearance3,4 of ADAMTS13 by autoantibodies leads to accumulation of ultra-large VWF in the circulation. These ultra-large VWF multimers can spontaneously bind platelets, causing the formation of microthrombi.2,5
ADAMTS13 is composed of multiple domains: a metalloprotease domain (M), a disintegrin-like domain (D), a first thrombospondin type-1 repeat (T1), a cysteine-rich domain, a spacer (S) domain, 7 additional thrombospondin type-1 (T2-T8) repeats, and 2 CUB (CUB1-2) domains.6 Epitope mapping of anti-ADAMTS13 autoantibodies in patients with iTTP revealed that the immune response is polyclonal and that almost all patients have autoantibodies against the cysteine/spacer (CS) domains.3,7-11 In healthy individuals, ADAMTS13 circulates in a folded conformation in which the central S domain interacts with the C-terminal CUB domains.12,13 We recently showed that ADAMTS13 adopts an open conformation in patients with iTTP and identified open ADAMTS13 as a novel biomarker for iTTP.14-16 This open conformation is characterized by a disrupted S-CUB interaction and an exposed cryptic epitope in the S domain.15,16 An ADAMTS13 conformation enzyme-linked immunosorbent assay (ELISA), based on our murine monoclonal antibody 1C4 that specifically recognizes the cryptic epitope in the S domain, allows detection of open ADAMTS13 in iTTP plasma but not folded ADAMTS13 in healthy donor plasma.15 Addition of purified immunoglobulin G antibodies (IgGs) from patients with iTTP to healthy donor plasma changed the conformation of folded ADAMTS13 to an open conformation.16
In this study, we investigated whether the anti-ADAMTS13 autoantibodies against the CS or CUB domains can disrupt the S-CUB interaction of folded ADAMTS13, thereby inducing an open ADAMTS13 conformation. Therefore, anti-CS and anti-CUB autoantibodies were purified from 13 patients with iTTP and tested using our ADAMTS13 conformation ELISA.
Methods
Purification of IgGs from plasma
We selected 13 iTTP plasma samples from our previous patient cohort10,15,16 in which we showed that their plasma contained open ADAMTS1315 and that their purified IgGs could open ADAMTS13.16 For this study, IgGs were again purified from 2 mL of citrated plasma from these 13 patients with acute iTTP using Protein G Sepharose 4 Fast Flow (GE Healthcare, Waukesha, WI) column chromatography, according to the manufacturer’s instructions. IgGs from 9 healthy donors16 were also purified. The study protocol was approved by the Ethics Committees of Marseille, France (Projet National de Recherche Clinique 2007, no. 2007/23) and KU Leuven, Belgium (no. S260220). Written informed consent was obtained from each plasma donor in accordance with the Declaration of Helsinki.
Purification of specific anti-CS and anti-CUB autoantibodies
Specific anti-CS and anti-CUB autoantibodies were purified from the total IgGs of the 13 patients with acute iTTP by affinity chromatography. Therefore, the recombinant CS and CUB domains, both fusion proteins with albumin domain 1 (AD1; AD1-CS10 and AD1–CUB1-210 ), were coupled to cyanogen bromide–activated Sepharose 4B beads (GE Healthcare, Chicago, IL), according to the manufacturer’s instructions. Approximately 10 mg of the individually purified IgGs was loaded onto the CS- or CUB-affinity columns. After washing with 15 column volumes of phosphate-buffered saline, bound antibodies were eluted with 100 mM glycine (pH 2.6) and collected in 1 M Tris-HCl (pH 10). A microtiter plate coated with CS or CUB (2.5 µg/mL) was incubated with the respective elution fractions (1:5 diluted). Bound antibodies were detected with horseradish peroxidase (HRP)-labeled polyclonal goat anti-human (Fc-specific) IgGs (1/10 000; Sigma-Aldrich, St. Louis, MO). The colorimetric reaction was initiated by addition of O-phenylenediamine and H2O2 and stopped with 4 M sulfuric acid. The absorbance was measured at 492 nm (optical density at 492 nm [OD492 nm]). Peak fractions were pooled and dialyzed against phosphate-buffered saline, and the concentration was measured via spectrophotometry. The specificity of the purified anti-CS or anti-CUB autoantibodies was evaluated further using ELISA. Autoantibodies purified against CS were diluted to 25 µg/mL and tested on coated CS and MDT (AD1-MDT10 ) (2.5 µg/mL). Autoantibodies purified against CUB were tested for binding to coated CUB and T2-T8 (AD1–T2-T810 ) (2.5 µg/mL). IgGs purified from 9 healthy donors were used to determine the background (mean OD + 3 standard deviations). Detection of bound antibodies and colorimetric development were performed as described above.
Identification of opening anti-ADAMTS13 autoantibodies
The purified anti-CS and anti-CUB autoantibodies were tested in the ADAMTS13 conformation ELISA that was based on our murine monoclonal antibody 1C4 that specifically recognizes the cryptic epitope in the S domain (1C4-ELISA), as described previously.15,16 Briefly, a 96-well microtiter plate was coated (5 µg/mL) with 1C4. The purified anti-CS or anti-CUB autoantibody fractions (30 µL) were individually preincubated with normal human plasma (NHP; final dilution 1:8) and transferred to the 1C4-coated plate. The presence of bound open ADAMTS13 was detected by addition of the biotinylated murine monoclonal antibody 3H917 (1.5 µg/mL), followed by HRP-labeled high-sensitivity streptavidin (1:10 000; Invitrogen, Carlsbad, CA). A twofold dilution series of NHP, preincubated with 2.5 µg/mL murine opening anti-CUB1 antibody 17G2,14,18 was added as a reference. The conformation index (CI) was calculated by normalizing the OD492 nm of the sample to the mean OD492 nm of the reference (ie, NHP [final dilution 1:8] preincubated with 2.5 µg/mL 17G2).15,16
Results and discussion
To investigate whether anti-CS or anti-CUB autoantibodies can disrupt the S-CUB interaction in folded ADAMTS13, we isolated anti-CS and anti-CUB autoantibodies from 13 patients with iTTP using CS- and CUB-affinity chromatography, respectively.
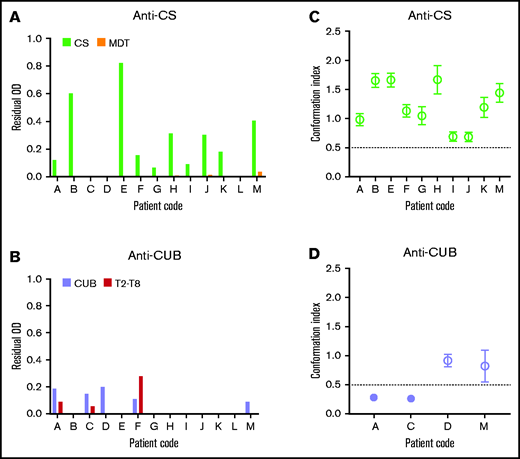
We first isolated the total IgGs from the plasma of 13 patients with iTTP. Next, IgGs were loaded on a CS- or CUB-coupled affinity column. Anti-CS and anti-CUB autoantibodies were successfully purified from 10 and 4 of the 13 patients, respectively (Table 1). Purified anti-CS and anti-CUB autoantibody fractions were specific for CS and CUB because they recognized CS and CUB, respectively, in ELISA, whereas little to no binding to MDT or T2-T8, respectively, was observed (Figure 1A-B). The anti-CUB autoantibody fraction from patient F, which also recognized T2-T8 well (Figure 1B), was considered not specific and was not tested further.
Overview of the purified anti-CS and anti-CUB autoantibodies from 13 iTTP patients
| Patient code . | ID . | Antibody concentration (µg/mL) . | |
|---|---|---|---|
| Anti-CS . | Anti-CUB . | ||
| A | MF-KB-TTP01-A | 64 | 108 |
| B | MF-KB-TTP02-A | 58 | |
| C | MF-KB-TTP03-A | 56 | |
| D | MF-KB-TTP05-A | 101 | |
| E | MF-KB-TTP09-A | 35 | |
| F | MF-KB-TTP10-A | 50 | 73* |
| G | MF-KB-TTP11-A | 114 | |
| H | MF-KB-TTP12-A | 124 | |
| I | MF-KB-TTP13-A | 75 | |
| J | MF-KB-TTP15-A | 30 | |
| K | MF-KB-TTP17-A | 51 | |
| L | MF-KB-TTP18-A | ||
| M | MF-KB-TTP20-A | 69 | 214 |
| Patient code . | ID . | Antibody concentration (µg/mL) . | |
|---|---|---|---|
| Anti-CS . | Anti-CUB . | ||
| A | MF-KB-TTP01-A | 64 | 108 |
| B | MF-KB-TTP02-A | 58 | |
| C | MF-KB-TTP03-A | 56 | |
| D | MF-KB-TTP05-A | 101 | |
| E | MF-KB-TTP09-A | 35 | |
| F | MF-KB-TTP10-A | 50 | 73* |
| G | MF-KB-TTP11-A | 114 | |
| H | MF-KB-TTP12-A | 124 | |
| I | MF-KB-TTP13-A | 75 | |
| J | MF-KB-TTP15-A | 30 | |
| K | MF-KB-TTP17-A | 51 | |
| L | MF-KB-TTP18-A | ||
| M | MF-KB-TTP20-A | 69 | 214 |
Patient code, patient ID, and the antibody concentration (µg/mL) of the anti-CS or anti-CUB autoantibodies after purification using CS- or CUB-affinity chromatography, respectively. Antibody concentrations were measured via spectrophotometry at optical density 280 nm (OD280 nm). Green or purple boxes indicate that autoantibodies were eluted from the CS- or CUB-affinity column, respectively, and that they consisted of anti-CS or anti-CUB autoantibodies because they showed specific binding to CS or CUB, respectively, in ELISA (Figure 1A-B), with the exception of the anti-CUB autoantibody fraction of patient F (*), which also recognized T2-T8 (Figure 1B). This anti-CUB autoantibody fraction was not studied further. Gray boxes indicate that no autoantibody was eluted from the CS- or CUB-affinity column (Figure 1A-B).
The presence of anti-CS autoantibodies that open ADAMTS13 is a common feature of the autoimmune response in patients with iTTP. (A-B) IgG fractions from 13 patients with acute iTTP were purified against CS (A) and CUB (B) using affinity chromatography. The specificity of the purified autoantibody fractions against CS (A) or CUB (B) was tested, using ELISA, on coated CS (light green) and MDT (orange) (A) and CUB (lavender) and T2-T8 (red) (B). Bound autoantibodies were detected using HRP-labeled polyclonal goat anti-human (Fc-specific) IgGs. Data are shown as the residual OD value that was calculated by subtracting the OD value of the sample on the fragment of interest (CS, MDT, CUB, or T2-T8), with the mean OD + 3 SD value of the binding of purified IgGs of 9 healthy donors to the corresponding fragment. Anti-CS (A) or anti-CUB (B) autoantibody fractions were used for further analysis if the residual OD value of the sample on CS(A, light green) or CUB (B, lavender), respectively, was positive, whereas the residual OD of binding to the other ADAMTS13 domains (MDT (A, orange) or T2-T8 (B, red)) was <50% of the binding to CS (A, light green) or CUB (B, lavender). Hence, only the anti-CUB autoantibody fraction from patient F was not used further. The anti-CS autoantibody fractions isolated from 10 patients (C) and the anti-CUB autoantibody fractions isolated from 4 patients (D) were individually preincubated with NHP containing closed ADAMTS13 and tested, using 1C4-ELISA, to investigate which autoantibodies could open ADAMTS13. Data are expressed as the mean CI ± SD (n = 3). A mean CI ≤ 0.50 (filled circle) represents closed ADAMTS13, whereas a mean CI > 0.50 (open circle) represents open ADAMTS13.15,16
The presence of anti-CS autoantibodies that open ADAMTS13 is a common feature of the autoimmune response in patients with iTTP. (A-B) IgG fractions from 13 patients with acute iTTP were purified against CS (A) and CUB (B) using affinity chromatography. The specificity of the purified autoantibody fractions against CS (A) or CUB (B) was tested, using ELISA, on coated CS (light green) and MDT (orange) (A) and CUB (lavender) and T2-T8 (red) (B). Bound autoantibodies were detected using HRP-labeled polyclonal goat anti-human (Fc-specific) IgGs. Data are shown as the residual OD value that was calculated by subtracting the OD value of the sample on the fragment of interest (CS, MDT, CUB, or T2-T8), with the mean OD + 3 SD value of the binding of purified IgGs of 9 healthy donors to the corresponding fragment. Anti-CS (A) or anti-CUB (B) autoantibody fractions were used for further analysis if the residual OD value of the sample on CS(A, light green) or CUB (B, lavender), respectively, was positive, whereas the residual OD of binding to the other ADAMTS13 domains (MDT (A, orange) or T2-T8 (B, red)) was <50% of the binding to CS (A, light green) or CUB (B, lavender). Hence, only the anti-CUB autoantibody fraction from patient F was not used further. The anti-CS autoantibody fractions isolated from 10 patients (C) and the anti-CUB autoantibody fractions isolated from 4 patients (D) were individually preincubated with NHP containing closed ADAMTS13 and tested, using 1C4-ELISA, to investigate which autoantibodies could open ADAMTS13. Data are expressed as the mean CI ± SD (n = 3). A mean CI ≤ 0.50 (filled circle) represents closed ADAMTS13, whereas a mean CI > 0.50 (open circle) represents open ADAMTS13.15,16
To investigate whether the purified anti-CS and anti-CUB autoantibodies can open the closed conformation of healthy donor ADAMTS13, purified anti-CS and anti-CUB autoantibodies were incubated with NHP, and the conformation of ADAMTS13 was tested in our ADAMTS13 conformation ELISA. Interestingly, the purified anti-CS autoantibody fraction from all patients opened ADAMTS13 (Figure 1C; 10/10). In contrast, only half of the purified anti-CUB autoantibody fractions opened ADAMTS13 (Figure 1D; 2/4).
Hence, our data show that the S-CUB interaction in folded ADAMTS13 can be disrupted by anti-CS and anti-CUB autoantibodies isolated from patients with iTTP, leading to exposure of the 1C4 cryptic epitope in the S domain. However, our data show that particularly anti-CS autoantibodies have the capacity to open ADAMTS13. This finding is in line with the observation that open ADAMTS13 is a biomarker for iTTP15,16 and that almost all patients with iTTP (80-90%) have anti-CS autoantibodies.3,7-11 Our finding that some purified anti-CUB autoantibody fractions could also open ADAMTS13 could explain the presence of open ADAMTS13 in the few patients with iTTP who had undetectable anti-CS autoantibodies.
In conclusion, we showed that all anti-CS autoantibodies and some anti-CUB autoantibodies isolated from patients with iTTP can open the ADAMTS13 conformation. The presence of anti-CS autoantibodies inducing an open ADAMTS13 conformation can be recognized as a common feature of the autoimmune response in iTTP.
Acknowledgments
This work was supported by The European Framework Program for Research and Innovation (Horizon2020 Marie Sklodowska Curie Innovative Training Network PROFILE Grant 675746) and Fonds voor Wetenschappelijk Onderzoek grant G090120N. A.C. received a travel grant from the Charles Nicolle Foundation.
Authorship
Contribution: L.D.W., E.R., and A.C. designed research, performed experiments, and analyzed data; L.D.W. and E.R. wrote the manuscript; K.K. provided recombinant ADAMTS13 fragments; E.T. and G.K. provided plasma samples from patients with iTTP; A.C., K.K., G.K., E.T., A.M., C.T., B.S.J., P.C., A.V., and S.F.D.M. provided helpful discussions and critically reviewed the manuscript; and K.V. supervised the study, designed research, analyzed data, and wrote and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karen Vanhoorelbeke, Laboratory for Thrombosis Research, IRF Life Sciences, KU Leuven Campus Kulak Kortrijk, Etienne Sabbelaan 53, 8500 Kortrijk, Belgium; e-mail: karen.vanhoorelbeke@kuleuven.be.
References
Author notes
Data sharing requests should be sent to Karen Vanhoorelbeke (karen.vanhoorelbeke@kuleuven.be).