Key Points
Baseline geriatric assessment measures are associated with survival among older AML patients treated with nonintensive chemotherapy.
Baseline global quality of life is associated with survival among older AML patients treated with nonintensive chemotherapy.
Abstract
Geriatric assessment (GA) predicts survival among older adults with acute myeloid leukemia (AML) treated intensively. We evaluated the predictive utility of GA among older adults treated with low-intensity therapy on a multisite trial. We conducted a companion study (CALGB 361101) to a randomized phase 2 trial (CALGB 11002) of adults ≥60 years and considered “unfit” for intensive therapy, testing the efficacy of adding bortezomib to decitabine therapy. On 361101, GA and quality of life (QOL) assessment was administered prior to treatment and every other subsequent cycle. Relationships between baseline GA and QOL measures with survival were evaluated using Kaplan-Meier estimation and Cox proportional hazards models. One-hundred sixty-five patients enrolled in CALGB 11002, and 96 (52%) of them also enrolled in 361101 (median age, 73.9 years). Among participants, 85.4% completed ≥1 baseline assessment. In multivariate analyses, greater comorbidity (hematopoietic cell transplantation-specific comorbidity index >3), worse cognition (Blessed Orientation-Memory-Concentration score >4), and lower European Organization for Research and Treatment of Cancer global QOL scores at baseline were significantly associated with shorter overall survival (P < .05 each) after adjustment for Karnofsky Performance Status, age, and treatment arm. Dependence in instrumental activities of daily living and cognitive impairment were associated with 6-month mortality (hazard ratio [HR], 3.5; confidence interval [CI], 1.2-10.4; and HR, 3.1; CI, 1.1-8.6, respectively). GA measures evaluating comorbidity, cognition, and self-reported function were associated with survival and represent candidate measures for screening older adults planned to receive lower-intensity AML therapies. This trial was registered at www.clinicaltrials.gov as #NCT01420926 (CALGB 11002).
Introduction
Acute myeloid leukemia (AML) is a disease most commonly diagnosed among older adults.1 Despite advances in therapy in recent years, outcomes remain poor.1 Clinical trial design and treatment decisions for older adults depend upon a determination of “fitness” or “unfitness” for therapy.2 Although treatment paradigms are shifting for older adults with recent drug approvals,3 a persistent gap in evidence is the lack of validated criteria for characterization of fitness or unfitness in the context of AML therapy. To date, chronologic age alone has remained a key criterion for determining “fitness.”3,4 Recent US Food and Drug Administration approvals for the use of venetoclax, ivosidinib, and glasdegib for AML define the eligible population by age >75 years or comorbidities that predict poor tolerance of intensive chemotherapy, and ivosidenib also used Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) ≥2 as a prerequisite for treatment of IDH1 mutant AML. Extensive research has shown that age and performance status are inadequate surrogates for fitness assessment for older adults.5-8 Identifying reliable criteria that enhance prediction of treatment tolerance and benefit in both the intensive and less-intensive settings is key for providing personalized care and advancing the field.
Geriatric assessment (GA) provides a standardized approach to assessing older adults who may have variable comorbidity, physical, and cognitive function despite the same chronologic age. GA is guideline recommended for older adults starting a new chemotherapy regimen8 because it can identify vulnerabilities not detected in routine oncologic care, predict treatment tolerance and benefit, and provide information that enhances communication and informs care.9,10
In the setting of AML, GA is feasible11,12 and provides information that informs prediction of survival and treatment tolerance.6,13-15 Among older adults treated intensively, GA measures made at diagnosis (ie, objectively measured physical function and cognition) and assessed at postremission evaluation (ie, objectively measured physical function and depressive symptoms) have been associated with survival. Measures that best discriminate tolerance to less-intensive therapies, however, may differ. In an observational study of older adults with AML and myelodysplastic syndrome treated nonintensively, dependence in activities of daily living, PS, and fatigue were independently associated with worse survival,14 leading to the development of a “fitness” score for testing in clinical trials.16 To date, there are no published studies utilizing geriatric assessment in therapeutic clinical trials testing lower-intensity therapies. Testing the utility of GA measures to predict treatment outcomes in the context of a nonintensively treated clinical trial populations is a next step to inform trial design and guide treatment decisions as well as improve supportive care for a growing proportion of older adults with AML.
Here, we report on an ancillary GA companion study to a phase 2 clinical trial that randomized older adults who were considered unfit for intensive chemotherapy to a 10-day decitabine regimen vs decitabine plus bortezomib (CALGB 11002).17 The objectives of the ancillary study were to (1) describe patient characteristics of older adults enrolled on 11002 using GA, (2) evaluate the association between GA measures and survival, and (3) explore the change in GA measures during treatment.
Methods
Cancer and Leukemia Group B (CALGB) 361101 was a prospective multi-site embedded companion study offered to patients enrolled on CALGB 11002 at the 18 participating CALGB institutions. CALGB 11002 was a phase 2 randomized study of adults 60 years of age or older with newly diagnosed AML, testing the safety and efficacy of adding bortezomib to a 10-day decitabine regimen vs decitabine alone, open for enrollment between 2011 and 2013.18 Treatment was administered in 28-day cycles; details were previously published.17 Any site participating in CALGB 11002 could enroll in 361001. This study, with the 361001 companion, was approved by the National Cancer Institute Central Institutional Review Board and by the institutional review board at each participating institution. CALGB is now part of the Alliance for Clinical Trials in Oncology.
Eligibility criteria
Patients were eligible to enroll in the GA companion study (CALGB 361101) if they met eligibility criteria for and enrolled in CALGB 11002. Briefly, eligibility criteria for the treatment and companion study included a new histologic diagnosis of AML (excluding acute promyelocytic leukemia) and age 60 years or older. Eligibility was not restricted by PS or organ function. Patients with FLT3 mutated or core binding factor AML were excluded unless aged ≥75 years and/or had an ECOG PS >2 or ejection fraction <40%.
Procedures
Before patients were enrolled in CALGB 361101, the study cochairs (H.D.K. and E.K.R.) trained research staff at each participating site (via telephone). Then, patients who were eligible to enroll in CALGB 11002 were offered the opportunity to enroll in CALGB 361101. All patients who chose to participate in the GA companion study (361101) completed institutional review board–approved, protocol-specific informed consent at the time of consent to the treatment trial. The GA and a quality-of-life questionnaire were completed at baseline (prior to initiation of chemotherapy) and after cycles 2, 4, 8, and 12, and then every 6 months while on study, either in the inpatient or outpatient setting. Patient registration, data collection, and statistical analyses were conducted by the Alliance Statistics and Data Management Center. Data quality was ensured by review of data by the Alliance Statistics and Data Management Center and by the study chairperson following Alliance policies. The study chair contacted site staff to obtain reasons for missing data when one was not provided.
Measures
The GA tool used in this study was developed for use in the National Clinical Trials Network (NCTN) setting, with a full description of measures previously reported.19,20 The GA includes validated measures assessing the domains of physical function, comorbid medical conditions, psychological state, social activities and support, nutritional status, cognitive function, and medications.19,20 Modifications to the GA tool for use in the AML setting included the addition of the Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI)21 and the substitution of the Mental Health Inventory-17 to assess psychological health.22 The same GA was used in a previously published companion study (CALGB 361006) that was open during the same time period for older adults enrolling on an intensive chemotherapy trial.12
The GA tool is organized into a health care provider (nurse or certified research associate)-administered assessment and a self-administered patient questionnaire. The health care provider–administered questionnaire included 5 brief assessments: (1) HCT-CI21,23,24 , (2) Karnofsky Performance Status (KPS), (3) Timed Up and Go (a performance based measure of physical function; time assessed in seconds for those who could complete the test or recorded as unable to perform)25 , (4) Blessed Orientation-Memory-Concentration test ([BOMC], in which higher scores indicate worse cognitive performance)26 , and (5) recording of height and weight (current and 6 months prior) to evaluate nutritional status including calculation of body mass index. Prior weight was collected by self-report if not recorded in the medical record.
The self-administered patient questionnaire included validated self-reported measures of physical function and activities (inclusive of activities of daily living, instrumental activities of daily living [IADLs], and mobility items), a patient-rated KPS, self-reported falls in the past 6 months, self-reported comorbid conditions and a rating of the degree to which each causes interference in activities, number and type of medications, assessment of psychological state (symptoms of anxiety and depression), social activity, and social support. A member of the health care team could assist those patients who needed help.
Health-related quality of life (QOL) was assessed using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30).27,28 Domains assessed included general physical symptoms, fatigue and malaise, and physical, social, and emotional functioning. Time points for assessment were the same as for the GA questionnaires.
Outcomes
Overall survival (OS) was estimated from the time of signing the consent form. Change in GA measures from baseline to the end of cycles 2 and 4 were evaluated.
Statistical analyses
Descriptive statistics were used to summarize GA measures at baseline and follow-up. Continuous variables were described by mean (standard deviation) and median (range) and categorical variables by frequency and percentage. Changes from baseline to follow-up time points were calculated using the Wilcoxon signed-rank test for continuous variables and McNemar’s test for categorical variables. Survival probabilities were estimated using the Kaplan-Meier estimator.29 Associations between baseline GA measures and OS were evaluated by comparing survival probabilities between groups (dichotomized by the median score or a clinically meaningful cutpoint if available) using the log-rank test. Cox proportional hazards models were used for multivariate survival analyses adjusting for age, KPS status at baseline, and treatment arm. All analyses were performed using SAS Version 9.3 (SAS Institute, Cary NC) and had a 2-sided α level of 0.05. Data lock for trial data were on 20January 2020. Due to the exploratory nature of this analysis, there was no adjustment for multiple comparisons.
Results
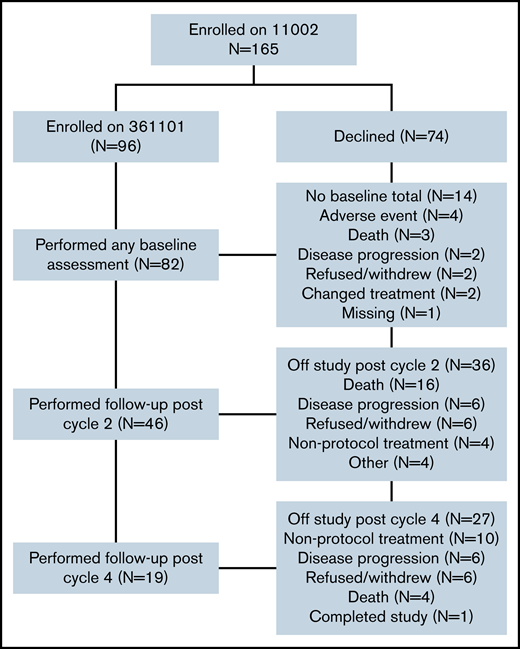
Among the 165 patients who enrolled in clinical trial CALGB 11002, 96 (52%) enrolled in the GA companion study (CALGB 361101), from 18 different institutions (Figure 1). Of these, 82 completed at least 1 baseline assessment (85%). Forty-six (48%) were assessed post–cycle 2, and 19 (20%) post–cycle 4. Most common reasons for attrition from enrollment through cycle 4 (N = 77) were death (N = 24), initiation of nonprotocol treatment (N = 20), disease progression (N = 15), and refusal of further study treatment (N = 13) (see Figure 1 for details).
Baseline characteristics are presented in Table 1. Nearly 65% of participants were aged 70 years and above (median age, 72.7; range, 60-92.4 years). Most were non-Hispanic, White males with ECOG PS 0-1 (74%). Mortality was 27% at 3 months and 43% at 6 months. Baseline demographics were similar to those published for the parent trial (CALGB 11002).17 Median OS was 8.0 months (95% confidence interval [CI], 0.0-72.1 months).
Baseline characteristics of older adults with AML on CALGB 361101 (Alliance) (n = 96)
| Baseline characteristics . | Median (range) or frequency (%) . |
|---|---|
| Demographics | |
| Age (years) median (range) | 72.7 (60.6-92.4) |
| % 60-64 | 14.6 |
| % 65-69 | 19.8 |
| % 70-74 | 24.0 |
| % 75-79 | 20.8 |
| % ≥80 | 20.8 |
| % female | 30.2 |
| % white | 90.6 |
| % non-Hispanic | 91.7 |
| % ECOG performance status ≤1 | 73.9 |
| Clinical onset of AML | |
| % De novo | 63.2 |
| % Therapy-related | 11.6 |
| % MDS/AHD | 25.2 |
| ELN classification | |
| % Normal | 27.4 |
| % Intermediate-II | 22.1 |
| % Adverse | 38.9 |
| % No diagnosis cytogenetics | 11.6 |
| Baseline characteristics . | Median (range) or frequency (%) . |
|---|---|
| Demographics | |
| Age (years) median (range) | 72.7 (60.6-92.4) |
| % 60-64 | 14.6 |
| % 65-69 | 19.8 |
| % 70-74 | 24.0 |
| % 75-79 | 20.8 |
| % ≥80 | 20.8 |
| % female | 30.2 |
| % white | 90.6 |
| % non-Hispanic | 91.7 |
| % ECOG performance status ≤1 | 73.9 |
| Clinical onset of AML | |
| % De novo | 63.2 |
| % Therapy-related | 11.6 |
| % MDS/AHD | 25.2 |
| ELN classification | |
| % Normal | 27.4 |
| % Intermediate-II | 22.1 |
| % Adverse | 38.9 |
| % No diagnosis cytogenetics | 11.6 |
AHD, antecedent hematologic disorder; AML, acute myeloid leukemia; ELN, European LeukemiaNet; MDS, myelodysplastic syndromes.
Table 2 describes GA measures and global QOL at baseline for the study cohort. Although the majority reported good performance status, mean scores on the self-reported daily activities and Timed Up and Go performance test indicate prevalent functional impairment. Notably, ∼1 in 5 was unable to perform the Timed Up and Go test, suggesting that the mean time underrepresents physical frailty in this population. Most participants screened negative for cognitive impairment, had no history of falls, had polypharmacy and moderate comorbidity, and reported adequate social support. Mean scores for global QOL were low. There were no significant differences in baseline GA scores or global EORTC QOL by treatment assignment (data not shown).
Baseline geriatric assessment and quality of life measures (n = 82)
| Domain . | Measure . | Range of scores . | N missing . | Mean ± SD median (range) . | N (%) impaired . |
|---|---|---|---|---|---|
| Functional status | Activities of daily living (subscale of MOS physical health) – impaired: <100 | 0-100 (higher score: better physical function) | 1 | 56.4 ± 29.9 55 (5-100) | 73 (90%) |
| IADL (subscale of the OARS) – impaired: <14 | 0-14 (higher score: less need for assistance) | 1 | 12.6 ± 2.3 14 (3-14) | 35 (43%) | |
| Karnofsky Self-Reported Performance Rating Scale – impaired: <80 | 40-100 (higher score: better function) | 2 | 78.1 ± 14.7 80 (40-100) | 33 (41%) | |
| Karnofsky Physician Performance Rating Scale – impaired: <80 | 0-100 (higher score: better function) | 2 | 79.9 ± 14.7 80 (50-100) | 50 (63%) | |
| Number of falls in last 6 mo – impaired: ≥1 | Higher score, worse performance | 9 | 0.4 ± 1.5 0 (0-12) | 13 (18%) | |
| Timed Up and Go (seconds) – impaired: ≥13.5 | Higher score: worse performance) | 23 | 15.9 ± 13.6 14 (6-110) | 30 (51%) | |
| Cognition | BOMC – ≥4 cognitive dysfunction ≥11 cut-point for dementia screen | 0-28 | 6 | 3.8 ± 4.6 2 (0-23) | ≥ 4: 30 (39%) ≥11: 5 (7%) |
| Comorbidity | OARS General Health Scale | 0-13 (higher score: greater comorbidity) | 0 | 2.5 ± 1.6 2.0 (0.0-8.0) | 1-2: 31 (38%) 3+: 40 (49%) |
| HCT-CI | 0-29 (higher score: greater comorbidity) | 1 | 2.9 ± 2.2 3.0 (0-10) | 1-2: 26 (32%) 3+: 41 (51%) | |
| Medications | Number of medications | Polypharmacy indicated by ≥ 5 medications | 10 | 5.9 ± 4.6 5.0 (0-22) | |
| Psychological state | Mental Health Inventory-17 | 0-100 (higher score: better mental health) | 2 | 76.4 ± 13.3 78.8 (37.6-94.1) | N/A |
| Social activity | MOS Social Activity Survey | 0-100 (higher score: better social activity) | 3 | 46.4 ± 21.9 41.7 (0-91.7) | N/A |
| Social support | MOS Social Support Survey: Emotional Information and Tangible Subscales | 0-100 (higher score: better social support) | 3 | 83.1 ± 26.4 93.8 (0-100) | N,A |
| Nutrition | Percent unintentional weight loss in last 6 mo | Higher indicates lower nutritional status | 12 | −3.3 ± 12.4 −1.2 (-56.8-18.6) | N/A |
| Body Mass Index | 10 | 29.8 ± 6.6 28.2 (19.8-60.5) | |||
| Global Quality of life | EORTC QLQ C30 Global | 0-100 (higher score: better QOL) | 7 | 56.7 ± 26.4 58.3 (0-100) | N/A |
| Domain . | Measure . | Range of scores . | N missing . | Mean ± SD median (range) . | N (%) impaired . |
|---|---|---|---|---|---|
| Functional status | Activities of daily living (subscale of MOS physical health) – impaired: <100 | 0-100 (higher score: better physical function) | 1 | 56.4 ± 29.9 55 (5-100) | 73 (90%) |
| IADL (subscale of the OARS) – impaired: <14 | 0-14 (higher score: less need for assistance) | 1 | 12.6 ± 2.3 14 (3-14) | 35 (43%) | |
| Karnofsky Self-Reported Performance Rating Scale – impaired: <80 | 40-100 (higher score: better function) | 2 | 78.1 ± 14.7 80 (40-100) | 33 (41%) | |
| Karnofsky Physician Performance Rating Scale – impaired: <80 | 0-100 (higher score: better function) | 2 | 79.9 ± 14.7 80 (50-100) | 50 (63%) | |
| Number of falls in last 6 mo – impaired: ≥1 | Higher score, worse performance | 9 | 0.4 ± 1.5 0 (0-12) | 13 (18%) | |
| Timed Up and Go (seconds) – impaired: ≥13.5 | Higher score: worse performance) | 23 | 15.9 ± 13.6 14 (6-110) | 30 (51%) | |
| Cognition | BOMC – ≥4 cognitive dysfunction ≥11 cut-point for dementia screen | 0-28 | 6 | 3.8 ± 4.6 2 (0-23) | ≥ 4: 30 (39%) ≥11: 5 (7%) |
| Comorbidity | OARS General Health Scale | 0-13 (higher score: greater comorbidity) | 0 | 2.5 ± 1.6 2.0 (0.0-8.0) | 1-2: 31 (38%) 3+: 40 (49%) |
| HCT-CI | 0-29 (higher score: greater comorbidity) | 1 | 2.9 ± 2.2 3.0 (0-10) | 1-2: 26 (32%) 3+: 41 (51%) | |
| Medications | Number of medications | Polypharmacy indicated by ≥ 5 medications | 10 | 5.9 ± 4.6 5.0 (0-22) | |
| Psychological state | Mental Health Inventory-17 | 0-100 (higher score: better mental health) | 2 | 76.4 ± 13.3 78.8 (37.6-94.1) | N/A |
| Social activity | MOS Social Activity Survey | 0-100 (higher score: better social activity) | 3 | 46.4 ± 21.9 41.7 (0-91.7) | N/A |
| Social support | MOS Social Support Survey: Emotional Information and Tangible Subscales | 0-100 (higher score: better social support) | 3 | 83.1 ± 26.4 93.8 (0-100) | N,A |
| Nutrition | Percent unintentional weight loss in last 6 mo | Higher indicates lower nutritional status | 12 | −3.3 ± 12.4 −1.2 (-56.8-18.6) | N/A |
| Body Mass Index | 10 | 29.8 ± 6.6 28.2 (19.8-60.5) | |||
| Global Quality of life | EORTC QLQ C30 Global | 0-100 (higher score: better QOL) | 7 | 56.7 ± 26.4 58.3 (0-100) | N/A |
MOS, Medical Outcomes Survey; N/A, not applicable (no consensus defined threshold for impairment); OARS, Older Americans Resources and Services; SD, standard deviation.
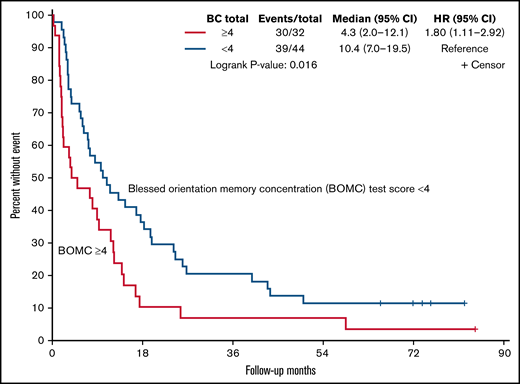
Baseline GA measures and global QOL were associated with survival, as reported in Table 3. Specifically, in univariate analysis dependence in IADLs, comorbidity (HCT-CI score ≥3), worse cognition (BOMC score ≥4), and lower EORTC global score were associated with inferior OS (P < .05 for each). For example, median OS was 10.4 months for those with better cognitive function (score <4) vs 4.3 months for those with a worse score on the cognitive screen (≥4, P = .02, Figure 2). After adjustment for age, physician-rated KPS, and treatment type, GA measures of comorbidity (HCT-CI) and cognition (BOMC ≥4) as well as EORTC global QOL remained independently associated with survival (P < .05 for each). When evaluating the relationship between baseline GA measures and QOL with 6-month mortality, only dependence in IADLs and cognitive impairment were associated in both univariate analysis and after adjustment for age, KPS, and treatment type (hazard ratio, 3.5; CI, 1.2-10.4; and hazard ratio, 3.1; CI, 1.1-8.6, respectively).
Multivariate analysis of baseline assessment, geriatric assessment measures, and mortality
| Characteristic . | Hazard ratio* (95% confidence interval) . | P value . |
|---|---|---|
| Mortality | ||
| HCT-CI | ||
| Reference: 0 | ||
| 1-2 | 1.8 (0.8-3.8) | .152 |
| ≥3 | 2.5 (1.2-5.2) | .018 |
| BOMC test score | ||
| Reference: < 4 | ||
| ≥4 | 1.7 (1-2.8) | .048 |
| EORTC global health score | 0.99 (0.9-1) | .050 |
| 6-Month Mortality (odds ratio) | ||
| IADL (subscale of the OARS) | ||
| Reference: 14 | ||
| <14 | 3.5 (1.2-10.4) | .025 |
| BOMC test score | ||
| Reference: < 4 | ||
| ≥4 | 3.14 (1.1-8.6) | .026 |
| Characteristic . | Hazard ratio* (95% confidence interval) . | P value . |
|---|---|---|
| Mortality | ||
| HCT-CI | ||
| Reference: 0 | ||
| 1-2 | 1.8 (0.8-3.8) | .152 |
| ≥3 | 2.5 (1.2-5.2) | .018 |
| BOMC test score | ||
| Reference: < 4 | ||
| ≥4 | 1.7 (1-2.8) | .048 |
| EORTC global health score | 0.99 (0.9-1) | .050 |
| 6-Month Mortality (odds ratio) | ||
| IADL (subscale of the OARS) | ||
| Reference: 14 | ||
| <14 | 3.5 (1.2-10.4) | .025 |
| BOMC test score | ||
| Reference: < 4 | ||
| ≥4 | 3.14 (1.1-8.6) | .026 |
OARS, Older Americans Resources and Services.
Adjusted for age, physician-rated Karnofsky Performance Status, and treatment arm.
Due to high attrition in this study, evaluation of change in GA measures and global QOL during treatment is limited. Here, we describe changes from baseline to first follow-up assessment (completion of 2 cycles of treatment; approximately 8 weeks). Analyses were stratified by treatment arm due to differential exposure to chemotherapeutic agents per protocol (supplemental Table 1). The only difference observed from baseline to first follow-up was a ∼20-point decline in the social activity scale reported for those enrolled on the combination therapy arm (P = .006). An increase in concomitant medication use was also noted in both arms (+1.5 decitabine vs +3.8 decitabine/bortezomib, P = .07 and .06, respectively). No changes in physical function, comorbidity, cognition, or emotional health were observed, although the follow-up sample was small due to attrition. EORTC Global QOL score was not different in either arm from baseline to post–cycle 2 assessment. Only 19 individuals remained on study after cycle 4, which precluded additional comparative analyses.
Discussion
This is the first multisite study to use GA to characterize older adults considered “unfit” for intensive chemotherapy on an NCTN AML treatment trial. There are several key take-home points from this study. First, use of validated GA measures enhances characterization of vulnerabilities such as high comorbidity burden and functional limitations that are not otherwise captured in routine clinical trial data collection. Second, GA measures, specifically comorbidity burden, self-reported physical limitations, and cognition, are independently associated with survival. Finally, this study highlights the challenges of attempting to characterize the impact of treatment on functional outcomes and QOL due to high attrition associated with morbidity, refractory disease, and early mortality.
The results of this study demonstrating the utility of GA to enhance characterization of older adults are consistent with a growing body of literature in both hematologic and nonhematologic malignancies.8,10 In the setting of AML, studies have shown that a GA performed at the time of initial treatment evaluation can provide information not routinely captured in oncology care in both the intensive and less-intensive treatment settings.6,11,12,14 This information is important for interpreting study results and understanding how to extrapolate results to patients in clinical practice. Comparing scores on GA measures collected in this study with identical measures collected in a published trial of intensively treated older adults,12 subjects on this study had a higher degree of comorbidity and lower physical function at baseline. Results from the GA confirm the study intent to accrue a “less fit” population.
Our study adds to the literature by providing evidence that GA measures can inform prediction of survival for older adults treated less intensively on a clinical trial. The measures associated with OS in this study, after accounting for age, performance status, and treatment type, were comorbidity burden, cognitive function, and global QOL. Comorbidity burden has been associated with survival in multiple other studies, although results in the literature are inconsistent, particularly when applied in an intensively treated patient cohort.6,14,30 -32 In this study, older adults with significant comorbidity were eligible and did enroll, as evidenced by the higher scores at baseline. Those patients are at higher risk of complications during treatment, likely reflecting the lower survival.
An important finding in this study is the relationship between cognitive function and survival. Evidence is emerging regarding the importance of cognitive function as a risk factor for shorter survival among older adults receiving intensive AML chemotherapy or stem cell transplantation.6,33,34 Varied cognitive measurements were used, but results are consistent. In a single-site cohort study of intensively treated older adults, those with impaired cognition, measured by the Modified Mini-Mental State Exam, had a 2.5-fold higher risk of death than those without impaired cognitive function in multivariate analysis.6 In a study using GA prior to hematopoietic cell transplantation, 330 subjects were included, with a median age of 63, and 17% were found to have cognitive dysfunction using the BOMC, with scores >7. In multivariate analysis, impaired cognition (BOMC ≥7) was independently associated with 1-year nonrelapse mortality and conferred an inferior 1-year OS.34 In a preplanned subgroup analysis in 224 patients aged >60 years, cognitive impairment remained the sole GA metric predictive of nonrelapse mortality.34 In our study, a lower threshold was used to define impairment (BOMC ≥4), based on evidence that this threshold is associated with chemotherapy toxicity among older adults.35 Our data are novel in 2 ways. First, our data suggest that milder cognitive dysfunction can be an independent predictor of poor outcome in AML patients because the threshold for impairment that was associated with survival in this study was lower than the threshold used clinically to trigger dementia screening. Second, our findings indicate that cognitive dysfunction is a relevant risk factor for shorter survival regardless of intensity of therapy planned. Our observations showing an association between cognitive function and both 6-month mortality and OS suggest that the presence of cognitive dysfunction at baseline may increase short-term toxicity and longer-term treatment tolerance. Further studies are need to validate these findings and elucidate mechanisms underlying these associations.
Self-reported physical function has been identified as a risk factor for worse OS in studies of older adults with hematologic and nonhematologic conditions.7,8,14,36 Among studies investigating AML treatment specifically, requiring assistance with activities of daily living has been associated with worse survival in a cohort of nonintensively treated patients.14 In the current analysis, requiring assistance with IADLs was associated with increased 6-month mortality. Functional limitations may be a consequence of the burden of disease, preleukemia functional impairment, or both. Regardless of etiology, impaired physical function increases risk of complications, which may be most evident earlier in the treatment course.
We evaluated health-related QOL along with GA measures in this study and found that lower baseline global QOL was also negatively associated with survival. QOL assessment provides complementary information to GA measures and may better reflect symptom burden at the time of treatment initiation.12 Other studies have shown that QOL measures assessing symptoms such as fatigue at baseline identify older adults at risk for worse outcomes during treatment.14,37 These data highlight the importance of capturing patient-reported outcomes at baseline to identify populations who may benefit from more aggressive supportive care during treatment.
In designing this study, we hoped to characterize the changes in GA measures and QOL during treatment among older AML patients treated less intensively. Understanding how patients feel and function during therapy is particularly important given the chronicity of treatment with hypomethylating agents and the importance of comparing functional survivorship between treatments. Although a baseline assessment was collected in the majority of patients, slightly less than half (48%) were assessed after cycle 2, and only a minority of patients were on study for assessment after cycle 4; the primary reasons were morbidity and mortality during this 4-month time period. Although the objective response rate of the parent trial was 39% (higher than in other multicenter trials of decitabine treatment), the median OS was 9.3 months. Conducting serial GA and QOL assessments in this sicker, multimorbid, high-risk population proved challenging. Those patients who provided data were likely very different from the informative missing majority. Although this challenge is not unique to this setting, it is more dramatic given the poor outcomes overall seen in this patient population. This experience suggests that future attempts to incorporate serial GA and QOL in this population should use additional strategies such as remote data collection, more frequent but shorter assessments, and end-of-study assessment to minimize missing data for those who went off study for progression or toxicity.
Strengths of this study include the use of a validated GA in a multisite NCTN trial controlling for treatment received. This is the largest published study evaluating GA in a therapeutic treatment trial testing lesser intensive therapies and was conducted at multiple sites, which enhances generalizability. This is one of the first reports to comprehensively characterize older adults treated less intensively with a complete GA. There were also several limitations to this study. One was that the GA was an optional substudy to the treatment study, and not all subjects participated. It is possible that those who consented to the GA may not have been representative of the group of AML patients as a whole; these patients may have differed in their physical or cognitive health. Study participants were mainly non-Hispanic White males; women and persons of color were underrepresented. Follow-up geriatric assessment was collected in person only for those who remained on study, resulting in a high proportion of missing follow-up data given the high morbidity and mortality rate in this study. The study sample size and attrition limited power to detect differences between treatment arms and change over time. Larger studies are also warranted to confirm observed associations between GA measures and survival and explore optimal cut-points for “impairment.” It is possible that findings may differ in clinical trials testing new low-intensity treatment regimens and warrant further study in these settings.
Despite limitations, this study achieved important objectives. Specifically, this is one of the first studies to show the utility of GA to characterize the heterogeneity of patients considered “unfit” for intensive therapy in the context of a therapeutic trial. Importantly, several brief GA measures including comorbidity, cognitive function, and self-reported functional limitations were associated with survival. Taken together with published studies in both intensive and nonintensive settings, these data support the inclusion of these core geriatric measures in pretreatment assessment and in future trial design to support treatment selection and inform targeted interventions to improve outcomes for older adults. Use of GA can guide treatment decisions to avoid “over- or under-treatment” for older adults with AML.38 Future studies evaluating the relationship between GA measures, disease burden, and toxicity outcomes will further inform management.
Acknowledgments
The following institutional networks participated in this study: Cancer Research Consortium of West Michigan NCORP, Grand Rapids, MI; Kathleen Yost, UG1CA189971, Dana-Farber/Partners Cancer Care LAPS, Boston, MA; Harold Burstein, UG1CA233180, Dartmouth College - Norris Cotton Cancer Center LAPS, Lebanon, NH; Konstantin Dragnev, UG1CA233323, Mount Sinai Hospital, New York, NY; Lewis Silverman, NYP/Weill Cornell Medical Center, New York, NY; Scott Tagawa, National Capital Area Minority Underserved NCORP, Washington, DC; Marcus Noel, UG1CA239758, NorthShore University Health System-Evanston Hospital, Evanston, IL; David Grinblatt, Northwell Health NCORP, Lake Success, NY; Jonathan Kolitz, The Ohio State University Comprehensive Cancer Center LAPS, Columbus, OH; Claire Verschraegen, UG1CA233331, Roswell Park Cancer Institute LAPS, Buffalo, NY; Ellis Levine UG1CA233191, Southeast Cancer Control Consortium CCOP, UNC Lineberger Comprehensive Cancer Center LAPS, Chapel Hill, NC; Matthew Milowsky, UG1CA233373, University of Chicago Comprehensive Cancer Center LAPS, Chicago, IL; Hedy Kindler, UG1CA233327, University of Iowa/Holden Comprehensive Cancer Center, Iowa City, IA; Umar Farooq, University of Missouri; Ellis Fischel, Columbia, MO; Puja Nistala, and Wake Forest University Health Sciences, Winston-Salem, NC, H.D.K.
Research reported in this publication was supported by the National Cancer Institute, National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), UG1CA233180, UG1CA233327, UG1CA233331, UG1CA189823, U10CA180833, U10CA180836, U10CA180850, U10CA180857, U10CA180867, and U10CA180888 and U10CA180819 (SWOG). Support to Alliance for Clinical Trials in Oncology and Alliance Foundation Trials programs is listed at https://acknowledgements.alliancefound.org. H.D.K. was funded by the American Society of Hematology (ASH); Association of Specialty Professors (ASP) Junior Faculty Scholar Award in Clinical and Translational Research (supported by ASH, Atlantic Philanthropies, John A. Hartford Foundation, Association of Specialty Professors); the Wake Forest University Claude D. Pepper Older Americans Independence Center (P30 AG-021332); Paul Beeson Career Development Award in Aging Research (K23AG038361, supported by National Institute on Aging, American Federation for Aging Research, John A. Hartford Foundation, and Atlantic Philanthropies); the Gabrielle’s Angel Foundation for Cancer Research; and also supported in part by Millennium.
Authorship
Contribution: H.D.K. and E.K.R. designed the research, performed research, interpreted analyses, and wrote and edited the manuscript; E.S., B.M., and J.L.R. conducted and interpreted analyses and edited the manuscript; and B.P., M.W., A.W., R.A.L., and G.J.R. performed research, interpreted analyses, and edited the manuscript.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Heidi D. Klepin, Section on Hematology and Oncology, Medical Center Blvd, Wake Forest School of Medicine, Winston-Salem, NC 27157; e-mail: hklepin@wakehealth.edu.
References
Author notes
E.K.R. and H.D.K contributed equally to this study.
For data sharing, send correspondence to the Alliance for Clinical Trials in Oncology publications team (publications@alliancenctn.org).
The full-text version of this article contains a data supplement.